Significance
Whether breast cancer will respond to the antiestrogen tamoxifen is determined by whether cellular proliferation is estrogen receptor (ER) α-mediated. As opposed to early ductal cancer, which is an ERα-rich, proliferating disease, in early lobular cancer both ERα and ERβ are abundantly expressed and proliferation is rare. In advanced lobular cancer, ERβ is lost, ERα is retained, and proliferation is high. Thus, tamoxifen may be an effective pharmaceutical in late but not early lobular cancer.
Keywords: ductal carcinoma in situ, invasive ductal carcinoma, invasive lobular carcinoma
Abstract
The role of estrogen receptor (ER) α as a target in treatment of breast cancer is clear, but those of ERβ1 and ERβ2 in the breast remain unclear. We have examined expression of all three receptors in surgically excised breast samples from two archives: (i): 187 invasive ductal breast cancer from a Japanese study; and (ii) 20 lobular and 24 ductal cancers from the Imperial College. Samples contained normal areas, areas of hyperplasia, and in situ and invasive cancer. In the normal areas, ERα was expressed in not more than 10% of epithelium, whereas approximately 80% of epithelial cells expressed ERβ. We found that whereas ductal cancer is a highly proliferative, ERα-positive, ERβ-negative disease, lobular cancer expresses both ERα and ERβ but with very few Ki67-positive cells. ERβ2 was expressed in 32% of the ductal cancers, of which 83% were postmenopausal. In all ERβ2-positive cancers the interductal space was filled with dense collagen, and cell nuclei expressed hypoxia-inducible factor 1α. ERβ2 expression was not confined to malignant cells but was strong in stromal, immune, and endothelial cells. In most of the high-grade invasive ductal cancers neither ERα nor ERβ was expressed, but in the high-grade lobular cancer ERβ was lost and ERα and Ki67 expression were abundant. The data show a clear difference in ER expression between lobular and ductal breast cancer and suggest (i) that tamoxifen may be more effective in late than in early lobular cancer and (ii) a potential role for ERβ agonists in preventing in situ ductal cancers from becoming invasive.
Despite decades of research, the etiology of breast cancer remains unclear. It is currently thought that most breast cancers occur in the normal terminal duct lobular unit and progress in a stepwise fashion over time (1). Ductal carcinoma in situ (DCIS) means the cancer has not spread beyond the duct into any normal surrounding breast tissue and is thought by some to be the direct precursor of invasive ductal carcinoma (IDC).
Estrogens play an important role in normal breast development as well as breast cancer progression (2). Most of the effects of estrogen are mediated through its two receptors: estrogen receptor α (ERα) and β (ERβ) (3). ERα is expressed in 50–80% of breast tumors, and its presence is the main indicator for antihormonal therapy (4). ERβ was first discovered in 1996, and its role in breast cancer is still being explored (5–7).
The first step in understanding the role of ERβ in breast cancer was to define the expression pattern of ERβ in the normal human breast and in various stages of cancer. Since its discovery, several laboratories have reported ERβ expression in clinical samples (8–28). Most of these studies investigated the expression of ERβ in invasive breast cancer samples (12–15, 17, 19, 21–23). Some studies have reported ERβ expression in invasive breast cancer and normal breast tissue (11, 18, 26–28), but few have compared the expression of ERβ in the normal tissue, DCIS, and IDC within the same sample. Usually tumor samples are taken from one patient and normal tissue from another patient (8–10). Samples taken from different patients have intrinsic limitation (i.e., they cannot account for variations between different patients). In addition, because tumors are heterogeneous, core biopsies do not fully reflect the histological and biological diversity of breast tumors (29).
The roles of ERβ1 and its splice variant ERβ2 in breast cancer are still unclear. As reviewed by Murphy and Leygue (30), some studies show a loss of ERβ1 as ductal cancer progresses, but others do not. Some studies show ERβ2 as a marker of bad prognosis (31), and others not (19). Some of these differences may be due to differences in antibody use and differences in tissue fixation and handling.
When ERα and ERβ are coexpressed in breast cancer it is unclear whether tamoxifen treatment will be successful. This is because tamoxifen acts as an agonist of ERβ at activator protein 1 (AP-1) sites (32) and thus should oppose the antiproliferative effects of the tamoxifen–ERα complex. Yan et al. (33) have found that expression of ERβ predicts tamoxifen benefit in patients with ERα-negative early breast cancer, whereas Esslimani-Sahla et al (23) have found that low ERβ level is an independent marker, better than ERα level, to predict tamoxifen resistance. Although apparently saying different things, these two results actually agree with each other: in ERα-negative breast cancer, estrogen is not driving proliferation, so tamoxifen via ERβ may interfere with another growth signaling pathway. In ERα-positive cancers whose proliferation is driven by E2, tamoxifen with ERβ would oppose the antiproliferative effects of the ERα–tamoxifen complex.
