Significance
The outer membrane (OM) of Gram-negative bacteria is composed of lipopolysaccharide (LPS) and glycerophospholipids (GPLs). Environmental regulation of LPS promotes bacterial resistance to host cationic antimicrobial peptides by altering surface charge and hydrophobicity. This work demonstrates that pathogenic Salmonella coordinately regulate GPL and LPS through the PhoPQ regulatory proteins and the OM palmitoyltransferase enzyme PagP. The broad conservation of PhoPQ and PagP in bacteria suggests that environmental regulation of OM GPL may be a conserved stress-response strategy for antimicrobial resistance. Improved understanding of OM GPL synthesis, transport, and modification could lead to new therapies that target the bacterial OM barrier.
Keywords: signal transduction, acyltransferase, lipid remodeling, pathogenesis
Abstract
Gram-negative bacteria have two lipid membranes separated by a periplasmic space containing peptidoglycan. The surface bilayer, or outer membrane (OM), provides a barrier to toxic molecules, including host cationic antimicrobial peptides (CAMPs). The OM comprises an outer leaflet of lipid A, the bioactive component of lipopolysaccharide (LPS), and an inner leaflet of glycerophospholipids (GPLs). The structure of lipid A is environmentally regulated in a manner that can promote bacterial infection by increasing bacterial resistance to CAMP and reducing LPS recognition by the innate immune system. The gastrointestinal pathogen, Salmonella Typhimurium, responds to acidic pH and CAMP through the PhoPQ two-component regulatory system, which stimulates lipid A remodeling, CAMP resistance, and intracellular survival within acidified phagosomes. Work here demonstrates that, in addition to regulating lipid A structure, the S. Typhimurium PhoPQ virulence regulators also regulate acidic GPL by increasing the levels of cardiolipins and palmitoylated acylphosphatidylglycerols within the OM. Triacylated palmitoyl-PG species were diminished in strains deleted for the PhoPQ-regulated OM lipid A palmitoyltransferase enzyme, PagP. Purified PagP transferred palmitate to PG consistent with PagP acylation of both lipid A and PG within the OM. Therefore, PhoPQ coordinately regulates OM acidic GPL with lipid A structure, suggesting that GPLs cooperate with lipid A to form an OM barrier critical for CAMP resistance and intracellular survival of S. Typhimurium.
Gram-negative bacteria have an outer membrane (OM) composed of an asymmetric bilayer of outer leaflet lipopolysaccharide (LPS) and inner leaflet glycerophospholipids (GPLs). The OM provides a robust permeability barrier and first line of defense against antibiotics and the immune system (1, 2). Hydrophobic interactions between lipid A, the membrane component of LPS, and GPL tether LPS to the bacterial surface. Gram-negative bacteria regulate lipid A structure in response to stressful environments to increase resistance to antibiotics and avoid host immune detection, promoting their survival and replication within host tissues (2).
Salmonellae are pathogenic Gram-negative bacteria that cause a variety of illnesses, including gastroenteritis and systemic febrile disease (3). The ability of Salmonellae to establish and maintain an intracellular replication niche is essential to their pathogenesis. Survival and proliferation within phagolysosomes require the induction of systems that promote resistance to cationic antimicrobial peptides (CAMPs), oxygen and nitrogen radicals, and other specific stresses, such as acidic pH (4). Among these sensory systems is the Salmonella Typhimurium PhoPQ virulence system, which regulates enzymes that modify lipid A to increase the OM permeability barrier to CAMP and promote bacterial resistance (1, 2, 4, 5).
The Salmonellae PhoPQ system regulates aminoarabinose, ethanolamine, and phosphate substitutions to lipid A and LPS-core sugars by activating transcription of genes encoding the PmrAB two-component regulators (2). Derivitization of LPS phosphates with cationic groups reduces the net-negative bacterial surface charge and results in decreased binding of CAMP to the OM. Concomitant with surface charge reduction, PhoPQ directly activates transcription and synthesis of PagP (6). PagP is a conserved OM phospholipase/palmitoyltransferase that removes sn-1 palmitate (C16:0) from GPL and transfers it to lipid A using an active site on the outer leaflet (7, 8). Activity of PagP confers specific bacterial resistance to amphipathic bilayer-damaging alpha-helical CAMP, such as mammalian LL-37 and C18G (9).
S. Typhimurium PagP activity is modulated by both PhoPQ activation and OM damage, indicating that the enzyme can function as part of an acute membrane repair response (9). Environmental stress like divalent cation limitation and CAMP insertion displaces outer-leaflet lipid A molecules, causing breaches in the OM permeability barrier. Upon membrane damage, inner-leaflet GPL molecules are hypothesized to flip into the OM outer leaflet to form what are predicted to be symmetrical lipid microdomains (1, 10). In addition to PagP, many Gram-negative bacteria also possess a highly conserved OM phospholipase A (OMPLA) that has broad substrate specificity and hydrolyzes GPL within the OM outer leaflet to maintain bilayer asymmetry during replication (11, 12). In addition to OMPLA, a dedicated system for the uptake and reacylation of the sn-1 lysophospholipids exists, and its function is linked to an acyl-acyl carrier protein on the cytoplasmic surface of the inner membrane (IM) (12, 13). Therefore, wholesale GPL transport and flipping is intrinsic to rapid restorative membrane repair.
