Abstract
Physiological pregnancy requires the maternal immune system to recognize and tolerate embryonic Ags. Although multiple mechanisms have been proposed, it is not yet clear how the fetus evades the maternal immune system. In this article, we demonstrate that trophoblast-derived thymic stromal lymphopoietin (TSLP) instructs decidual CD11c+ dendritic cells (dDCs)with increased costimulatory molecules; MHC class II; and Th2/3-type, but not Th1-type, cytokines. TSLP-activated dDCs induce proliferation and differentiation of decidual CD4+CD25− T cells into CD4+CD25+FOXP3+ regulatory T cells (Tregs) through TGF-β1. TSLP-activated dDC–induced Tregs display immunosuppressive features and express Th2-type cytokines. In addition, decidual CD4+CD25+FOXP3+ Tregs promote invasiveness and HLA-G expression of trophoblasts, resulting in preferential production of Th2 cytokines and reduced cytotoxicity in decidual CD56brightCD16− NK cells. Of interest, decreased TSLP expression and reduced numbers of Tregs were observed at the maternal–fetal interface during miscarriage. Our study identifies a novel feedback loop between embryo-derived trophoblasts and maternal decidual leukocytes, which induces a tolerogenic immune response to ensure a successful pregnancy.
Introduction
Physiological pregnancy may be considered a successful embryo allograft (1), in which the maternal immune system recognizes but does not reject paternal Ags expressed in the embryo. Several mechanisms for evading rejection by the maternal immune system have been proposed (2, 3), and the following mechanisms are generally accepted. Trophoblasts do not express classical MHC molecules. However, nonclassical MHC molecules HLA-G, HLA-C, HLA-E, and HLA-F are expressed on most trophoblast populations (4). HLA-G binds to the killing inhibitory receptor on NK cells and protects trophoblasts from NK cell–mediated attack. HLA-G may also protect the fetus from an allogeneic T cell response. The Th2 cytokine environment at the maternal–fetal interface protects trophoblast functions. In addition, immunoregulatory molecules, including IDO, IL-10, and TGF-β, are expressed at high levels at the maternal–fetal interface. Accumulating evidence supports the concept that regulatory T cells (Tregs) play important roles in establishing and maintaining active immune tolerance during pregnancy (3, 5–8). Tregs expand in the periphery and especially at the maternal–fetal interface in human and murine pregnancy (8). The proportion of systemic and decidual Tregs (dTregs) was significantly lower in specimens from women with recurrent miscarriages compared with that in specimens from women with a normal pregnancy (8). However, the source and function of Tregs during pregnancy remain unclear.
Generation and maintenance of Tregs require TGF-β–dependent de novo FOXP3 expression and function by target Ags (9–11). Induction of Tregs by tolerogenic DCs has recently received more attention. Thymic stromal lymphopoietin (TSLP), a member of the IL-7 cytokine family, is selectively expressed in thymic epithelial cells of Hassall’s corpuscles (12). TSLP-activated dendritic cells (TSLP-DCs) induce differentiation of CD4+FOXP3− thymocytes into CD4+FOXP3+ Tregs (12, 13). Generating CD4+CD25+ Tregs can be regarded as the “third function” of the thymus. However, thymic function is degraded in fertile women and further inhibited by steroid hormones during pregnancy. Thus, the thymus is unlikely to be the source of the high numbers of peripheral and dTregs found in pregnancy. Extrathymic generation of Tregs has been proposed and is involved in maternal–fetal tolerance (14). First-trimester human trophoblasts secrete TSLP, and decidual CD11c+ DCs express the TSLP receptor (TSLPR) (15). Thus, decidual DCs (dDCs) may be instructed by trophoblasts via TSLP–TSLPR interactions to induce differentiation of CD4+CD25− T cells into CD4+CD25+FOXP3+ Tregs.
Maternal immune cells must tolerate invading extravillous cytotrophoblasts to allow adequate placental growth and development. HLA-G is thought to play a key role in placenta development by controlling trophoblast invasiveness and maintaining a local immunosuppressive state through interaction with decidual CD56brightCD16− NK cells (16, 17). Secretion of soluble HLA-G by early-stage embryos appears to be necessary for successful pregnancies and may be a marker for increased pregnancy rates by in vitro fertilization (16, 17).
In this study, using different cell cocultures, we investigated the origin of dTregs during early pregnancy in human beings. We identified a novel regulatory loop between embryo-derived trophoblasts and maternal immune cells in the decidua, which induces immune tolerance to facilitate a successful pregnancy.