Investigation of the expression pattern of ERβ in normal tissue, DCIS, and IDC is important to understand the function of this receptor in the progression of breast cancer. We have a set of samples obtained from surgical excision of breast tumors from women before pharmacological intervention. The cohorts include lobular cancer, which has not yet been thoroughly studied for ERβ expression. Lobular cancer is an ERα-positive form of breast cancer characterized by loss of E-cadherin and relatively low proliferation rate. It is accompanied by a resistance to anoikis (34). It accounts for 10–15% of diagnosed breast cancer, and there are still many questions about the optimal therapeutic approach to this cancer. We have explored the changes in expression of the two ERs using identical protocols and reagents in different developmental stages of breast cancer within each patient.
Results
Comparison of ERα and ERβ in Normal Breast Epithelium, DCIS, and IDC.
The ERβ antibody for the immunohistochemistry is ERβ 503 IgY antibody, which has been used previously (35). Representative staining is shown in Fig. 1A. There were 94 patients who had both normal tissue and DCIS, 115 patients in whose sections there was both DCIS and IDC, and 94 patient samples in which there was normal breast tissue, DCIS, and IDC.
Fig. 1.
Expression of ERα and ERβ in normal tissue, DCIS, and IDC. (A) Representative immunohistochemical staining of ERα and ERβ in normal breast tissue, DCIS, and IDC. (Scale bars, 25 µm.) (B) Box chart of Allred scores of ERα and ERβ (n = 94) staining. Bottoms and tops of the boxes are the 25th and 75th percentiles, respectively; the lines across the boxes are the median values; the ends of the whiskers represent 10th and 90th percentiles; asterisks represent the minimum and maximum of all of the data (*P < 0.0001). (C) Box chart of Allred scores of ERα and ERβ staining (n = 115). Description of box chart is the same as in B (*P < 0.0001, **P = 0.01). (D) Box chart of Allred scores of ERα and ERβ (n = 94) staining. Description of the box chart is the same as in B (*P < 0.0001, **P = 0.03).
In normal breast tissue there was robust expression of ERα in approximately 10% of epithelial cells (Fig. 1 A, a), whereas ERβ was expressed in more than 70% of epithelial cells with intermediate to strong signals (Fig. 1 A, d). In DCIS the percentage of ERα-positive cells was markedly increased (Fig. 1 A, b), whereas the percentage of ERβ-positive cells was significantly decreased (Fig. 1 A, e). Using the Allred score, we quantified the staining of the two ERs and made a box chart. Statistical analysis showed that the changes in expression levels of ERα and ERβ, which occur in the transition between normal epithelium and DCIS, were significant (Fig. 1B, Left and Right, *P < 0.0001).
Progression from in situ to invasive carcinoma is the first step in malignancy. As shown in Fig. 1 A, b and e, ERα expression was high and the ERβ level was low in DCIS, as assessed by both the percentage of positively staining cells and the staining intensity. On average, the 115 patients had mean Allred score values of 4.7 for ERα and 1.1 for ERβ in DCIS (Fig. 1C). In the invasive carcinoma, ERα expression was reduced and ERβ was completely lost in most of the samples (Fig. 1 A, c and f). The mean values for ERα were 2.9 and 0.59 for ERβ (Fig. 1C). These changes were statistically significant as lesions progressed from DCIS to IDC (Fig. 1C, Left, *P < 0.0001; Fig. 1C, Right, **P = 0.01).
In the 94 patient samples containing normal breast tissue, DCIS, and IDC, the mean values for ERα in normal epithelium, DCIS, and IDC were 2.7, 4.5, and 2.2, respectively (Fig. 1D), whereas the mean values for ERβ in normal epithelium, DCIS, and IDC were 3.9, 1.2, and 0.58, respectively (Fig. 1D). These differences in expression of ERs between the different regions were statistically significant (Fig. 1D, *P < 0.0001 and **P = 0.03).
Correlation Between ERs with Clinical and Tumor Characteristics.