Like all Enterobacteriaceae, S. Typhimurium synthesize three major GPLs: phosphatidylethanolamine (PE), phosphatidylglycerol (PG), and diphosphatidylglycerol, also known as cardiolipin (CL) (14). Glycerophospholipids are substituted with a particular combination of C14, C16, or C18 fatty acids, and diversity is generated by the combinatorial properties of different acyl substitutions, which, for tetra-acylated families like CL, can be quite extensive (15). Bacterial GPL are synthesized on the inner leaflet of the IM by defined biosynthetic mechanisms (16). However, unlike lipid A, which is enzymatically flipped and transported to the OM by specialized protein machinery (17), our current understanding of the mechanism of GPL transport to the OM remains rudimentary by comparison (12), and specific regulation of OM GPL has not been demonstrated. Therefore, we explored the hypothesis that the S. Typhimurium PhoPQ virulence system regulates OM GPL content. We provide evidence for this hypothesis and define PagP as a bifunctional palmitoyltransferase that palmitoylates both PG and lipid A within the S. Typhimurium OM.
Results
PhoQcHAMP Increases Transcription of PhoP-activated Genes and S. Typhimurium Antimicrobial Peptide Resistance More than Other Activated PhoQ Alleles.
S. Typhimurium strains with mutations in phoQ that have variable expression of PhoP-activated genes (pag) were constructed to study GPL regulation. The highest level of constitutive pag expression (Fig. 1A) was obtained with a strain containing a glutamate-to-lysine substitution at amino acid 232 (Table S1), termed PhoQcHAMP. This allele was isolated by domain-targeted random mutagenesis of the PhoQ HAMP domain found in histidine kinases, adenylyl cyclases, methyl-accepting chemoreceptors and phosphatases and blue/white colony screening for maximal PhoPQ-regulated gene activation. The phenotypes of bacteria expressing this allele indicated that it conferred high levels of resistance to C18G (Fig. 1B) and polymyxin B (Fig. 1C) greater than either wild type, or strains expressing a mutant PhoQ enzyme that also directs constitutive pag activation in repressing conditions (Fig. 1A) (9, 18). The phoQcHAMP allele conferred robust C18G resistance between one and two orders of magnitude greater than phoQT48I (Fig. 1B). Therefore, specific OM remodeling identified by use of strains expressing these alleles should reflect PhoPQ-mediated effects.
Fig. 1.
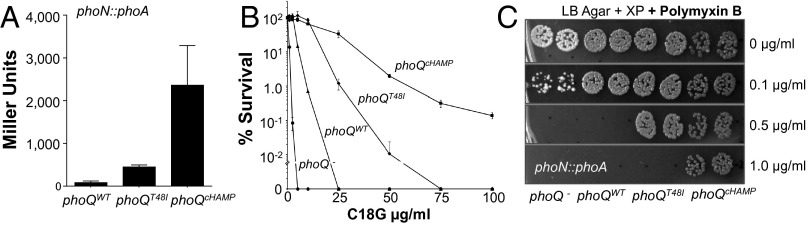
PhoQcHAMP activates pag expression and antimicrobial peptide resistance more than other constitutively activated PhoQ enzymes. (A) A phoN reporter measured pag activation in phoQ deletion mutants expressing phoQ alleles in trans. (B) Bacteriocidal activity of C18G against S. Typhimurium phoQ deletion mutants expressing vector alone (●), phoQWT (▲), phoQT48I (◆), phoQcHAMP (■). (C) Antimicrobial activity of polymyxin B against bacteria on solid media.
PhoPQ Activation Increases OM Cardiolipins and Acylphosphatidylglycerols.
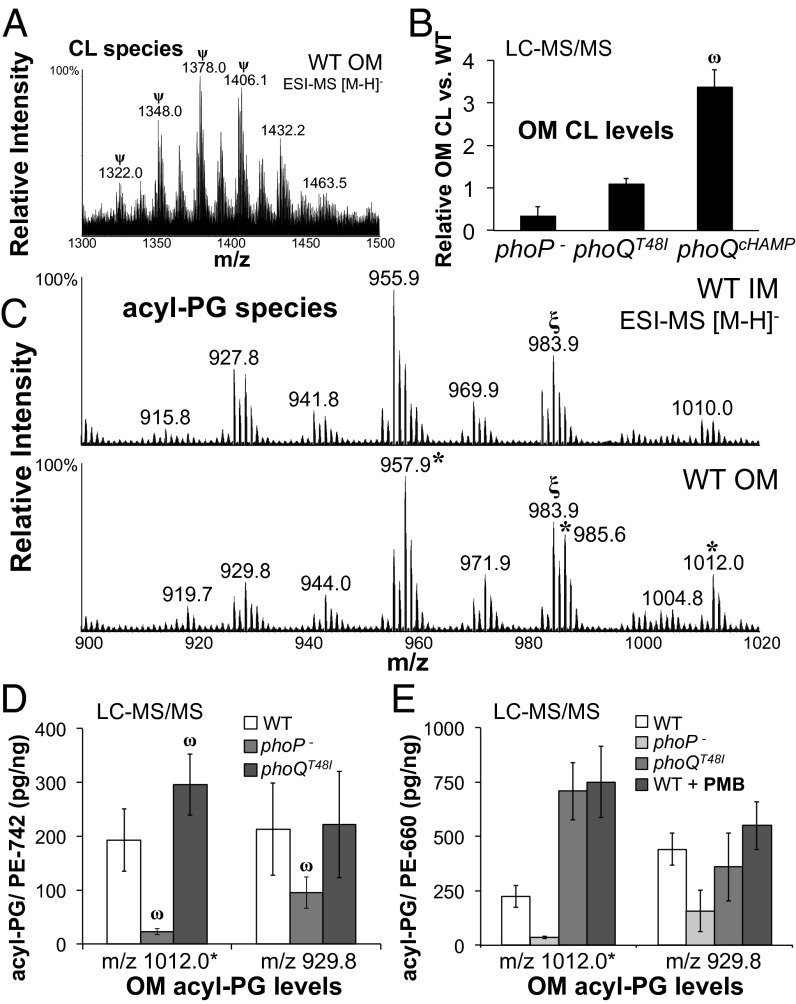
S. Typhimurium glycerophospholipids were extracted from isolated membrane fractions (Fig. 2A). Purity of the membranes was assessed by immunoblotting and enzymatic assay (Fig. S1), indicating that the inner and outer membranes were separated. Membrane lipids were then assessed by TLC (Fig. 2B and Fig. S2), matrix-assisted-laser-desorption-ionization time-of-flight mass-spectrometry (MALDI-MS) (Fig. 2C), and electrospray-ionization time-of-flight mass-spectrometry (ESI-MS) (Figs. S2 and S3). TLC and ESI-MS indicated that different states of PhoPQ activation had minor impact on IM GPL content (Fig. S2). In contrast to the IM, PhoPQ activation dramatically increased two distinct GPL families within the OM, the cardiolipins (diphosphatidylglycerols) and the acylphosphatidylglycerols (Fig. 2 A and B). Acylphosphatidylglycerols are triacylated GPLs whose synthesis and function are not defined (19). These modified PG molecules are acylated with C14 or C16 fatty acids by transesterification at sn-3′ on the polar head group (Fig. 2A). Genetic and biochemical evidence suggests synthesis of acyl-PG involves the IM transacylase, PldB (13). Reduced retention of acyl-PG to a silica matrix reflected the decreased polarity and increased hydrophobicity of this lipid family (Fig. 2B).
