Abstract
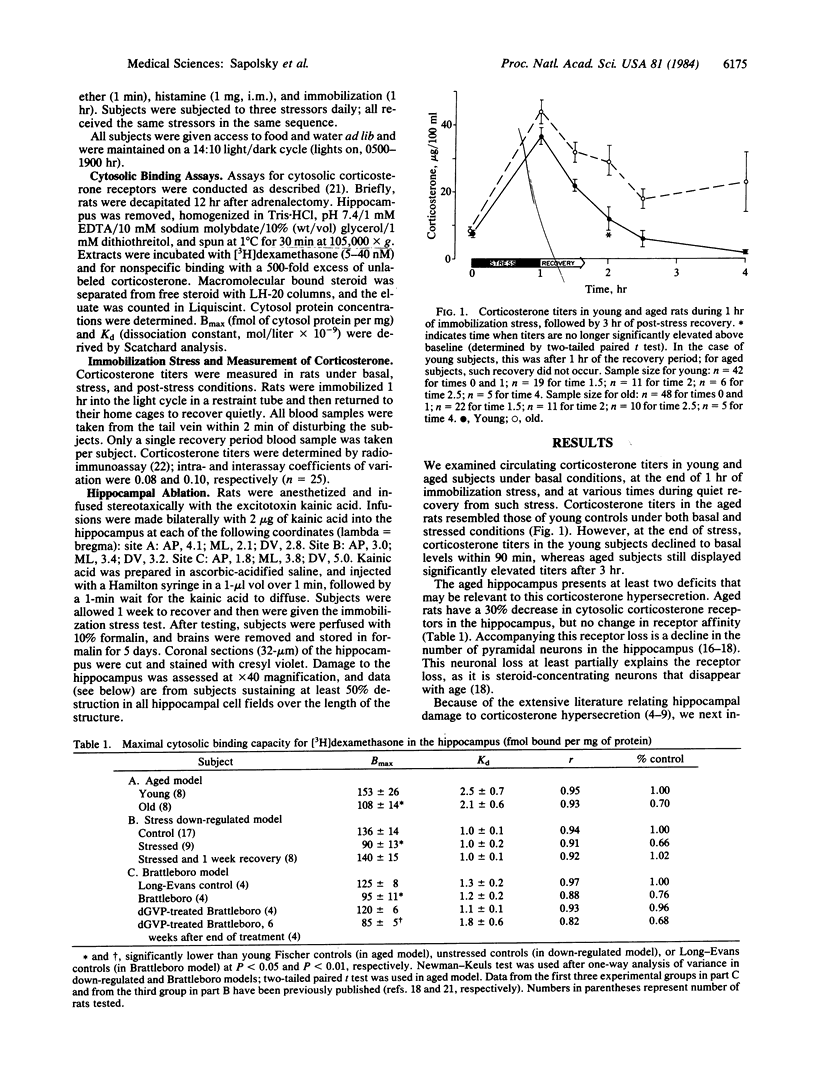
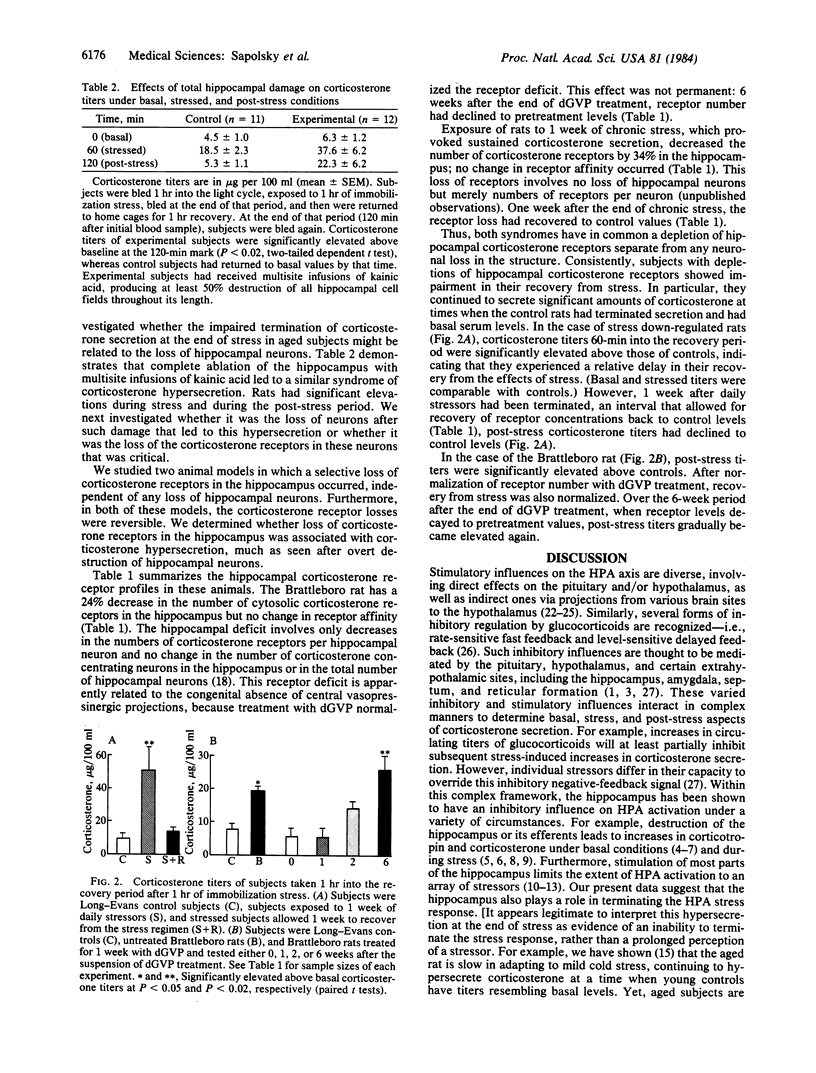
The hippocampus is the principal target site in the brain for adrenocortical steroids, as it has the highest concentration of receptor sites for glucocorticoids. The aged rat has a specific deficit in hippocampal glucocorticoid receptors, owing in large part to a loss of corticoid-sensitive neurons. This deficit may be the cause for the failure of aged rats to terminate corticosterone secretion at the end of stress, because extensive lesion and electrical stimulation studies have shown that the hippocampus exerts an inhibitory influence over adrenocortical activity and participates in glucocorticoid feedback. We have studied whether it is the loss of hippocampal neurons or of hippocampal glucocorticoid receptors in the aged rat that contributes most to this syndrome of corticosterone hypersecretion. To do this, we used two model systems for producing reversible glucocorticoid receptor depletion in the hippocampus, and we found that depletion of receptors without inducing cell loss results in corticosterone hypersecretion. Furthermore, correction of the receptor deficit results in normalization of corticosterone secretion. These results focus attention on the hippocampus as an important glucocorticoid sensor in relation to the stress response. They also provide important new physiological correlates for the remarkable plasticity of the hippocampal glucocorticoid receptor system, which is under independent control by corticosterone and by vasopressin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conforti N., Feldman S. Effects of dorsal fornix section and hippocampectomy on adrenocortical responses to sensory stimulation in the rat. Neuroendocrinology. 1976;22(1):1–7. doi: 10.1159/000122607. [DOI] [PubMed] [Google Scholar]
- Dallman M. F., Yates F. E. Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Ann N Y Acad Sci. 1969 Apr 21;156(2):696–721. doi: 10.1111/j.1749-6632.1969.tb14008.x. [DOI] [PubMed] [Google Scholar]
- Dunn J. D., Orr S. E. Differential plasma corticosterone responses to hippocampal stimulation. Exp Brain Res. 1984;54(1):1–6. doi: 10.1007/BF00235813. [DOI] [PubMed] [Google Scholar]