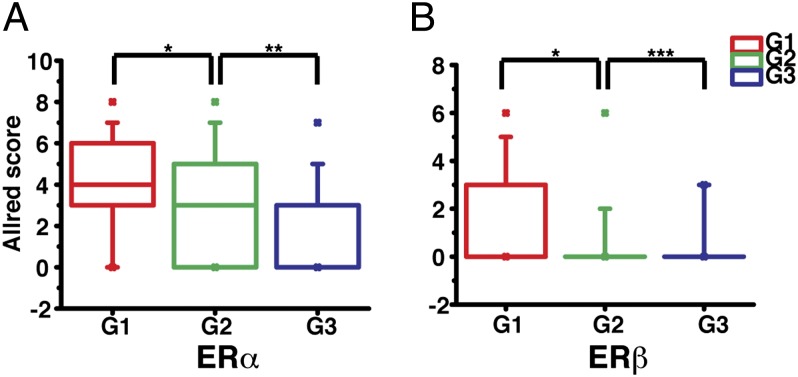
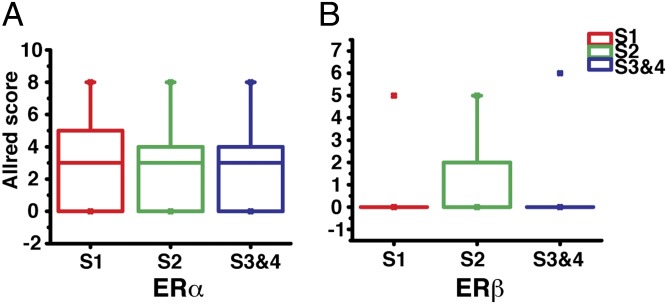
The histological grade of breast cancer is an important prognostic index and it is therefore used as one of the parameters to develop an individual treatment strategy. Using a three-tier grading system, the 187 patients included 39 grade 1, 111 grade 2, and 37 grade 3 (Table 1). We found that both ERα and ERβ expression levels were higher in grade 1 than in grade 2 invasive breast cancer (Fig. 2, *P < 0.001). In grade 3 IDC ERα expression was even lower than in grade 2 (Fig. 2, **P < 0.05), but ERβ was barely detected. Thus, it seems that ERα may influence both low- and high-grade breast cancers, but ERβ may only influence early development of breast cancer. There were 20 IDC samples that were both ERα and ERβ positive, but the two receptors were not colocalized in any nuclei. We also compared the expression of ERα and ERβ in different cancer stages. Using the American Joint Committee on Cancer TNM system, the 187 samples contained 111 stage 1, 45 stage 2, 14 stage 3, 7 stage 4, and 10 at undetermined stage. Statistical analysis showed that there are no significant differences of ERα and ERβ expression between different cancer stages (Fig. 3).
Table 1.
Clinicopathological characteristics of 187 patients and primary ductal carcinomas
| Characteristic | Patients, n (%)* |
| Menopausal status | |
| Premenopausal | 20 (10.7) |
| Postmenopausal | 122 (65.2) |
| Undefined | 45 (24.1) |
| Histological grade | |
| 1 | 39 (20.8) |
| 2 | 111 (59.4) |
| 3 | 37 (19.8) |
| Stage | |
| 1 | 111 (59.4) |
| 2 | 45 (24.1) |
| 3 | 14 (7.5) |
| 4 | 7 (3.7) |
| Undefined | 10 (5.3) |
| Tumor size (diameter, d) | |
| d <2 cm | 57 (30.5) |
| 2 cm ≤ d <5 cm | 109 (58.3) |
| d ≥ 5 cm | 21 (11.2) |
| Patient age (y) | |
| Range | 27–89 |
| Median | 57 |
| <50 | 60 (32.1) |
| 50 ≤ y <70 | 94 (50.3) |
| ≥70 | 33 (17.6) |
Values are n (%) except where noted otherwise.
Fig. 2.
Correlation of ERα and ERβ expression with histological grades in IDC. The Allred scores of ERα (A) and ERβ (B) in IDC were sorted according to breast tumor histological grades (G1 to G3) and their expression differences between each grade were determined by statistical analysis (*P < 0.001, **P < 0.05, ***P = 0.61). The description of the box chart is the same as in Fig. 1B.
Fig. 3.
Correlation of ERα and ERβ expression with cancer stages in IDC. The Allred scores of ERα (A) and ERβ (B) in IDC were sorted according to breast cancer stages (S1, S2, and S3&4). Statistical analysis showed that there is no significant difference between each group. The description of the box chart is the same as in Fig. 1B.
As shown in Table 2, the ERβ status significantly correlated with ERα status, but exhibited no significant relationship with progesterone receptor (PR) or human epidermal growth factor receptor 2 (HER-2) status. Of the 187 patients, 20 were premenopausal and 122 postmenopausal. Although menopausal status did not affect expression patterns of ERα and ERβ, the premenopausal sample size is too small to draw firm conclusions.
Table 2.
Relationship between ERβ and clinicopathological factors in invasive ductal breast cancer
| Factor | Patients, n | ERβ expression | ||
| Positive | Negative | P* | ||
| ERα status | 0.02 | |||
| Positive | 108 | 20 | 88 | |
| Negative | 79 | 5 | 74 | |
| PR status | 0.40 | |||
| Positive | 114 | 16 | 98 | |
| Negative | 62 | 6 | 56 | |
| HER-2 status | 0.91 | |||
| Positive | 44 | 6 | 38 | |
| Negative | 139 | 18 | 121 | |
PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.
Pearson χ2 test.
ERβ2 Expression in Breast Cancer.