Fig. 2.
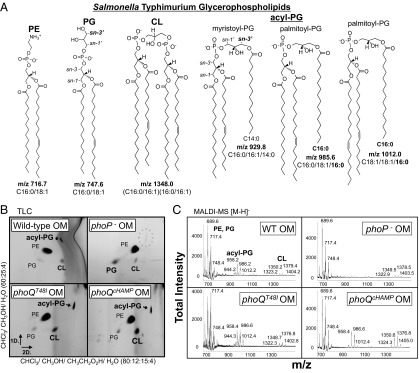
PhoPQ increases cardiolipin and acylphosphatidylglycerol content of the OM. (A) Chemical structures, molecular formulas, and mass-to-charge ratios (m/z) of GPL in negative ionization mode [M-H]−. (B) The 2D TLC of OM GPL. (C) MALDI-MS spectra of OM GPL were acquired with a 50-Hz repletion rate, and 3,000 shots were accumulated to generate the total intensities depicted. Relative intensity reflects the intensity of a peak relative to the highest peak in the range. sn- specifies the carbon to which a particular fatty acid is esterified.
Although present in both bilayers, S. Typhimurium acyl-PG species appeared to be more abundant within the OM (Fig. 2B and Fig. S2). By TLC and ESI-MS, PhoP-null mutants showed a modest reduction in acyl-PG content within the IM (Fig. S2). Consistent with a specific role for PhoPQ in promoting OM GPL levels, the OM fractions of PhoP-null mutant bacteria were severely diminished for acyl-PG (Fig. 2 B and C and Fig. S3). Further regulation of OM acyl-PG was demonstrated using strains with varying levels of PhoPQ-activity: low (wild type) moderate (phoQT48I), and high (phoQcHAMP) (Fig. 1A). Low activity was sufficient for OM acyl-PG production because wild-type and phoQT48I mutant bacteria were similar (Fig. 2 B and C and Fig. S3). For CL, moderate PhoQ activity prompted modest yet reproducible increases by TLC. High activity induced large increases in OM CL species detectable by both TLC and MALDI-MS (Fig. 2 B and C). Therefore, the S. Typhimurium PhoPQ system regulates increases in OM acyl-PG and CL coordinately with modification of lipid A and increased resistance to CAMP.
Increasing PhoQ Activity Increases Cardiolipin Levels Within the OM.
To measure lipids from membrane extracts, GPLs were separated and quantified using normal-phase liquid-chromatography and collision-induced-dissociation mass spectrometry (LC-MS/MS). Parent ion scans detected several predicted CL molecules within the OM (Fig. 3A) (20). Membrane lipids were separated, and distinct CL species were quantified as picograms (pg) of CL/nanogram (ng) of PE within samples (Fig. 3B, Fig. S4, and Table S2). Turnover of cellular PE is minimal in enteric bacteria and did not vary here (14). Therefore, each CL molecule was normalized to three PE species (Table S2). Consistent with TLC and MALDI-MS, quantitative LC-MS/MS of phoQT48I mutants revealed modest yet significant increases in OM CL relative to wild-type S. Typhimurium (Table S2). Wild-type bacteria produced CL species with mass-to-charge ratios (m/z) of 1348.0 and 1374.0 measuring 29 and 48 pg/ng whereas phoQT48I mutants produced 51 and 77 pg/ng of these species, respectively. High PhoQ activity prompted further significant increases, and phoQcHAMP bacteria produced 95 and 150 pg/ng of these CL molecules (Table S2). Statistical analyses indicated that some CL species were even significantly increased in phoQcHAMP mutants relative to phoQT48I mutants, supporting the idea that increasing PhoQ activity increases the CL content of the OM.
Fig. 3.
PhoPQ activation increases the levels of CL and palmitoyl-PG species within the OM. (A) ESI-MS of OM CL. ψSpecies was quantified by LC-MS/MS (Table S2). (B) The average fold difference ± SD for all CL/PE comparisons from four experiments. ωThe average pg/ng value is significantly greater than wild type by an unpaired Student t test. Unpaired t tests were used throughout the study where significance is indicated. (C) ESI-MS of IM and OM acyl-PG. *Denotes a palmitoyl-PG (21). ξm/z 983.9 reflects two distinct acyl-PG species. (D) LC-MS/MS determined the acyl-PG/ PE (pg/ng) in OM extracts (Table S3). Shown is the average value from three experiments ± SD ωThe value is significantly different from wild type. (E) S. Typhimurium was treated with polymyxin B (PMB), and acyl-PG species were quantified by LC-MS/MS. The average value from two experiments ± SD is shown alongside the values obtained for mutant bacteria (Table S3).