- Dupont A., Bastarache E., Endröczi E., Fortier C. Effect of hippocampal stimulation on the plasma thyrotropin (THS) and corticosterone responses to acute cold exposure in the rat. Can J Physiol Pharmacol. 1972 Apr;50(4):364–367. doi: 10.1139/y72-054. [DOI] [PubMed] [Google Scholar]
- Feldman S., Conforti N. Amygdalectomy Inhibits adrenocortical responses to somatosensory and olfactory stimulation. Neuroendocrinology. 1981 Jun;32(6):330–334. doi: 10.1159/000123182. [DOI] [PubMed] [Google Scholar]
- Feldman S., Conforti N., Chowers I. The role of the medial forebrain bundle in mediating adrenocortical responses to neurogenic stimuli. J Endocrinol. 1971 Dec;51(4):745–749. doi: 10.1677/joe.0.0510745. [DOI] [PubMed] [Google Scholar]
- Feldman S., Conforti N. Participation of the dorsal hippocampus in the glucocorticoid feedback effect on adrenocortical activity. Neuroendocrinology. 1980;30(1):52–55. doi: 10.1159/000122974. [DOI] [PubMed] [Google Scholar]
- Fischette C. T., Komisaruk B. R., Edinger H. M., Feder H. H., Siegel A. Differential fornix ablations and the circadian rhythmicity of adrenal corticosteroid secretion. Brain Res. 1980 Aug 18;195(2):373–387. doi: 10.1016/0006-8993(80)90073-6. [DOI] [PubMed] [Google Scholar]
- KIM C., KIM C. U. Effect of partial hippocampal resection on stress mechanism in rats. Am J Physiol. 1961 Aug;201:337–340. doi: 10.1152/ajplegacy.1961.201.2.337. [DOI] [PubMed] [Google Scholar]
- KNIGGE K. M. Adrenocortical response to stress in rats with lesions in hippocampus and amygdala. Proc Soc Exp Biol Med. 1961 Oct;108:18–21. doi: 10.3181/00379727-108-26832. [DOI] [PubMed] [Google Scholar]
- Krey L. C., Lu K. H., Bulter W. R., Hotchkiss J., Piva F., Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. II. GH and cortisol secretion. Endocrinology. 1975 May;96(5):1088–1093. doi: 10.1210/endo-96-5-1088. [DOI] [PubMed] [Google Scholar]
- Landfield P. W., Baskin R. K., Pitler T. A. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981 Oct 30;214(4520):581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Landfield P. W., Waymire J. C., Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978 Dec 8;202(4372):1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- Mandell A. J., Chapman L. F., Rand R. W., Walter R. D. Plasma Corticosteroids: Changes in Concentration after Stimulation of Hippocampus and Amygdala. Science. 