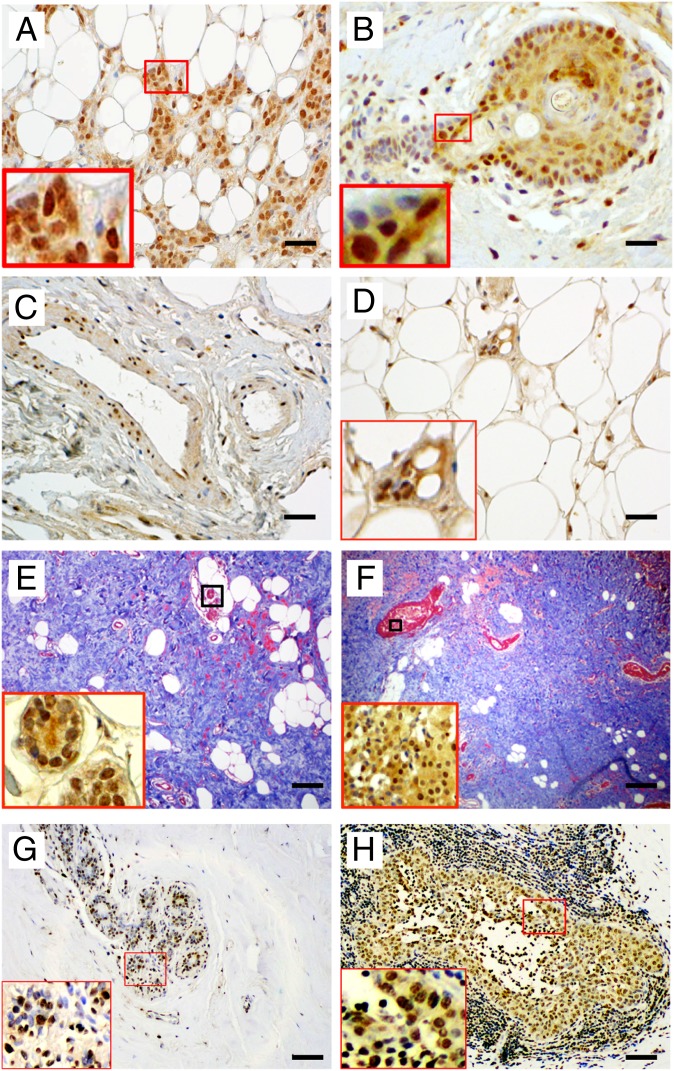
Of the samples stained for ERβ2, 32% were ERβ2 positive. ERβ2 was expressed in nuclei of the breast cancer epithelium (Fig. 4 A–F), particularly those surrounding the necrotic cores of dense tumor masses, as well as expression in the epithelium of normal ducts, stroma, endothelium, and adipocytes (Fig. 4 C–E) in dense breasts. This widespread expression led us to the conclusion that ERβ2 is not a marker of malignant cells but rather a splice variant that is induced by hypoxic environment of tumors. Hypoxia-inducible factor 1α (HIF-1α) staining confirmed that these areas are hypoxic (Fig. 4 G and H).
Fig. 4.
ERβ2 expression in invasive breast cancers. (A–D and the Insets of E and F) Representative immunohistochemical staining of ERβ2 showing ERβ2 expression in cells from invading tumor edge (A); in cells surrounding necrotic centers (B); in stroma and in endothelium and muscle layers of the vessel wall (C); and in adipocytes (D). (E and F) Representative trichrome staining to show collagen in dense breasts (Insets are ERβ2 staining). (G and H) Representative HIF-1α staining. (Scale bars, 50 µm in A–D; 100 µm in E–H.)
Eighty-three percent of the ERβ2-positive samples were from postmenopausal women, and in all of these breasts there was abundant dense collagen, a condition that we have previously shown is correlated with mammographic diagnosis of dense breasts (36) (Fig. 4 E and F). Of the 20 premenopausal women, 10 expressed ERβ2, and these were also collagen rich. ERβ2 was not coexpressed in nuclei with either ERα or ERβ and had no effect on expression of E-cadherin.
Lobular Cancer.
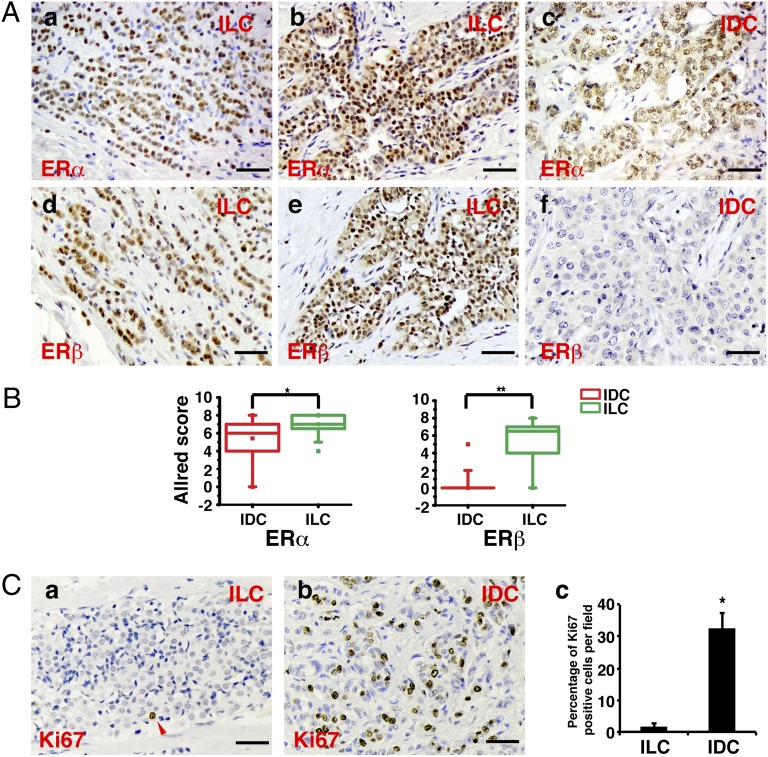
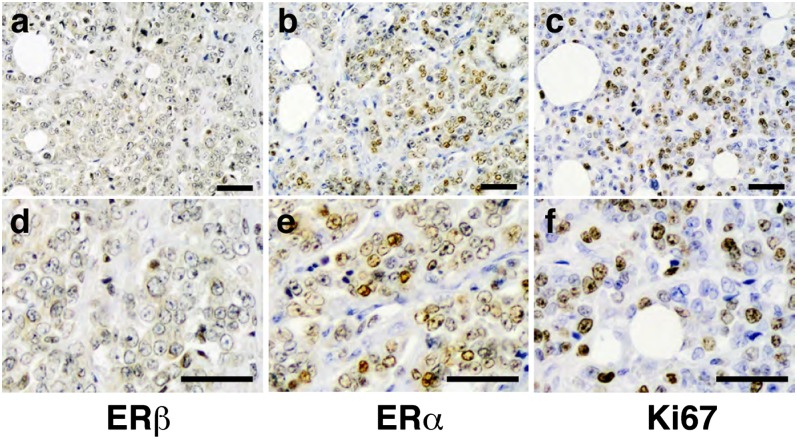
The 24 IDC and 20 invasive lobular breast cancer (ILC) samples from Imperial College were stained for ERα, ERβ, E-cadherin, and Ki67. Results showed that all lobular cancers, except for one grade 3 ILC and one grade 2 ILC of solid variant form, strongly expressed both ERα and ERβ (Fig. 5A), but as in the Japanese cohort, there was no evidence of ERβ expression in most of IDC (Fig. 5 A, f). Quantitation using Allred score shows that the difference in ERβ expression between IDC and ILC was significant (Fig. 5B). The proliferation marker Ki67 revealed that unlike ductal cancers, proliferating cells were very rare in ILC (Fig. 5 C, a–c) and, as expected, E-cadherin was absent. There are some areas in ILC where the number of proliferating cells was high, and these areas were negative for ERβ and positive for ERα (Fig. 6). It seems that early lobular cancer is a disease of anoikis, not proliferation, but in later stages it becomes a proliferative disease.