PhoPQ Activation Is Required for Synthesis of OM Acylphosphatidylglycerols with Palmitate at the sn-3′ Position.
Previous structural characterizations of S. Typhimurium acyl-PG suggested tremendous species complexity (21). To examine the extent of variations in IM and OM content, acyl-PG parent ions were scanned and analyzed. Compared with the IM, the OM contained acyl-PG species with obvious variations in relative abundance (Fig. 3C). Species abundant within the IM included those with m/z of 955.9, predicted to carry C14:0 or C14:1 at the sn-3′ position, and 983.9 predicted to carry C16:1 or C16:0 at sn-3′ (21). Although present, these acyl-PG species were less abundant within the OM. Predominant OM acyl-PG structures included those with an m/z of 957.9, 985.6, and 1012.0, each predicted to harbor C16:0 at sn-3′ (Fig. 3C) (21). Therefore, the OM appears to be enriched with acyl-PG species that are palmitoylated (Fig. 2A). Synthesis of OM palmitoyl-PG molecules was regulated by the PhoPQ system because palmitoyl-PG species were diminished in PhoP-null mutants relative to wild-type and PhoPQ-activated bacteria (Fig. S3). In contrast, IM acyl-PG species showed minimal specific variation (Fig. S2), consistent with the hypothesis that PhoPQ regulates an acyltransferase that specifically increases the level of palmitoyl-PG within the OM.
Reversed-phase LC-MS/MS was developed to quantify acyl-PG molecules. Normal-phase LC was unsuitable because acyl-PG molecules are poorly retained by silica in apolar solvent systems (Fig. 2B and Figs. S2, S4, and S5) (14, 19). Using LC-MS/MS (Fig. S4), acyl-PG species with or without palmitate at sn-3′ were quantified (Fig. 3D and Table S3). Consistent with TLC, MALDI-MS, and ESI-MS, quantitative LC-MS/MS showed that PhoP controls synthesis of several OM acyl-PG species. Specifically, wild-type bacteria totaled 213 pg/ng of the myristoyl-PG, m/z 929.8 (Fig. 2A) whereas PhoP-null mutants measured 95 pg/ng of this species, less than half the level of wild type (Fig. 3D and Table S3). Therefore, PhoPQ may play a minor role in regulating some nonpalmitoylated acyl-PG species, perhaps by controlling their transport to the OM after PldB-dependent synthesis. In contrast to the myristoyl-PG species, palmitoyl-PG species were severely reduced yet still measurable within the OM of PhoP-null bacteria. For example, wild-type bacteria measured 193 pg/ng of the palmitoyl-PG, m/z 1012.0, whereas PhoP-null mutants measured 23 pg/ng of this molecule, nearly ten times less than wild type and fifteen times less than activated mutants, which totaled 296 pg/ng (Fig. 3D, Table S3, and Note S1). The fact that palmitoyl-PG species were diminished in PhoP-null mutants indicated that the majority of S. Typhimurium PG transacylase activity is PhoPQ-regulated under these conditions (13).
Exposure of S. Typhimurium to Polymyxin B Increases the Levels of Palmitoyl-PG Species Within the OM.
Growth of S. Typhimurium with subinhibitory CAMP activates the PhoPQ system (22). To test whether polymyxin B (PMB) stimulates synthesis of OM palmitoyl-PG molecules, wild-type bacteria were grown with PMB, and acyl-PG species were quantified. As predicted, PMB prompted increases in the palmitoyl-PG, m/z 1012.0, but not the myristoyl-PG, m/z 929.8, compared with untreated control bacteria (Fig. 3E). Consistent with the prediction that PMB induces palmitoyl-PG synthesis through PhoPQ, PMB-treated bacteria increased the palmitoyl-PG, m/z 1012.0, to levels similar to those of PhoPQ-activated bacteria (Fig. 3E). Therefore, S. Typhimurium increases OM palmitoyl-PG levels in response to an environmental signal that activates PhoPQ.
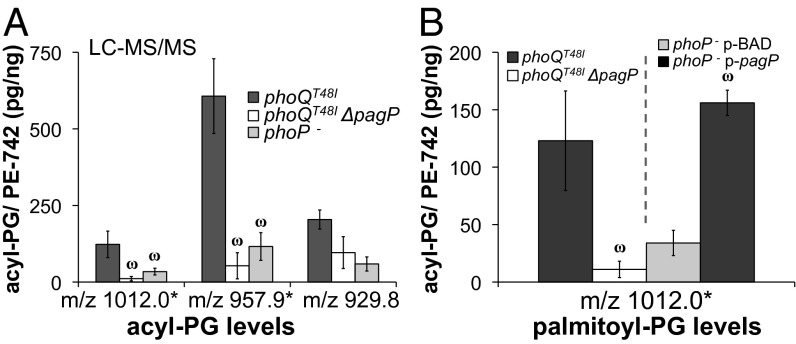
PagP Is Necessary and Sufficient for PhoPQ-Regulated Production of Palmitoyl-PG Molecules.