1963 Mar 22;139(3560):1212–1212. doi: 10.1126/science.139.3560.1212. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Weiss J. M., Schwartz L. S. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968 Nov 30;220(5170):911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Riegle G. D., Hess G. D. Chronic and acute dexamethasone suppression of stress activation of the adrenal cortex in young and aged rats. Neuroendocrinology. 1972;9(3):175–187. doi: 10.1159/000122048. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., McEwen B. S. Corticosterone receptors decline in a site-specific manner in the aged rat brain. Brain Res. 1983 Dec 19;289(1-2):235–240. doi: 10.1016/0006-8993(83)90024-0. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., McEwen B. S., Rainbow T. C. Do vasopressin-related peptides induce hippocampal corticosterone receptors? Implications for aging. J Neurosci. 1984 Jun;4(6):1479–1485. doi: 10.1523/JNEUROSCI.04-06-01479.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., McEwen B. S. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984 Jan;114(1):287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., McEwen B. S. The adrenocortical stress-response in the aged male rat: impairment of recovery from stress. Exp Gerontol. 1983;18(1):55–64. doi: 10.1016/0531-5565(83)90051-7. [DOI] [PubMed] [Google Scholar]
- Veldhuis H. D., de Kloet E. R. Vasopressin-related peptides increase the hippocampal corticosterone receptor capacity of diabetes insipidus (Brattleboro) rats. Endocrinology. 1982 Jan;110(1):153–157. doi: 10.1210/endo-110-1-153. [DOI] [PubMed] [Google Scholar]
- Wilson M. M., Greer S. E., Greer M. A., Roberts L. Hippocampal inhibition of pituitary-adrenocortical function in female rats. Brain Res. 1980 Sep 22;197(2):433–441. doi: 10.1016/0006-8993(80)91128-2. [DOI] [PubMed] [Google Scholar]
- de Kloet E. R., Veldhuis H. D. The hippocampal corticosterone receptor system of the homozygous diabetes insipidus (Brattleboro) rat. Neurosci Lett. 1980 Feb;16(2):187–192. doi: 10.1016/0304-3940(80)90342-0. [DOI] [PubMed] [Google Scholar]