Materials and Methods
Collection of human placental and decidual tissues during the first trimester of pregnancy
This study was approved by the ethics committee of the Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China. Signed written consent was obtained from each participant. First-trimester human villous tissues were obtained from 84 women with clinically normal pregnancies (age: 28.64 ± 5.99 y; gestational age at sampling: 53.35 ± 7.6 d [mean ± s.d.]), which were terminated for nonmedical reasons. The decidual tissues were obtained from 24 women with clinically normal pregnancies (age: 28.21 ± 5.54 y; gestational age at sampling: 52.79 ± 7.2 d [mean ± s.d.]). All pregnancies were confirmed by ultrasound and blood tests, and were excluded from endocrine, anatomical, and genetic abnormalities, as well as infection. Tissues were immediately collected for the isolation of trophoblasts and decidual lymphocytes.
Immunohistochemistry
Immunohistological staining was performed as previously described (12). Human deciduas were labeled with mouse anti-CD11c and sheep anti-TSLP Abs (R&D Systems). Slides were incubated with mouse anti-human TSLP overnight at 4°C followed by anti-CD11c staining.
Isolation and culture of human trophoblasts
Trophoblasts were isolated by trypsin–DNase I digestion and discontinuous Percoll gradient centrifugation from pooled villi obtained from four to six different pregnancies, as previously described (15). Cells were then seeded in tissue culture plates for further purification based on differential adherent velocity to eliminate adherent fibroblast cells and unattached leukocytes. This method supplies a 95% purity of trophoblast cells, as assessed by immunocytochemistry and flow cytometry (FCM) for cytokeratin 7 positivity, HLA-G positivity, and vimentin negativity (data not shown). Purified trophoblasts were seeded in a 24-well plate at 1 × 106 cells per milliliter. Supernatants from trophoblast culture were collected after 48 h, centrifuged at 2000 × g, and stored at −80°C.
Isolation and culture of decidual CD4+ T cells, DCs, and NKs
Decidual tissues (4–6 g) were cut and digested in RPMI 1640 supplemented with collagenase type IV (1.0 mg/ml, CLS-1; Worthington Biomedical) in 1% FBS for 80 min at 37°C with gentle agitation. The suspension was filtered and enriched by discontinuous Percoll gradient centrifugation, as previously described (15). Decidual CD4+ T cells, CD4+CD25+ T cells, or CD4+CD25− T cells were isolated by MACS using CD4+ and CD4+CD25+ bead kits (Miltenyi Biotec). dDCs were isolated by MACS with the BDCA-1 kit (Miltenyi Biotec). dDC purity was 86.8 ± 7.8%, as determined by FCM with PE-CD11c. CD56brightCD16− decidual NK (dNK) cells were purified with microbeads conjugated to anti-human CD56 mAb (Miltenyi Biotec). dNK purity was 95 ± 6.5%, as measured by FCM with FITC-CD56, CD3-PE, and CD16-allophycocyanin.
dDCs were cultured for 24 h in media containing control, recombinant human (rh) TSLP (20 ng/ml; R&D Systems), rhIL-7 (50 ng/ml; PeproTech), LPS (10 μg/ml; Sigma-Aldrich), or trophoblast-derived supernatant. Neutralizing anti-TSLP Ab (50 μg/ml; R&D Systems) was added to some culture.
DC and CD4+T cell coculture
dDCs were cultured for 24 h in media containing rhIL-7 (50 ng/ml; R&D Systems), rhTSLP (20 ng/ml; R&D Systems), or supernatant with or without neutralizing anti-TSLP Ab (50 μg/ml; R&D Systems). Cells were collected and washed to remove soluble molecules, and then cocultured with 2 × 104 freshly isolated autologous decidual CD4+CD25− T cells in round-bottom 96-well culture plates for 7 d. Neutralizing TGF-β1 (20 μg/ml; R&D Systems) and/or IL-10 (2 μg/ml; R&D Systems) Abs or isotype control abs were added to individual wells. Increasing ratios of DCs to T cells were evaluated, and a 1:6 ratio was selected. In parallel experiments, isolated T cells were cultured with plate-bound anti-CD3 Ab (OKT-3; 5 μg/ml), soluble anti-CD28 (28.2; 1 μg/ml), and 20 U/ml IL-2 for 7 d. In addition, decidual CD4+CD25− and CD4+CD25+ subpopulations were cultured for 7 d with dDCs instructed by media or TSLP. Cells were collected and analyzed by FCM for CD4, CD25, and FOXP3. In another experiment, decidual CD4+ T cells were separated into CD4+CD25− and CD4+CD25+ subpopulations, which were then labeled with CFSE (Molecular Probes, Carlsbad, CA) or PKH26 (Sigma-Aldrich, St. Louis, MO), as previously described (13). CFSE-labeled CD4+CD25+ Tregs, PKH26-labeled CD4+CD25− T cells, and a mixture of the two populations at an original 1:9 CD25+CD25− cell ratio were cultured for 6 d with TSLP-dDCs. Cells were stained for CD4 and FOXP3 and analyzed by FCM.