Fig. 5.
ERα, ERβ, and Ki67 in IDC vs. ILC. (A) Representative immunohistochemical staining of ERα (a–c) and ERβ (d–f) expression in ILC and IDC. (Scale bar, 50 μm.) (B) Box chart of Allred scores of ERα (Left) and ERβ (Right) staining. The description of the box chart is the same as in Fig. 1B (*P = 0.01, **P < 0.0001). (C) Comparison of Ki67 staining in IDC vs. ILC. Representative immunohistochemical staining of Ki67 in ILC and IDC is shown in a and b, respectively; c represents the quantitation analysis of the percentage of Ki67-positive cells (*P < 0.01).
Fig. 6.
Expression of (A and D) ERβ, (B and E) ERα, and (C and F) Ki67 in high-grade, advanced-stage ILC. (Scale bar, 50 µm.)
Discussion
In the present study we used surgically excised samples of human breast cancer, both ductal and lobular samples, to compare the expression of ERα, ERβ, and ERβ2 in normal tissue and different stages of carcinoma within each patient sample, and we also examined the correlation of these two ERs with tumor grade, menopausal status, HER-2, and PR.
We found that in ductal cancer ERβ expression markedly decreased from expression in 80% of cells in normal tissue to very few in DCIS and IDC. This ERβ expression pattern, decreasing from normal to tumor tissue, seems to be common for all estrogen-dependent tumors (37). A similar expression profile has been reported in other tissues, like ovary (38), prostate (39), and colon cancer (40).
The expression of ERβ in invasive carcinoma is negatively correlated with histological grade 1 to grade 2 and 3. Loss of ERβ is an early event in breast cancer progression, and there is no significant correlation between grade 2 and grade 3. One possible reason for the early loss of ERβ is that the cells in which breast cancer arises are the ERα-positive cells. The ERα-positive cells have a growth advantage, which allows clonal expansion. In agreement with this view, there are few, if any, breast cancer cell lines that have any significant expression of ERβ. Such an interpretation would explain why the percentage of cells expressing ERα increases from normal breast tissue to DCIS.
ERα expression was lost in the very high-grade cancers. The reason for the loss of ERα remains to be explained. One possibility, which has been suggested by Platet et al. (2), is that ERα has a positive role in breast cancer cell proliferation and a negative role in invasion. There are several reports indicating that ERα negatively regulates the invasive activity of breast cancer cell lines (2, 41). This explanation is compatible with the notion that loss of ERα in breast cancer patients indicates invasiveness and poor prognosis (42).
