Abstract
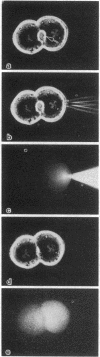
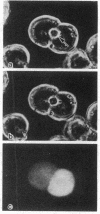
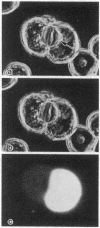
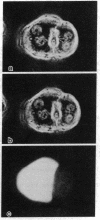
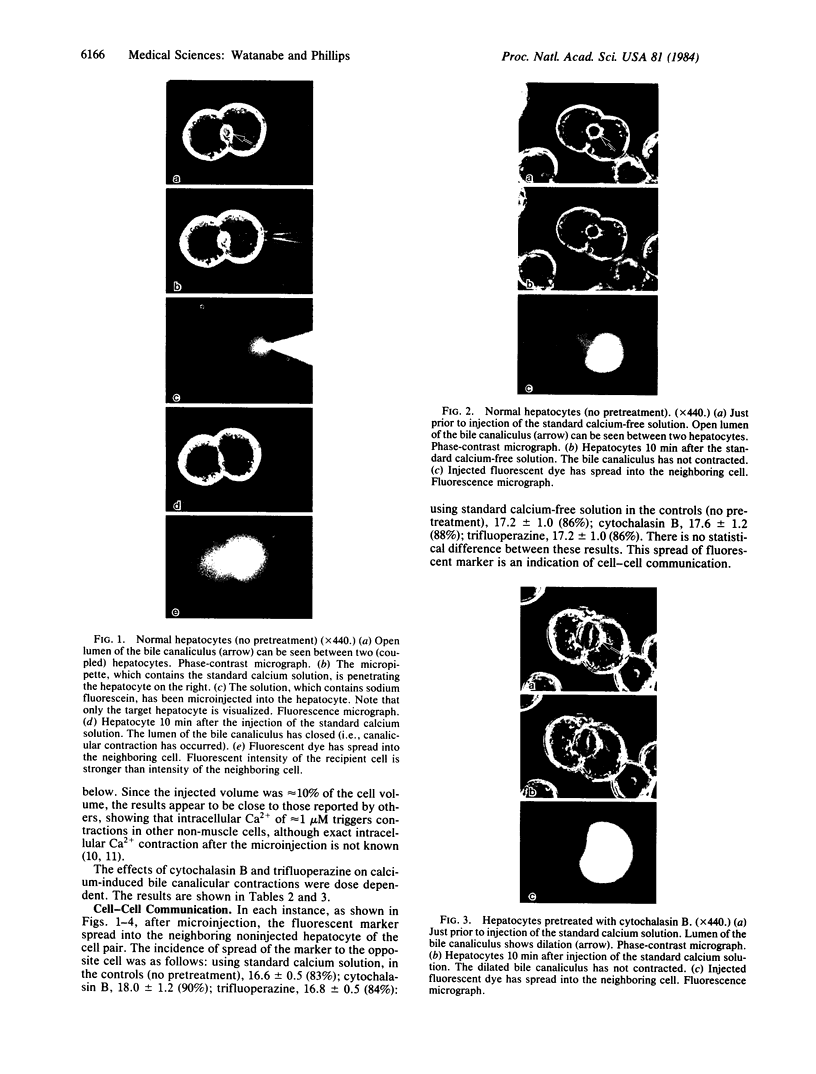
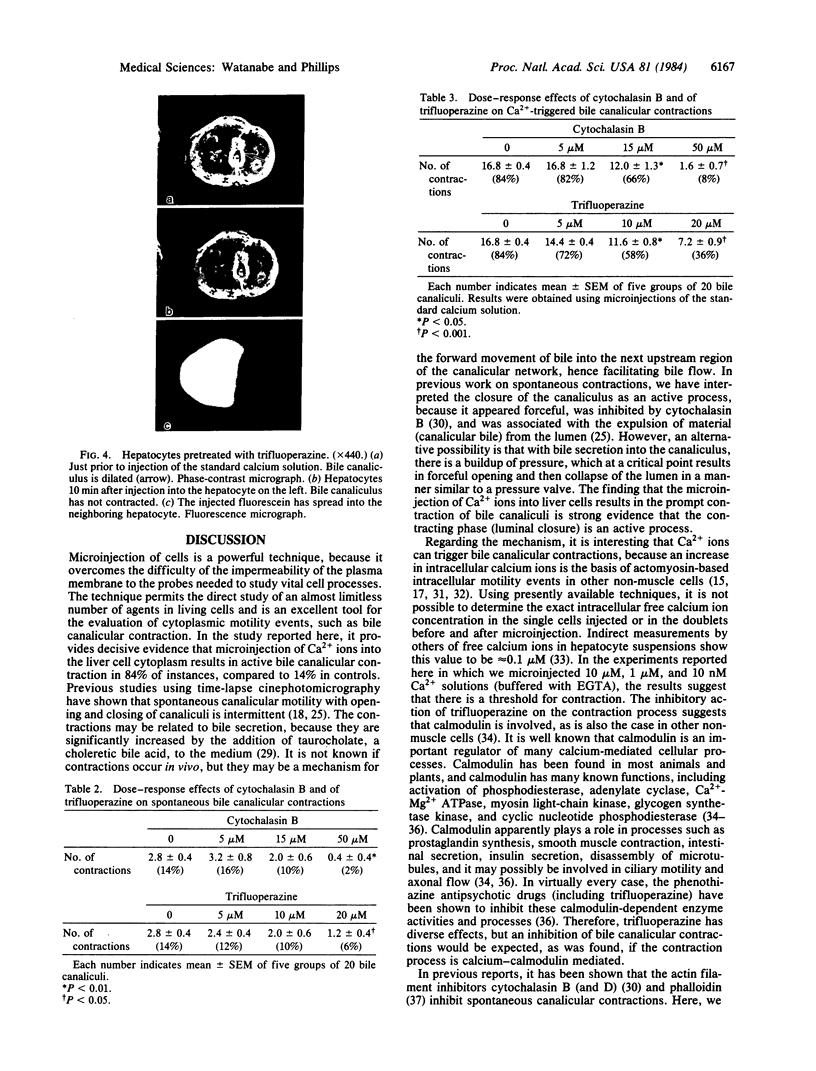
Cytoplasmic microinjection of Ca2+ triggers contraction of bile canaliculi in freshly isolated monolayer cultures of rat hepatocytes. Unseparated paired hepatocytes were used to demonstrate this motility-based phenomenon. Only one cell of the pair was injected, but fluorescein spread from the target cell to the opposite cell; also, the contractions were always uniform, equally involving both hepatocytes that form the canaliculus, indicating that communication exists between the cell pairs. Inhibitors of calmodulin and actin filaments, trifluoperazine and cytochalasin B, respectively, inhibited the Ca2+-induced contractions. Hence, the mechanism of contraction has features in common with actin-myosin based cytoplasmic motility behavior found in other non-muscle cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Sellers J. R., Conti M. A., Pato M. D., de Lanerolle P. Regulation of smooth muscle contractile proteins by calmodulin and cyclic AMP. Fed Proc. 1982 Oct;41(12):2873–2878. [PubMed] [Google Scholar]
- Brandon D. L. The identification of myosin in rabbit hepatocytes. Eur J Biochem. 1976 May 17;65(1):139–146. doi: 10.1111/j.1432-1033.1976.tb10398.x. [DOI] [PubMed] [Google Scholar]
- Broschat K. O., Stidwill R. P., Burgess D. R. Phosphorylation controls brush border motility by regulating myosin structure and association with the cytoskeleton. Cell. 1983 Dec;35(2 Pt 1):561–571. doi: 10.1016/0092-8674(83)90190-3. [DOI] [PubMed] [Google Scholar]
- Burgess D. R. Reactivation of intestinal epithelial cell brush border motility: ATP-dependent contraction via a terminal web contractile ring. J Cell Biol. 1982 Dec;95(3):853–863. doi: 10.1083/jcb.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Elias E., Hruban Z., Wade J. B., Boyer J. L. Phalloidin-induced cholestasis: a microfilament-mediated change in junctional complex permeability. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2229–2233. doi: 10.1073/pnas.77.4.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French S. W., Davies P. L. Ultrastructural localization of actin-like filaments in rat hepatocytes. Gastroenterology. 1975 Apr;68(4 Pt 1):765–774. [PubMed] [Google Scholar]