NK and human primary trophoblast coculture
Human primary trophoblasts were cultured with or without CD4+CD25+ dTregs at a 1:4 Treg/trophoblast ratio. Media and CD4+CD25+ dTregs were removed after 24 h of culture. The remaining trophoblasts were cocultured with 2 × 105 CD56brightCD16− dNK cells. Increasing ratios were evaluated, and a 1:1 ratio of NK/Tro cells was selected for future experiments. A neutralizing HLA-G Ab (20 μg/ml, clone 87G; BioLegend) or isotype control was added to individual wells. dNK cells were harvested for FCM or NK cytotoxic assay after 3 d of coculture.
Flow cytometry
Cells were washed, treated with Fc-block (anti-CD16/CD32 Abs) for 15 min, and incubated with the appropriate fluorochrome-conjugated Abs for 30 min at 4°C for cell surface staining. The Abs in this study were FITC-conjugated mAbs against CD14, CD56, and CD80; PE-conjugated anti-CD3, CD86, and CD83; PerCP-conjugated anti-CD4; allophycocyanin-conjugated anti-CD16, HLA-DR, and OX-40L; and PerCy5.5-conjugated anti-CD40 (eBioscience). FITC-conjugated anti–HLA-G (clone MEM-G9) was from Abcam. Cells were fixed and labeled according to the manufacturer’s protocol for intracellular FOXP3, GATA3, HLA-G5, and cytokine staining. FITC-conjugated mAbs against human IFN-γ, Alexa Fluor 488–conjugated anti-human FOXP3, PE-conjugated anti-human TNF-α, GATA3, and CD25, allophycocyanin-conjugated anti-human FOXP3, IL-4, IL-5, and IL-10, PerCy5.5-conjugated anti-human IL-4 Abs, or their corresponding isotype controls were used (eBioscience). HLA-G5–specific Ab 5A6G7 was obtained from Abcam. FCM analysis was performed on a FACScan machine with CellQuest Software (BD Biosciences). Postacquisition FACS data were analyzed with FCS Express version 3 (De Novo Software).
ELISA
Supernatants were collected from each group, centrifuged at 2000 × g, and stored at −80°C. IL-10, TGF-β1, IFN-γ, TNF-α, and IL-12p70 concentrations were quantified in cell culture supernatants with cytokine-specific ELISA kits following the manufacturer’s instructions (R&D Systems).
Suppressive functional assays
Decidual native CD4+CD25− T cells, CD4+CD25+ T cells, or CD4+CD25+ T cells generated in culture with trophoblast-instructed DCs, or their mixtures, were seeded in triplicate in round-bottom 96-well plates. Cultures were stimulated for 7 d in 5 μg/ml anti-CD3, 1 μg/ml anti-CD28, and 20 IU/ml rhIL-2. Cellular proliferation was assessed by [3H]-thymidine incorporation, as previously described (11). Individual supernatants were collected to measure secreted IFN-γ concentrations.
Matrigel invasion assay
Trophoblast invasion across Matrigel was evaluated objectively in an invasion chamber based on our previous procedure (18). Cell culture inserts were precoated with Matrigel and placed in a 24-well plate. Trophoblasts (2 × 105 in 200 μl DMEM with 2% FBS) were plated in the upper chamber. Decidual CD4+CD25+ Tregs (in different cell ratios of T/Tros) were plated in the upper chamber (direct cell contact) or in the lower chamber (indirect cell contact). Cells were preincubated with neutralizing Abs against TGF-β1 (20 μg/ml) or IL-10 (2 μg/ml) in some experiments. TGF-β1 (20 μg/ml) or IL-10 (2 μg/ml) Abs alone were used as controls. The lower chamber was filled with 500 μl DMEM with 10% FBS, and cells were incubated at 37°C for 48 h.
Inserts were removed, and noninvading cells with Matrigel were removed from the upper surface of the filter by wiping with a cotton bud. The inserts were fixed and stained with hematoxylin. Cells were observed with an Olympus BX51tDP70 fluorescence microscope. Cells that migrated to the lower surface were counted at a magnification of ×200. Each experiment was carried out in triplicate and repeated three times independently.
Western blot
Purified primary trophoblasts were cultured in six-well plates alone or with native CD4+CD25+ dTregs (Tregs/trophoblasts at a 1:4 ratio). Trophoblasts were harvested after 48 h of treatment. A total of 50 μg protein from each treatment group was separated on a 10% NaDodSO4 polyacrylamide gel and transferred to polyvinyl difluoride membranes, which were blocked and then immunoblotted with mouse anti–HLA-G (1:500, clone 87G; BioLegend) and mouse anti-GAPDH (1:10000, KangChen) overnight at 4°C. Bound Abs were visualized with peroxidase-conjugated secondary Abs (Santa Cruz Biotechnology), followed by detection with an ECL Kit (Pierce) on Las-300 (Fujifilm).