In the present study we quantified the average ER staining score of the cells in each stage to represent the three most accepted steps of breast cancer progression. This ER expression pattern at each tumor stage within one patient gives valuable information about the role of the two ERs in breast cancer progression. One intrinsic limitation of the study is that within each stage (normal tissue, DCIS, and IDC) cells are still heterogeneous. This heterogeneity raises a very basic question about the identity of cells from which breast cancer arises. Because in the breasts of women with high-grade invasive cancer there are also normal regions as well as early stages of cancer, it is unlikely that cancer arises from expansion of a single clone. It seems to be a continuous, ongoing process.
As was shown recently (36), density of collagen is a histological marker of breast density. In the present study there was a marked correlation between expression of ERβ2 and breast density as measured by collagen staining. ERβ2 was also expressed in cells surrounding necrotic centers of dense tumor nests. These results suggest that ERβ2 is induced in the hypoxic areas of some cancers. This is the opposite of the action of ERβ, which promotes degradation of HIF-1α (43) and consequently a more differentiated phenotype in the epithelium of cancer cells. Surprisingly, in view of current thinking about the role of ERβ2 as a regulator of ERα (44), ERβ2 was not colocalized in nuclei with ERα and did not affect the expression of the two ERα-regulated genes PR and E-cadherin. Our results therefore indicate that ERβ2 is not a marker of malignancy but is an indication of the degree of hypoxia of the cancer.
ILC represents approximately 5–15% of diagnosed breast cancers (45). The incidence of ILC is increasing (46), and the need to find better ways to treat this type of breast cancer has become more pressing. As reported earlier (34), we confirmed that lobular cancer does not express E-cadherin, whereas in ductal cancer E-cadherin expression was positively correlated with ERα expression. In addition to the high expression of ERβ in early lobular cancer, another marked difference between lobular and ductal cancer was the lack of proliferating cells in lobular cancer: Ki67-positive cells were very abundant in ductal cancer but very rare in lobular cancer. Thus, our data support the previous conclusion (47, 48) that lobular cancer is a disease resulting from resistance to anoikis and not one of proliferation. What was surprising in the present study is the finding that high-grade lobular cancer is characterized by loss of ERβ expression, high ERα level, and high proliferation. Our finding of an increase in proliferation upon loss of ERβ supports the idea that ERβ has an antiproliferative role in the breast (49).
In summary, ERβ agonists may be useful in preventing progression of ductal cancer from low to higher grades and, although ERβ is extensively expressed in lobular cancers, loss of ERβ in late stages of lobular cancer leads to a highly proliferative ERα-positive disease, which may respond to tamoxifen.
Methods
Patients and Breast Cancer Samples.
Information for breast cancer specimens from 187 female patients who underwent surgery at Nagoya City University Hospital (Nagoya, Japan) between 1992 and 2000 is shown in Table 1. No patients had received any preoperative chemotherapy or endocrine treatment. Another set of breast cancer samples containing 20 ILC and 24 IDC were from Imperial College London. The Reporting Recommendations for Tumor Marker Prognostic Studies criteria were followed throughout this study.
Immunohistochemistry.
The specificities of chicken polyclonal ERβ 503 IgY and sheep polyclonal ERβ2 antibodies have been tested previously (23, 35). Anti-ERα antibody was from Dako (clone 1D5). Anti-Ki67 antibody (ab15580) was purchased from Abcam. Anti-HIF1α (610958) was purchased from BD Biosciences. Paraffin sections were deparaffinized in xylene, rehydrated through graded alcohol (two times in 100%; once in 95%, 70%, and 50%). Antigen retrieval was done by PreTreatment Module (Thermo Fisher Scientific Inc.). Slides were heated at 97 °C for 10 min in citric buffer (pH 6.0). After quenching the endogenous peroxidase activity with 0.6% hydrogen peroxidase for 30 min, sections were prevented from nonspecific binding with 3% (wt/vol) BSA for 30 min. Then sections were incubated with monoclonal anti-ERα antibody (1:200 dilution in 3% BSA), anti-ERβ antibody (1:100 dilution in 3% BSA), anti-ERβ2 antibody (1:100 dilution in 3% BSA), anti-HIF1α (1:400 dilution in 3% BSA), or anti-Ki67 antibody (1:1,000 dilution in 3% BSA) overnight at 4 °C. For ERβ staining, sections were incubated for 1 h with biotinylated goat anti-chicken IgY antibody (Abcam; 1:200 dilution in 3% BSA). For ERβ2 staining, sections were incubated for 1 h with biotinylated rabbit anti-sheep IgG antibody (Zymed; 1:200 dilution in 3% BSA). For ERα, HIF-1α, and Ki67 staining, sections were incubated for 1 h with biotinylated goat anti-mouse IgG antibody (Invitrogen; 1:200 dilution in 3% BSA). After washing in PBS, sections were incubated with Vectastain ABC (Vector Laboratories) and developed using the DAB method (Dako). Slides were then counterstained with hematoxylin, dehydrated, and mounted. Masson’s trichrome staining was performed according to the Electron Microscopy Sciences manufacturer’s protocol.
Immunohistochemical Scoring.