The PhoPQ-regulated PagP enzyme is a very specific OM palmitoyltransferase that deacylates GPL and transfers palmitate to lipid A, generating hepta-acylated lipid A species (7). However, it was not known whether PagP could use cellular PG as a palmitate acceptor. Therefore, to ask whether PagP was responsible for the increased levels of OM palmitoyl-PG species promoted by PhoPQ, PagP-null mutants and PagP overexpression strains were analyzed. Parent ion scans of OM acyl-PG molecules showed that palmitoyl-PG species were diminished in PhoPQ-activated PagP-null mutant bacteria and increased in PhoP-null mutants overexpressing PagP (Fig. S5). Quantitative LC-MS/MS confirmed that PagP was necessary for production of palmitoyl-PG because PhoPQ-activated PagP+ bacteria measured significantly greater levels of m/z 957.9 and 1012.0 relative to PhoPQ-activated PagP-null mutant S. Typhimurium. These predominant OM palmitoyl-PG molecules totaled 607 and 123 pg/ng in PhoPQ-activated PagP+ membranes compared with PagP-null mutants, which measured only 53 and 11 pg/ng, respectively (Fig. 4A and Table S4). Modest differences in nonpalmitoylated acyl-PG species were also detected, suggesting possible minor off-target effects of pagP gene disruption. Overexpression of PagP in PhoP-null mutants was sufficient for production of OM palmitoyl-PG because arabinose induction dramatically increased the concentration of the palmitoyl-PG, m/z 1012.0. This OM lipid increased from 34 pg/ng to 156 pg/ng upon PagP overexpression (Fig. 4B and Table S5). Minimal differences in myristoyl-PG species were observed upon overexpression consistent with specificity of the PagP hydrocarbon ruler (23). These genetic data indicate that PagP has dual acceptor substrate specificity within the OM and transfers palmitate to both PG and lipid A during PhoPQ-regulated OM barrier remodeling.
Fig. 4.
PhoPQ activates pagP to increase the levels of palmitoyl-PG molecules within the S. Typhimurium OM. (A) LC-MS/MS determined the levels of acyl-PG/ PE (pg/ng) in OM GPL extracts (Table S4). Shown is the average from three experiments ± SD ωIndicates a value significantly different from that of phoQT48I pagP + bacteria. (B) Average acyl-PG/PE (pg/ng) for m/z 1012.0 in three experiments ± SD ωIndicates that the value is significantly different from phoQT48I pagP + or phoP − bacteria expressing an empty vector (pBAD) (Tables S4 and S5).
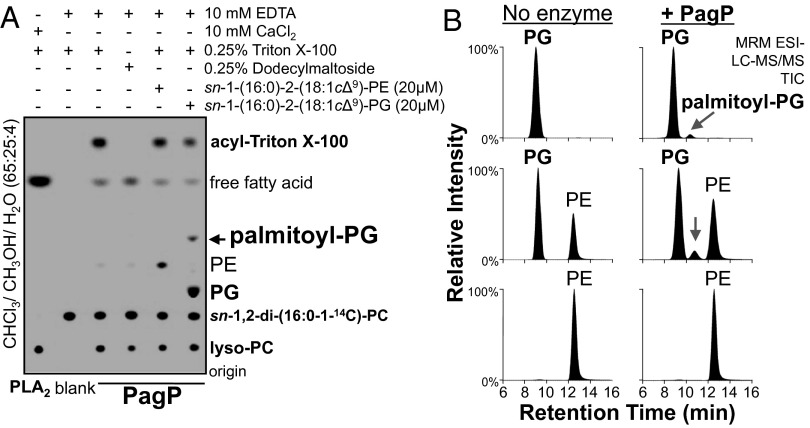
Purified PagP Transfers Palmitate to PG to Produce Palmitoyl-PG.
Given genetic support for dual substrate specificity of PagP, it was important to determine whether purified PagP could transpalmitoylate to PG sn-3′-OH moieties. To test this hypothesis, PagP was incubated with a labeled palmitate donor and an unlabeled GPL acceptor, and transesterification was monitored by autoradiography (Fig. 5A). Consistent with the hypothesis that PhoPQ activates PagP to increase the levels of palmitoyl-PG species within the OM, PagP-PG reactions yielded a product resembling acyl-PG (Fig. 5A). To confirm that this product was palmitoyl-PG, reactions with unlabeled lipid substrates were extracted and scanned for the predicted parent ion (Fig. S6). Preparative TLC and targeted LC-MS/MS determined that the lipid product was palmitoyl-PG, m/z 985.6 (Fig. S6). Further analytical mass spectrometry demonstrated that the palmitoyl-PG was not a contaminant of the PagP preparation and was specifically produced from PagP–PG reactions (Fig. 5B). Phosphatidylethanolamine cannot function as a PagP acceptor substrate, and parent ion scans did not reveal candidate acyl-PE products (Fig. S6). Therefore, PagP transfers palmitate specifically to the PG polar head group in vitro. These findings support a model whereby host signals activate PhoPQ to increase PagP levels and induce palmitoylation of PG coordinately with lipid A within the S. Typhimurium OM.
Fig. 5.
Purified PagP transfers palmitate to PG but not PE. (A) PagP was incubated with unlabeled PE, m/z 716.7, and PG, m/z 747.6, acceptor lipids and a radiolabeled palmitate donor lipid phosphatidylcholine (PC). Phospholipase A2 (PLA2) and Triton X-100 were used as positive controls for phospholipase and palmitoyltransferase activity, respectively. Label incorporation into PE and PG at sn-1 and sn-2 causing their detection by autoradiography is a result of acyl exchange reactions that occur for transacylase enzymes in vitro (40). (B) PagP-GPL reactions with PE, m/z 716.7, and PG, m/z 747.6 were analyzed by LC-MS/MS to separate, target, and detect the palmitoyl-PG product, m/z 985.6.