NK cytotoxicity assay
NK cytotoxicity was determined with the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (G1780; Promega) following the manufacturer’s instructions. dNK cells (effector cells; 100 μl) at concentrations of 2.0 × 106/ml, 1.0 × 106/ml, and 0.50 × 106/ml were mixed with 100 μl K-562 cells (target cells) at a concentration of 5.0 × 105/ml, resulting in three E:T ratios of 40:1, 20:1, and 10:1, respectively. Each E:T ratio was examined in triplicate experiments. The percentage of cytotoxicity was calculated after correcting for LDH release from NK cells with the following formula: Percent Cytotoxicity = 100 × (Experimental – Culture Medium Background)/(Maximum LDH Release – Culture Medium Background). LDHexperimental represented LDH release activity from cocultures of effector and target cells. LDHCulture Medium Background represented LDH release from K-562 cells. LDHmaximal represented LDH release from K-562 cells that were lysed by sonication.
Statistical analysis
All data are presented as mean ± SEM. Significance of differences between two groups was determined by two-tailed t tests. Multiple groups were analyzed with GraphPad Prism version 5 by one-way or two-way ANOVA with Bonferroni posttests. For all statistical tests, p values < 0.05 were considered statistically significant.
Results
Trophoblasts activate dDCs by secreting TSLP
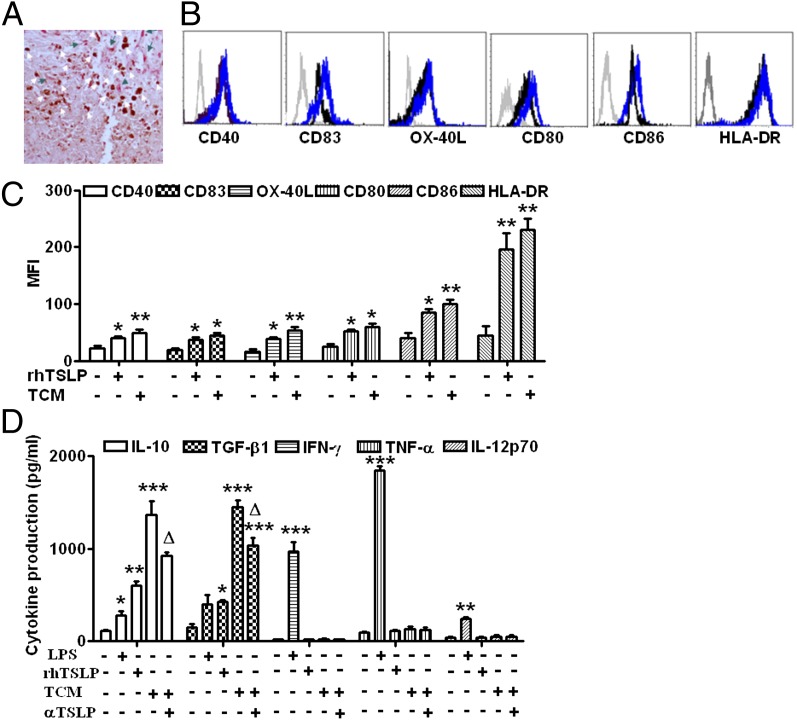
To investigate the action of trophoblasts on dDCs, we first examined the localization of CD11c+ dDCs and trophoblasts within human decidua. We have found that clusters of dDCs are present at the decidual basalis around the spiral artery and in decidual gland epithelial cells where large amounts of invasive trophoblasts are present. This finding provides anatomical evidence that TSLP-expressing trophoblasts are in close association with dDCs at the maternal–fetal interface (Fig. 1A).
FIGURE 1.
Trophoblast-derived TSLP activates decidual CD11c+ DCs. (A) The close association between TSLP+ trophoblasts (pink cells indicated by white arrows) and CD11c+ DCs (brown cells indicated by green arrows) is shown by TSLP and CD11c+ DC double staining in human decidua. (B and C) CD40, CD83, OX-40L, CD80, CD80, CD86, and HLA-DR expression was determined by FCM with gating on CD11c+ cells (B) after dDCs were stimulated with TSLP, trophoblast culture media, or media alone (control) for 24 h. The numbers in the histograms (C) indicate MFI. (D) Production of IL-10, TGF-β1, IFN-γ, TNF-α, and IL-12p70 (the heterodimeric biologically active form of IL-12) by dDCs was determined by ELISA. Results are shown from three independent experiments with three different samples from 3 decidua and 15 villi. Data are shown as mean ± SE. A representative graph is shown. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with control; △p < 0.05 compared with TCM. One-way ANOVA with Bonferroni posttests was used for (C), and two-way ANOVA with Bonferroni posttests was used in (D). αTSLP, neutralizing Ab against TSLP.