The staining of ERs in epithelial cells was evaluated and quantified using the Allred score. Briefly, each slide was inspected under light microscope. First the positively stained epithelial cells were counted and scored by the percentage of stained cells to the total epithelial cells in each stage (0, no positive cell; 1, <1%; 2, 1–10%; 3, 11–33%; 4, 34–66%; and 5, 67–100%). Then the average intensity of positively stained cells was scored with 0–3 (0, not stained; 1, weak; 2, intermediate; and 3, strong). The percentage score and the intensity score were added together to get the total Allred score, which ranged from 0 to 8. Allred scores larger than or equal to 3 were considered ER positive. More than six representative fields of each slide were analyzed for determining ER expression levels.
Statistical Analysis.
The statistical analyses were done with Origin Pro 8.6 software (OriginLab Corporation). The significance between two sets of values was calculated using Student’s t test. A P value of <0.05 was considered statistically significant.
Acknowledgments
This study was supported by grants from the Swedish Cancer Society, the Robert A. Welch Foundation (Grant E-0004), and the Emerging Technology Fund of Texas under Agreement 300-9-1958.
Footnotes
The authors declare no conflict of interest.
References
- 1.Wellings SR, Jensen HM. On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst. 1973;50(5):1111–1118. doi: 10.1093/jnci/50.5.1111. [DOI] [PubMed] [Google Scholar]
- 2.Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: A dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51(1):55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Warner M, Nilsson S, Gustafsson JA. The estrogen receptor family. Curr Opin Obstet Gynecol. 1999;11(3):249–254. doi: 10.1097/00001703-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Osborne CK. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat. 1998;51(3):227–238. doi: 10.1023/a:1006132427948. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmieri C, et al. Estrogen receptor beta in breast cancer. Endocr Relat Cancer. 2002;9(1):1–13. doi: 10.1677/erc.0.0090001. [DOI] [PubMed] [Google Scholar]
- 7.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11(8):597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 8.Shaaban AM, et al. Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am J Surg Pathol. 2003;27(12):1502–1512. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Bozkurt KK, Kapucuoğlu N. Investigation of immunohistochemical ERα, ERβ and ERβcx expressions in normal and neoplastic breast tissues. Pathol Res Pract. 2012;208(3):133–139. doi: 10.1016/j.prp.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Skliris GP, et al. Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol. 2003;201(2):213–220. doi: 10.1002/path.1436. [DOI] [PubMed] [Google Scholar]
- 11.Skliris GP, Carder PJ, Lansdown MRJ, Speirs V. Immunohistochemical detection of ERbeta in breast cancer: Towards more detailed receptor profiling? Br J Cancer. 2001;84(8):1095–1098. doi: 10.1054/bjoc.2001.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuqua SA, et al. Estrogen receptor beta protein in human breast cancer: Correlation with clinical tumor parameters. Cancer Res. 2003;63(10):2434–2439. [PMC free article] [PubMed] [Google Scholar]
- 13.Järvinen TA, Pelto-Huikko M, Holli K, Isola J. Estrogen receptor β is coexpressed with ERalpha and PR and associated with nodal status, grade, and proliferation rate in breast cancer. Am J Pathol. 2000;156(1):29–35. doi: 10.1016/s0002-9440(10)64702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruvberger-Saal SK, et al. Estrogen receptor β expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res. 2007;13(7):1987–1994. doi: 10.1158/1078-0432.CCR-06-1823. [DOI] [PubMed] [Google Scholar]
- 15.Miller WR, Anderson TJ, Dixon JM, Saunders PTK. Oestrogen receptor β and neoadjuvant therapy with tamoxifen: Prediction of response and effects of treatment. Br J Cancer. 2006;94(9):1333–1338. doi: 10.1038/sj.bjc.6603082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omoto Y, et al. Evaluation of oestrogen receptor β wild-type and variant protein expression, and relationship with clinicopathological factors in breast cancers. Eur J Cancer. 2002;38(3):380–386. doi: 10.1016/s0959-8049(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill PA, et al. Wild-type oestrogen receptor beta (ERbeta1) mRNA and protein expression in Tamoxifen-treated post-menopausal breast cancers. Br J Cancer. 2004;91(9):1694–1702. doi: 10.1038/sj.bjc.6602183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speirs V, et al. Coexpression of estrogen receptor alpha and beta: Poor prognostic factors in human breast cancer? Cancer Res. 1999;59(3):525–528. [PubMed] [Google Scholar]
- 19.Sugiura H, et al. Expression of estrogen receptor β wild-type and its variant ERbetacx/β2 is correlated with better prognosis in breast cancer. Jpn J Clin Oncol. 2007;37(11):820–828. doi: 10.1093/jjco/hym114. [DOI] [PubMed] [Google Scholar]
- 20.Roger P, et al. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61(6):2537–2541. [PubMed] [Google Scholar]