- Gabbiani G., Montesano R., Tuchweber B., Salas M., Orci L. Phalloidin-induced hyperplasia of actin filaments in rat hepatocytes. Lab Invest. 1975 Nov;33(5):562–569. [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANZON V. Liver cell secretion under normal and pathologic conditions studied by fluorescence microscopy on living rats. Acta Physiol Scand Suppl. 1952;28(101):1–268. [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T. C., 3rd, Mooseker M. S. Ca++-calmodulin-dependent phosphorylation of myosin, and its role in brush border contraction in vitro. J Cell Biol. 1982 Dec;95(3):943–959. doi: 10.1083/jcb.95.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Laishes B. A., Williams G. M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. 1. The effect of insulin. In Vitro. 1976 Jul;12(7):521–532. doi: 10.1007/BF02796495. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Mechanism of action of cytochalasin B on actin. Cell. 1980 Jun;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. Structure and function of the brush-border cytoskeleton. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):845–854. doi: 10.1101/sqb.1982.046.01.079. [DOI] [PubMed] [Google Scholar]
- Mooseker M. S., Bonder E. M., Grimwade B. G., Howe C. L., Keller T. C., 3rd, Wasserman R. H., Wharton K. A. Regulation of contractility, cytoskeletal structure, and filament assembly in the brush border of intestinal epithelial cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):855–870. doi: 10.1101/sqb.1982.046.01.080. [DOI] [PubMed] [Google Scholar]
- Oda M., Price V. M., Fisher M. M., Phillips M. J. Ultrastructure of bile canaliculi, with special reference to the surface coat and the pericanalicular web. Lab Invest. 1974 Oct;31(4):314–323. [PubMed] [Google Scholar]
- Osborn M., Weber K. Damage of cellular functions by trifluoperazine, a calmodulin-specific drug. Exp Cell Res. 1980 Dec;130(2):484–488. doi: 10.1016/0014-4827(80)90033-6. [DOI] [PubMed] [Google Scholar]
- Oshio C., Phillips M. J. Contractility of bile canaliculi: implications for liver function. Science. 1981 May 29;212(4498):1041–1042. doi: 10.1126/science.7015506. [DOI] [PubMed] [Google Scholar]
- Phillips M. J., Oshio C., Miyairi M., Katz H., Smith C. R. A study of bile canalicular contractions in isolated hepatocytes. Hepatology. 1982 Nov-Dec;2(6):763–768. doi: 10.1002/hep.1840020603. [DOI] [PubMed] [Google Scholar]