We then examined the phenotypes of dDCs treated with rhTSLP or trophoblast-derived supernatant. As expected, dDCs expressed relatively low levels of cell surface molecules, such as CD40, CD83, CD80, CD86, HLA-DR, and OX-40L (Fig. 1B). TSLP or trophoblast supernatant induced increased expression of these molecules above (Fig. 1B, 1C). In addition, TSLP and trophoblast supernatants also stimulated dDCs to produce high levels of IL-10 and TGF-β1 instead of proinflammatory cytokines, such as TNF-α, IFN-γ, and IL-12p70 (Fig. 1D), which was a unique feature of TSLP-mediated activation of DCs. Pretreatment with anti-TSLP neutralizing Ab significantly decreased the production of IL-10 and TGF-β1, which suggests that trophoblast-mediated activation of dDCs and subsequent production of IL-10 and TGF-β1 occur via TSLP secretion. Because trophoblasts themselves secrete high levels of IL-10 and TGF-β1 independently of TSLP signaling, pretreatment with anti-TSLP neutralizing Ab only partially decreased IL-10 and TGF-β1 secretion from supernatant-treated DCs (Fig. 1D).
TSLP-dDCs induce differentiation of dCD4+CD25− T cells into CD4+CD25+FOXP3+ Tregs through TGF-β1
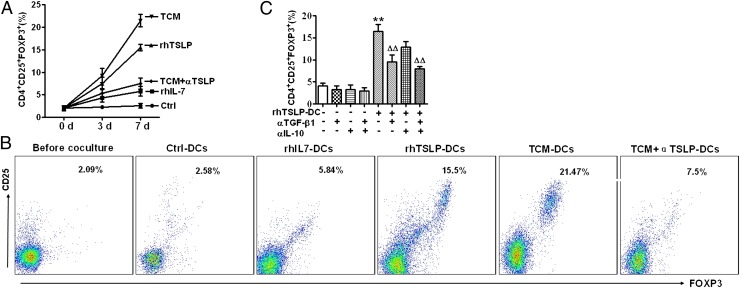
TSLP-DCs in the thymic medulla are critical for stimulating differentiation of high-affinity autoreactive developing T cells into Tregs (11). Therefore, we investigated whether TSLP-instructed DCs in decidua induced Treg differentiation. As shown in Fig. 2A, decidual DCs treated by TSLP or trophoblast culture media (TCM) (human trophoblast–derived supernatant) for 24 h induced significantly higher percentages (7.53 ± 2.4% and 9.27 ± 2.7%, respectively) of CD4+CD25+FOXP3+ Tregs in comparison with untreated and IL-7–treated DCs (2.27 ± 0.25% and 4.3 ± 1.7%, respectively) after 3 d of culture. The percentage of CD4+CD25+FOXP3+ Tregs increased even further after 7 d of culture with dDCs treated for 24 h by TSLP or TCM (15.5 ± 2.1% and 21.47 ± 2.34%, respectively) (Fig. 2B). In contrast, stimulation of CD4+CD25− T cells with anti-CD3, anti-CD28, and IL-2 induced vigorous expansion, but not differentiation of CD4+CD25− cells into FOXP3+ cells (Supplemental Fig. 1). Importantly, treatment of dDC with the anti-TSLP neutralizing Ab abolished the increase in CD4+CD25+FOXP3+ Tregs that were induced by the supernatant-treated dDCs (Fig. 2B). These results suggest that trophoblast-secreted TSLP instructs dDCs to induce conversion of CD4+CD25− T cells into CD4+CD25+FOXP3+ Tregs. However, stimulation by anti-CD3, anti-CD28, and IL-2 is not sufficient to induce differentiation of human CD4+CD25− T cells into FOXP3+ cells. Of note, induction of CD4+CD25+FOXP3+ Tregs was abrogated by treatment with an anti–TGF-β1 neutralizing Ab. In contrast, an anti–IL-10 neutralizing Ab did not have this effect (Fig. 2C). Thus, TGF-β1 signaling, but not IL-10 signaling, appears to be involved in the induction of Tregs by TSLP-dDCs.
FIGURE 2.
TSLP-DCs induce expansion of CD4+CD25+FOXP3+ T cells from decidual CD4+CD25− T cells in vitro. (A) Purified CD4+CD25− T cells were incubated with DCs pretreated with PBS, rhIL-7, rhTSLP, or TCM or with TCM combined with αTSLP-DCs at a 1:6 ratio of DCs to T cells for 3 or 7 d. Cells were analyzed by FCM gating of CD4+ cells. (A) Percentages of CD4+CD25+FOXP3+ T cells are indicated. (B) Representative FCM data after 7 d of culture are presented. (C) Percentages of CD4+CD25+FOXP3+ T cells determined by FCM following 7-d coculture of decidual CD4+CD25− T cells with or without rhTSLP-DCs in the presence or absence of anti–TGF-β1 or anti–IL-10 neutralizing Abs or isotype control. Results are shown from three independent experiments with three different deciduas and 17 villi. Data are shown as mean ± SE. A representative graph is shown. *p < 0.05 and **p < 0.01 compared with control; △△p < 0.01 compared with rhTSLP-DC. Two-way ANOVA with Bonferroni posttests was used in (C). αIL-10, neutralizing Ab against IL-10; αTGF-β1, neutralizing Ab against TGF-β1; Ctrl, control.