Discussion
This work demonstrates that acidic glycerophospholipids are regulated components of the OM barrier. S. Typhimurium PhoPQ coordinately regulates OM GPL with lipid A structures that contribute to resistance to CAMP. In this regard, the susceptibility of pagP mutants to alpha-helical CAMP previously attributed to palmitoylation of lipid A may also involve palmitoylation of PG (9). Palmitoylation reduces PG polarity and increases hydrophobicity and saturation of the bilayer, properties that, for lipid A, are known to prevent surface association and membrane intercalation of amphipathic alpha-helical CAMP (2). Although these dual functions of PagP cannot yet be separated, our data allow calculations to estimate the percentage of OM PG molecules that are palmitoylated during PhoPQ activation (Note S2). These estimates predict that combined PG, acyl-PG, and di-PG, commonly known as cardiolipin, constitute ∼11% of the wild-type OM lipid bilayer, assuming that lipid A constitutes 50% (1). Our estimates predict that, during PhoPQ activation, ∼31% of the total OM PG is palmitoylated at sn-3′. Previous measurements determined that roughly 20% of S. Typhimurium lipid A was palmitoylated under similar conditions (24). Therefore, the number of palmitoyl-PG molecules is estimated to be a similar order of magnitude to the number of palmitoylated lipid A molecules, supporting a biological function to the activity of PagP on PG. PagP structural studies support a model wherein palmitoylation targets are localized within the OM outer leaflet (25, 26). Therefore, PG likely migrates to the outer leaflet when PhoPQ is activated, and PagP activity on PG could contribute to local barrier function at specific sites within the OM outer leaflet (1).
Compared with palmitoyl-PG, the functional significance and mechanism of regulated increases in OM CL are unknown. Previous studies of the PhoPQ transcriptome have not identified a known CL synthase or obvious lipid transport system as being PhoPQ-regulated (22). Therefore, the effects observed may involve a posttranscriptional mechanism. Earlier biochemical studies indicated that CL was a minor component of the S. Typhimurium OM constituting roughly 2% of the total GPL. Cardiolipin was more abundant within the IM, constituting nearly 7% (27). This variation could raise a concern that the increase in OM CL content observed upon PhoPQ activation is a result of incomplete membrane separation. However, OM fractions were consistently and equivalently devoid of IM material, suggesting a specific increase in OM CL content (Fig. S1). Nevertheless, it is possible that PhoPQ increases CL at sites of cell division, such as polar septa, that may have a propensity to fractionate with the OM.
Considerable evidence suggests that CL molecules form membrane microdomains that may confer negative curvature to the inner plasma membrane during membrane fission for cell division and sporulation processes (28–31). It is tempting to speculate that modified lipid A structures require specific increases in CL to maintain proper membrane curvature during bacterial replication. Cardiolipin species are also known to interact with cationic amphipathic helical protein segments on bilayer surfaces (32). Therefore, CL molecules may direct CAMP, or cell division components, to specific microdomains to increase bacterial resistance, or coordinate membrane fission with OM lipid A and PG remodeling. In support of a role for CL in CAMP resistance, vancomycin-resistant Gram-positive Enterococcus faecalis can clinically acquire daptomycin resistance by sequestering CAMP to distinct negatively charged CL microdomains within the plasma membrane (33). In addition, electron micrographs of antimicrobial peptides interacting with bacterial surfaces have captured initial membrane-active events (34). Images suggest that CAMPs form specific holes and cause acute membrane blistering at polar septa rather than dissolution of the entire cell envelope as would be expected if insertion was random or uniform. Therefore, it is plausible that CL directs antimicrobial peptides to specific sites within the OM to facilitate S. Typhimurium resistance.
The PhoPQ system and its regulation of the OM barrier are a highly conserved mechanism important for Gram-negative pathogens of plants, animals, and insects (4, 35). The broad conservation of PhoPQ and PagP suggests that environmental regulation of OM GPL may be a conserved stress-response strategy for a variety of species. This work adds to our understanding of the components of the OM regulated by S. Typhimurium PhoPQ and suggests that many phenotypes promoted by this system, including resistance to CAMP, increased OM barrier function, and the ability to cause systemic disease, likely involve coordinate regulation of glycerophospholipids, lipid A, and envelope proteins. Improved understanding of OM GPL synthesis, transport, and modification could lead to new therapies that target the bacterial OM barrier.
Materials and Methods
Bacterial Strains and Culturing Conditions.
S. Typhimurium (Table S1) was grown in Luria-Bertani (LB) broth at 37 °C until the exponential phase (E-phase) (OD600 0.6–0.8). Bacteria with pBAD were grown in 100 μg/mL ampicillin (amp) and 0.2% glucose (36). For expression of phoQcHAMP and pagP, cells were washed to remove glucose, back diluted, induced with 0.2% arabinose, and cultured to E-phase (∼2.5 h). Bacteria exposed to polymyxin B (PMB) (USB) were cultured to OD600 = 0.1 in LB and treated with 1 μg/mL PMB (5, 22) for an additional 1.5 h at 37 °C before harvest.
Alkaline phosphatase activity was measured according to standard protocol. Bacteria were grown overnight in N-minimal medium with amp and 10 mM MgCl2 to demonstrate constitutive activation of PhoQcHAMP under conditions that repress wild-type PhoQ.
C18G sensitivity and polymyxin B sensitivity were tested using cells grown in LB to E-phase. Typically, 1 × 105 CFU were exposed to between 0 and 100 μg/mL of C18G (Abgent) for 1 h at 25 °C in PBS with 0.5% tryptone. For PMB sensitivity 10 μL droplets with ∼50 cfu were spotted onto LB agar with amp, XP (5-bromo-4-chloro-3-indolyl phosphate) (Sigma), and between 0 and 1 μg/mL PMB. The subinhibitory concentration of PMB for S. Typhimurium on solid medium is substantially lower than in liquid medium.
OM Isolation, GPL Extraction, and TLC.