- Phillips M. J., Oshio C., Miyairi M., Smith C. R. Intrahepatic cholestasis as a canalicular motility disorder. Evidence using cytochalasin. Lab Invest. 1983 Feb;48(2):205–211. [PubMed] [Google Scholar]
- Pollard T. D. Cytoplasmic contractile proteins. J Cell Biol. 1981 Dec;91(3 Pt 2):156s–165s. doi: 10.1083/jcb.91.3.156s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature. 1975 Mar 20;254(5497):250–252. doi: 10.1038/254250a0. [DOI] [PubMed] [Google Scholar]
- Simons T. J. A method for estimating free Ca within human red blood cells, with an application to the study of their Ca-dependent K permeability. J Membr Biol. 1982;66(3):235–247. doi: 10.1007/BF01868498. [DOI] [PubMed] [Google Scholar]
- Stockem W., Weber K., Wehland J. The influence of microinjected phalloidin on locomotion, protoplasmic streaming and cytoplasmic organization in Amoeba proteus and Physarum polycephalum. Cytobiologie. 1978 Oct;18(1):114–131. [PubMed] [Google Scholar]
- Toh B. H., Yildiz A., Sotelo J., Osung O., Holborow E. J., Fairfax A. Distribution of actin and myosin in muscle and non-muscle cells. Cell Tissue Res. 1979 Jun 8;199(1):117–126. doi: 10.1007/BF00237731. [DOI] [PubMed] [Google Scholar]
- Tonelli Q. J., Sorof S. Epidermal growth factor requirement for development of cultured mammary gland. Nature. 1980 May 22;285(5762):250–252. doi: 10.1038/285250a0. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Miyairi M., Oshio C., Smith C. R., Phillips M. J. Phalloidin alters bile canalicular contractility in primary monolayer cultures of rat liver. Gastroenterology. 1983 Aug;85(2):245–253. [PubMed] [Google Scholar]
- Weiss B., Prozialeck W., Cimino M., Barnette M. S., Wallace T. L. Pharmacological regulation of calmodulin. Ann N Y Acad Sci. 1980;356:319–345. doi: 10.1111/j.1749-6632.1980.tb29621.x. [DOI] [PubMed] [Google Scholar]
- Yerna M. J., Dabrowska R., Hartshorne D. J., Goldman R. D. Calcium-sensitive regulation of actin-myosin interactions in baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):184–188. doi: 10.1073/pnas.76.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef I. M., Murray R. K. Studies on the preparation of rat liver plasma membrane fractions and on their polypeptide patterns. Can J Biochem. 1978 Jul;56(7):713–721. doi: 10.1139/o78-107. [DOI] [PubMed] [Google Scholar]