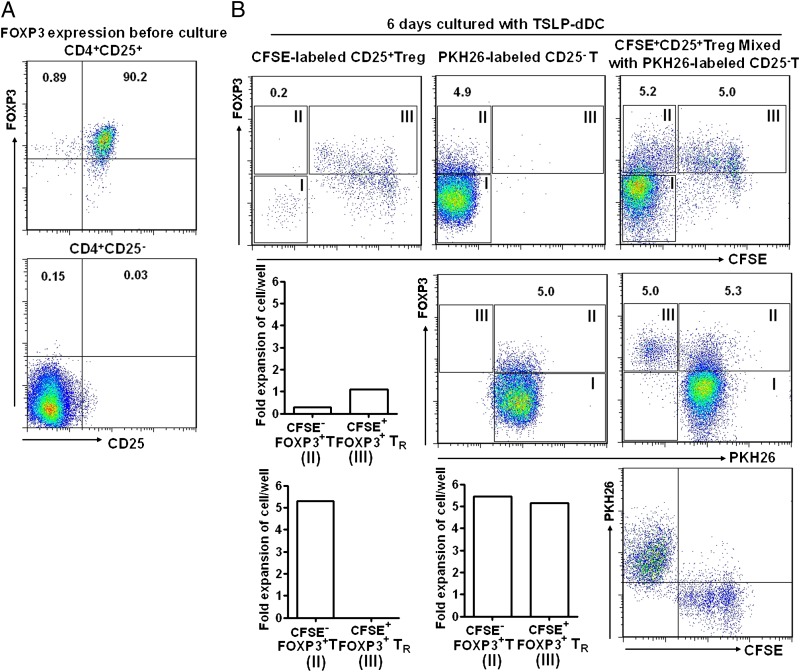
We then examined whether TSLP-dDCs induced differentiation of CD4+CD25−FOXP3− cells into CD4+CD25+FOXP3+ cells, or TSLP-dDCs induced expansion of a small number of contaminating CD4+CD25+FOXP3+ Tregs. We performed mixed cell culture with CFSE-labeled CD4+CD25+ Tregs and PKH26-labeled CD4+CD25− cells (12). Sorted CD4+CD25+ T cells were labeled with CFSE and mixed with PKH26-labeled CD4+CD25− T cells at a 1:9 ratio. This ratio was selected because the original decidual CD4+ T population contained CD25+ and CD25− cells at approximately a 1:9 ratio (data not shown). These mixed cells were cultured together with TSLP-dDCs, and proliferation of the two populations was compared (Fig. 3A, 3B). We found that decidual CD4+CD25+ Tregs expanded more than 5-fold in the mixed culture, which was similar to the level of expansion observed for CD4+CD25− cells. It seems unlikely that contaminating CD4+CD25+FOXP3+ Tregs, accounting for < 0.2% of CD4+CD25− in the cell culture, can expand to 5.2% in the cell culture. In addition, there is no difference in the cell viability of the different T cell populations under TSLP-dDC treatment (Supplemental Fig. 2). These data indicate that TSLP-dDCs preferentially induce CD4+CD25− T cells to differentiate into CD4+CD25+FOXP3+ T cells. This finding was further confirmed by data showing that TSLP-dDCs induced CD4+CD25− cells to proliferate and differentiate into CD4+CD25+ T cells in large numbers, whereas TSLP-DCs induced only limited proliferation and expansion of CD4+CD25+ T cells (Supplemental Fig. 3).
FIGURE 3.
TSLP-dDCs induce generation of CD4+FOXP3+ Tregs from CD4+CD25−FOXP3− T cells, but not from contaminating CD4+CD25+FOXP3+ cells in sorted CD4+CD25− T cells. (A) FCM analysis of FOXP3 expression on sorted CD4+CD25+ and CD4+CD25− T cells before culture. The numbers in dot plots indicate the percentages of FOXP3+ T cells. (B) CFSE-labeled CD4+CD25+ T cells, PKH26-labeled CD4+CD25− T cells, and a mixture of the two populations were cocultured with TSLP-dDCs at a 1:9 CD25+:CD25− cell ratio. After 6 d of culture, FOXP3 expression on each population was analyzed by FCM. The numbers in dot plots indicate the percentages of PKH26-labeled FOXP3+ cells (gate II). The dot plot at the bottom right was gated from FOXP3+ cells in a mixture of the CFSE-labeled CD4+CD25+ T cells, PKH26-labeled CD4+CD25− T cells at a 1:9 CD25+:CD25− cell ratio. Fold expansions are shown for PKH26-labeled FOXP3+ cells (gate II) and CFSE-labeled FOXP3+ cells (gate III) in the bar graph. Data are representative of three experiments with three different deciduas.