Subcellular membrane fractionation was achieved using a lysozyme/EDTA spheroplasting method (37) and homogenization (AVESTIN). Western blots for SecA (IM) and OmpA (OM), along with NADH dehydrogenase (IM) activity assays, were used to assess purity of the membranes (Fig. S1). Glycerophospholipids were extracted by Bligh-Dyer (38). TLC was performed on silica gel 60 plates. Lipids were visualized by iodine vapor and identified using commercial standards (Avanti). To identify acyl-PG, the silica were scraped, extracted in 1:1 (vol/vol) CHCl3:CH3OH, and analyzed by reversed-phase LC-MS/MS (Fig. S4).
MALDI-TOF-MS.
Extracts were spotted onto an MTP 384 target plate (Bruker) and mixed with 2',4',6'-trihydroxyacetophenone 5-chloro-2-mercaptobenzothiazole (THAP) and 20 mM EDTA in (3:1) CHCl3:CH3OH and analyzed in negative-ion [M-H]-1 mode on an AutoFlex Analyzer (Bruker). Instrument calibration and tuning parameters were optimized using HP Calmix (Sigma-Aldrich). Data were acquired and processed using FlexAnalysis software (Bruker Daltonics).
ESI-TOF-MS.
Lipid extracts were directly injected (10–50 µL) onto a Waters Synapt G1 Time-of-Flight Mass Spectrometer (TOF-MS). Analytes were ionized by electrospray in [M-H]-1 mode. Instrumental settings are found in SI Materials and Methods.
Normal-phase LC-MS/MS was performed in conjunction with ESI-MS/MS by diluting lipid extracts in mobile phase A and injecting 1–10 µL using a Waters Acquity H-class uHPLC SDS system interfaced with an Agilent Zorbax Rx-SIL column (2.1 × 100 mm, 1.8 μm). Lipids were ionized in [M-H]-1 mode on a Waters Premier-XE tandem mass spectrometer. The gradient and instrumental settings for multiple reaction monitoring (MRM) are in SI Materials and Methods.
Reversed-phase LC-MS/MS was performed in conjunction with ESI-MS/MS by injecting 1–10 µL using the Waters Acquity interfaced with a Phenomenex phenyl security guard cartridge (4 × 2.0 mm). The gradient and instrumental settings for MRM are in SI Materials and Methods.
Palmitoyltransferase Assay.
For radioactive experiments, PagP was purified as previously described (7). PagP activity was assessed in a volume of 25 μL with di-[1-14C]-16:0-PC to achieve a final concentration 20 μM (4,000 cpm/μL). The lipid was dried under N2 (g) and dissolved in 22.5 μL of reaction buffer containing 0.1 M Tris⋅HCl (pH 8.0), 10 mM EDTA, and 0.25% n-dodecyl β-d-maltoside (DDM). Reactions were started by addition of 2.5 μL of PagP (1 μg/mL). Reactions were incubated at 30 °C for 1 h and terminated by adding 12.5 μL to 22.5 μL of 1:1 (vol/vol) CHCl3/CH3OH; 5 μL of the lower organic phase provided 10,000 cpm for spotting onto a TLC plate. Plates were exposed overnight to a PhosphorImager screen and developed with a Molecular Dynamics Typhoon 9200. The PLA2 control (4 mU/μL) was incubated with 10 mM CaCl2 in place of EDTA. For palmitoyltransferase reactions with unlabeled PG and PE (Fig. 5B), PagP was purified as described (39).
Supplementary Material
Acknowledgments
Z.D.D. was supported by National Institutes of Health (NIH) Ruth L. Kirschstein National Research Service Award F32AI096820. S.M. was supported by a fellowship from Fundacao para a Ciencia e a Tecnologia, Portugal (SFRH / BPD / 42392 / 2007) cofunded by Programa Operacional Potencial Humano and Fundo Social Europeu. Work was also funded by the NIH and Grant R01AI030479 (to S.I.M.). R.E.B. was supported by Canadian Institutes of Health Research Grant MOP-125979.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316901111/-/DCSupplemental.
References
- 1.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67(4):593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Needham BD, Trent MS. Fortifying the barrier: The impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol. 2013;11(7):467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6(1):53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 4.Prost LR, Miller SI. The Salmonellae PhoQ sensor: Mechanisms of detection of phagosome signals. Cell Microbiol. 2008;10(3):576–582. doi: 10.1111/j.1462-5822.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 5.Murata T, Tseng W, Guina T, Miller SI, Nikaido H. PhoPQ-mediated regulation produces a more robust permeability barrier in the outer membrane of Salmonella enterica serovar typhimurium. J Bacteriol. 2007;189(20):7213–7222. doi: 10.1128/JB.00973-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunn JS, Belden WJ, Miller SI. Identification of PhoP-PhoQ activated genes within a duplicated region of the Salmonella typhimurium chromosome. Microb Pathog. 1998;25(2):77–90. doi: 10.1006/mpat.1998.0217. [DOI] [PubMed] [Google Scholar]
- 7.Bishop RE, et al. Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria. EMBO J. 2000;19(19):5071–5080. doi: 10.1093/emboj/cdd507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang PM, et al. Solution structure and dynamics of the outer membrane enzyme PagP by NMR. Proc Natl Acad Sci USA. 2002;99(21):13560–13565. doi: 10.1073/pnas.212344499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo L, et al. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell. 1998;95(2):189–198. doi: 10.1016/s0092-8674(00)81750-x. [DOI] [PubMed] [Google Scholar]
- 10.Jia W, et al. Lipid trafficking controls endotoxin acylation in outer membranes of Escherichia coli. J Biol Chem. 2004;279(43):44966–44975. doi: 10.1074/jbc.M404963200. [DOI] [PubMed] [Google Scholar]
- 11.Dekker N. Outer-membrane phospholipase A: Known structure, unknown biological function. Mol Microbiol. 2000;35(4):711–717. doi: 10.1046/j.1365-2958.2000.01775.x. [DOI] [PubMed] [Google Scholar]