- 21.Saunders PT, et al. Expression of oestrogen receptor beta (ERbeta1) protein in human breast cancer biopsies. Br J Cancer. 2002;86(2):250–256. doi: 10.1038/sj.bjc.6600035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC. Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. Br J Cancer. 2006;95(5):616–626. doi: 10.1038/sj.bjc.6603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esslimani-Sahla M, et al. Estrogen receptor beta (ER beta) level but not its ER beta cx variant helps to predict tamoxifen resistance in breast cancer. Clin Cancer Res. 2004;10(17):5769–5776. doi: 10.1158/1078-0432.CCR-04-0389. [DOI] [PubMed] [Google Scholar]
- 24.Miyoshi Y, Taguchi T, Gustafsson JA, Noguchi S. Clinicopathological characteristics of estrogen receptor-beta-positive human breast cancers. Jpn J Cancer Res. 2001;92(10):1057–1061. doi: 10.1111/j.1349-7006.2001.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw JA, et al. Oestrogen receptors alpha and beta differ in normal human breast and breast carcinomas. J Pathol. 2002;198(4):450–457. doi: 10.1002/path.1230. [DOI] [PubMed] [Google Scholar]
- 26.Iwao K, Miyoshi Y, Egawa C, Ikeda N, Noguchi S. Quantitative analysis of estrogen receptor-beta mRNA and its variants in human breast cancers. Int J Cancer. 2000;88(5):733–736. doi: 10.1002/1097-0215(20001201)88:5<733::aid-ijc8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered estrogen receptor alpha and beta messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998;58(15):3197–3201. [PubMed] [Google Scholar]
- 28.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Expression of estrogen receptor beta1, beta2, and beta5 messenger RNAs in human breast tissue. Cancer Res. 1999;59(6):1175–1179. [PubMed] [Google Scholar]
- 29.Cavaliere A, et al. Biopathologic profile of breast cancer core biopsy: Is it always a valid method? Cancer Lett. 2005;218(1):117–121. doi: 10.1016/j.canlet.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 30.Murphy LC, Leygue E. The role of estrogen receptor-β in breast cancer. Semin Reprod Med. 2012;30(1):5–13. doi: 10.1055/s-0031-1299592. [DOI] [PubMed] [Google Scholar]
- 31.Saji S, et al. Expression of ER betacx protein in ERalpha-positive breast cancer: Specific correlation with PgR. Cancer Res. 2002;62:4849–4853. [PubMed] [Google Scholar]
- 32.Paech K, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277(5331):1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 33.Yan Y, et al. Expression of both estrogen receptor-beta 1 (ER-beta1) and its co-regulator steroid receptor RNA activator protein (SRAP) are predictive for benefit from tamoxifen therapy in patients with estrogen receptor-alpha (ER-alpha)-negative early breast cancer (EBC) Ann Oncol. 2013;24(8):1986–1993. doi: 10.1093/annonc/mdt132. [DOI] [PubMed] [Google Scholar]
- 34.Dabbs DJ, et al. Lobular neoplasia of the breast revisited with emphasis on the role of E-cadherin immunohistochemistry. Am J Surg Pathol. 2013;37(7):e1–11. doi: 10.1097/PAS.0b013e3182918a2b. [DOI] [PubMed] [Google Scholar]
- 35.Saji S, et al. Estrogen receptors alpha and beta in the rodent mammary gland. Proc Natl Acad Sci. 2000;97(1):337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng G, et al. Effects of short-term estradiol and norethindrone acetate treatment on the breasts of normal postmenopausal women. Menopause. 2013;20(5):496–503. doi: 10.1097/GME.0b013e318276c4ea. [DOI] [PubMed] [Google Scholar]
- 37.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11(3):537–551. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutherford T, et al. Absence of estrogen receptor-beta expression in metastatic ovarian cancer. Obstet Gynecol. 2000;96(3):417–421. doi: 10.1016/s0029-7844(00)00917-0. [DOI] [PubMed] [Google Scholar]
- 39.Leav I, et al. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159(1):79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res. 2000;60(2):245–248. [PubMed] [Google Scholar]
- 41.Thompson EW, et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150(3):534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 42.Herynk MH, Fuqua SA. Estrogen receptors in resistance to hormone therapy. Adv Exp Med Biol. 2007;608:130–143. doi: 10.1007/978-0-387-74039-3_10. [DOI] [PubMed] [Google Scholar]
- 43.Mak P, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17(4):319–332. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao C, et al. Estrogen receptor beta2 negatively regulates the transactivation of estrogen receptor alpha in human breast cancer cells. Cancer Res. 2007;67(8):3955–3962. doi: 10.1158/0008-5472.CAN-06-3505. [DOI] [PubMed] [Google Scholar]
- 45.Korhonen T, Huhtala H, Holli K. A comparison of the biological and clinical features of invasive lobular and ductal carcinomas of the breast. Breast Cancer Res Treat. 2004;85(1):23–29. doi: 10.1023/B:BREA.0000021038.97593.8b. [DOI] [PubMed] [Google Scholar]
- 46.Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289(11):1421–1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 47.Derksen PW, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10(5):437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 48. Vlug E, Ercan C, van der Wall E, van Diest PJ, & Derksen PW (2013) Lobular breast cancer: Pathology, biology, and options for clinical intervention. Arch Immunol Ther Exp (Warsz), 10.1007/s00005-013-0251-0. [DOI] [PubMed]
- 49.Ström A, et al. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA. 2004;101(6):1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]