TSLP-dDC–induced CD4+CD25+FOXP3+ T cells display immune regulatory features
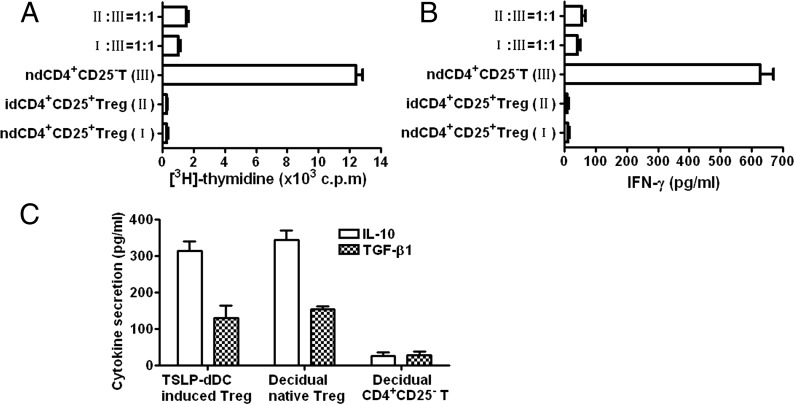
To confirm that CD4+CD25+FOXP3+ T cells induced by TSLP-dDCs were functional Tregs, we investigated the in vitro capacity of these cells to inhibit proliferation and IFN-γ production from CD4+CD25− T cells. In vivo-derived CD4+CD25+ T cells and in vitro-generated CD4+CD25+FOXP3+ T cells showed minimal responses to anti-CD3 and anti-CD28 treatments (Fig. 4A). In contrast, decidual CD4+CD25− T cells displayed vigorous proliferation in response to anti-CD3 and anti-CD28 treatments. Proliferation was inhibited by decidual CD4+CD25+ T cells and by CD4+CD25+FOXP3+ T cells produced from TSLP-dDC–stimulated decidual CD4+CD25− T cells. These findings suggest that newly generated CD4+CD25+FOXP3+ T cells display key features of suppressor T cells and exert suppressive effects on IFN-γ release (Fig. 4B).
FIGURE 4.
TSLP-DC–induced CD4+CD25+FOXP3+ T cells display features of Tregs. TSLP-DC–induced CD4+CD25+FOXP3+ T cells, decidual naive CD4+CD25+ T cells, CD4+CD25− cells, or their mixtures were restimulated with anti-CD3 and anti-CD28 Abs. (A) Suppressive function assessed by [3H]-thymidine incorporation. (B) Quantification of IFN-γ secreted by CD4+ T cells. (C) Quantification of IL-10 and TGF-β1 secreted by different CD4+ T cells as indicated. Results are shown from three independent experiments with three different deciduas. Data are shown as mean ± SE. A representative graph is shown.
Tregs are characterized by high levels of IL-10 and TGF-β1 production. Therefore, we investigated whether TSLP-dDC–induced dCD4+CD25+ T cells displayed the same functional phenotypes. As expected, TSLP-dDC–induced CD4+CD25+FOXP3+ T cells produced high levels of TGF-β1 and IL-10 compared with decidual CD4+CD25− T cells. Secretion of IL-10 and TGF-β1 by TSLP-dDC–induced CD4+CD25+FOXP3+ Tregs was similar to that of the native CD4+CD25+ dTregs (Fig. 4C).
We previously showed that decidual T cells acquired a Th2 phenotype in response to TSLP-exposed dDCs (15). We then determined if the induced dTregs also produced Th2 cytokines. Thus, we stained CD4+ T cells with IL-4, IL-5, GATA3, and FOXP3 Abs. More than 60% of CD4+FOXP3+ T cells expressed IL-5, and ∼ 10% of CD4+FOXP3+ T cells were IL-4+ (Fig. 5). GATA3, which is a specific marker of the Th2 phenotype and a critical transcription factor for Th2-type cell differentiation, was also expressed in ∼ 4% of CD4+FOXP3+ T cells. In the parallel experiment, we analyzed the percentage of GATA3, IL-5, and IL-4 in CD4+ T cells that had been polarized to Th2 in vitro. The data in Supplemental Fig. 4 showed that polarization of Th2 was characterized by increased GATA3 expression (accounting for 8% in CD4+ T cells), which was accompanied by high percentages of IL-4 (∼29%) and IL-5 (∼74%) in CD4+ T cells. Distinct from the induction of FOXP3 expression in CD4+ T cells by TSLP-dDCs, the Th2-polarized CD4+ T cells expressed a very low percentage of FOXP3. Combining the current results with our previous study (15), TSLP-DCs thus appear to induce the differentiation of naive T cells into Th2/Tregs.
FIGURE 5.