- 12.Malinverni JC, Silhavy TJ. An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proc Natl Acad Sci USA. 2009;106(19):8009–8014. doi: 10.1073/pnas.0903229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu L, Jackowski S, Rock CO. Isolation and characterization of Escherichia coli K-12 mutants lacking both 2-acyl-glycerophosphoethanolamine acyltransferase and acyl-acyl carrier protein synthetase activity. J Biol Chem. 1991;266(21):13783–13788. [PubMed] [Google Scholar]
- 14.Ames GF. Lipids of Salmonella typhimurium and Escherichia coli: Structure and metabolism. J Bacteriol. 1968;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett TA, Kordestani R, Raetz CR. Quantification of cardiolipin by liquid chromatography-electrospray ionization mass spectrometry. Methods Enzymol. 2007;433:213–230. doi: 10.1016/S0076-6879(07)33012-7. [DOI] [PubMed] [Google Scholar]
- 16.Parsons JB, Rock CO. Bacterial lipids: Metabolism and membrane homeostasis. Prog Lipid Res. 2013;52(3):249–276. doi: 10.1016/j.plipres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Z, White KA, Polissi A, Georgopoulos C, Raetz CR. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J Biol Chem. 1998;273(20):12466–12475. doi: 10.1074/jbc.273.20.12466. [DOI] [PubMed] [Google Scholar]
- 18.Gunn JS, Miller SI. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 1996;178(23):6857–6864. doi: 10.1128/jb.178.23.6857-6864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen RW, Ballou CE. Acyl phosphatidylglycerol: A new phospholipid from Salmonella typhimurium. J Biol Chem. 1971;246(10):3305–3313. [PubMed] [Google Scholar]
- 20.Hsu FF, Turk J. Characterization of cardiolipin from Escherichia coli by electrospray ionization with multiple stage quadrupole ion-trap mass spectrometric analysis of [M - 2H + Na]- ions. J Am Soc Mass Spectrom. 2006;17(3):420–429. doi: 10.1016/j.jasms.2005.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu FF, Turk J, Shi Y, Groisman EA. Characterization of acylphosphatidylglycerols from Salmonella typhimurium by tandem mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom. 2004;15(1):1–11. doi: 10.1016/j.jasms.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Bader MW, et al. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol Microbiol. 2003;50(1):219–230. doi: 10.1046/j.1365-2958.2003.03675.x. [DOI] [PubMed] [Google Scholar]
- 23.Ahn VE, et al. A hydrocarbon ruler measures palmitate in the enzymatic acylation of endotoxin. EMBO J. 2004;23(15):2931–2941. doi: 10.1038/sj.emboj.7600320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo L, et al. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276(5310):250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 25.Cuesta-Seijo JA, et al. PagP crystallized from SDS/cosolvent reveals the route for phospholipid access to the hydrocarbon ruler. Structure. 2010;18(9):1210–1219. doi: 10.1016/j.str.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan MA, Bishop RE. Molecular mechanism for lateral lipid diffusion between the outer membrane external leaflet and a beta-barrel hydrocarbon ruler. Biochemistry. 2009;48(41):9745–9756. doi: 10.1021/bi9013566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osborn MJ, Gander JE, Parisi E, Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium: Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972;247(12):3962–3972. [PubMed] [Google Scholar]
- 28.Koppelman CM, Den Blaauwen T, Duursma MC, Heeren RM, Nanninga N. Escherichia coli minicell membranes are enriched in cardiolipin. J Bacteriol. 2001;183(20):6144–6147. doi: 10.1128/JB.183.20.6144-6147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renner LD, Weibel DB. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc Natl Acad Sci USA. 2011;108(15):6264–6269. doi: 10.1073/pnas.1015757108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renner LD, Weibel DB. MinD and MinE interact with anionic phospholipids and regulate division plane formation in Escherichia coli. J Biol Chem. 2012;287(46):38835–38844. doi: 10.1074/jbc.M112.407817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doan T, et al. FisB mediates membrane fission during sporulation in Bacillus subtilis. Genes Dev. 2013;27(3):322–334. doi: 10.1101/gad.209049.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J Lipid Res. 2008;49(8):1607–1620. doi: 10.1194/jlr.R700018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran TT, et al. Daptomycin-resistant Enterococcus faecalis diverts the antibiotic molecule from the division septum and remodels cell membrane phospholipids. MBio. 2013;4(4) doi: 10.1128/mBio.00281-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmann M, et al. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother. 2010;54(8):3132–3142. doi: 10.1128/AAC.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop RE. The lipid A palmitoyltransferase PagP: Molecular mechanisms and role in bacterial pathogenesis. Mol Microbiol. 2005;57(4):900–912. doi: 10.1111/j.1365-2958.2005.04711.x. [DOI] [PubMed] [Google Scholar]
- 36.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177(14):4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castanié-Cornet MP, Cam K, Jacq A. RcsF is an outer membrane lipoprotein involved in the RcsCDB phosphorelay signaling pathway in Escherichia coli. J Bacteriol. 2006;188(12):4264–4270. doi: 10.1128/JB.00004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 39.Khan MA, et al. Gauging a hydrocarbon ruler by an intrinsic exciton probe. Biochemistry. 2007;46(15):4565–4579. doi: 10.1021/bi602526k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Homma H, Kudo I, Inoue K, Nojima S. Characteristics of phospholipid transacylase of Escherichia coli. J Biochem. 1987;101(4):1033–1039. doi: 10.1093/oxfordjournals.jbchem.a121945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.