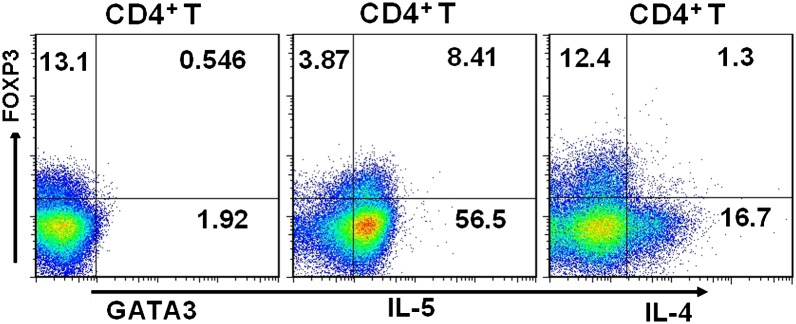
TSLP-dDC–induced decidual CD4+CD25+FOXP3+ T cells produce Th2 cytokines. Decidual CD4+FOXP3+ T cells generated by TSLP-DC were processed for FCM analysis after staining for IL-4 or IL-5, CD4, FOXP3, and GATA3. Decidual CD4+CD25− T cells were cultured with TSLP-dDCs for 7 d. Cultured cells were then stimulated with PMA and ionomycin. Expression levels of cell surface CD4 and intracellular IL-4, IL-5, FOXP3, and GATA3 at the single-cell level were analyzed by FCM. In parallel experiments, the polarizations of Th1 and Th2 were induced as the positive and negative controls for the positivity gates identifying GATA3+ cells, IL-5+ cells, and IL-4+ cells. Results are shown from three independent experiments with three different deciduas. The dot plots are representative of three experiments with three different deciduas.
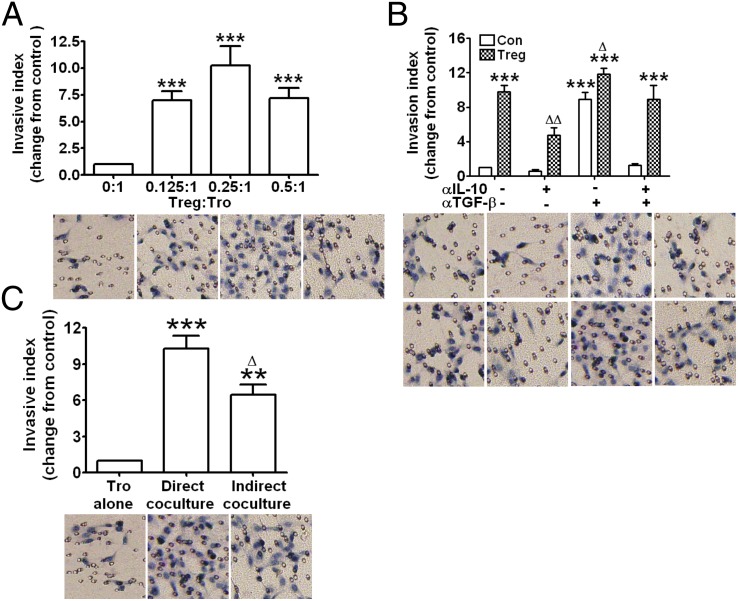
TSLP-dDC–induced CD4+CD25+ Tregs increase invasiveness of human trophoblasts
To investigate the possible regulatory role of TSLP-instructed dTregs in maternal–fetal crosstalk, primary trophoblasts were obtained and cocultured with dTregs at different ratios (E:T ratio). Proliferation of trophoblasts was not significantly affected after 48 h of coculture with dTregs (data not shown). In contrast, Tregs significantly enhanced invasiveness of primary trophoblasts at 1:0.125, 1:0.25, and 1:0.5 ratios of responder trophoblasts to dTregs compared with trophoblasts alone. This enhancement was greatest with the 1:0.25 cell ratio (Fig. 6A). Because secretion of soluble cytokines, including IL-10 and TGF-β1, is one of the most important mechanisms involved in human Treg functions, we examined whether these cytokines were key regulators of trophoblast invasiveness. Addition of anti–IL-10 neutralizing Ab significantly inhibited Treg-mediated invasion of trophoblasts (Fig. 6B). Surprisingly, pretreatment with the anti–TGF-β1 neutralizing Ab remarkably increased trophoblast invasiveness. These results indicate that Tregs might induce trophoblast invasiveness by secreting IL-10 and may prevent excessive invasion of trophoblasts by secreting TGF-β1.
FIGURE 6.
TSLP-dDC—induced decidual CD4+CD25+FOXP3+ T cells improve invasiveness of human trophoblasts. The invasiveness of primary trophoblasts in different treatment conditions for 48 h was evaluated by Transwell invasion assay. The invasion index of human primary trophoblasts cocultured with different ratios of Tregs and/or with neutralizing Abs for IL-10 and TGF-β1 was normalized to that of control trophoblasts alone. (A) Trophoblast invasiveness was enhanced by TSLP-dDC–induced CD4+FOXP3+ Tregs in a ratio-dependent manner. (B) Addition of IL-10 neutralizing Ab inhibited basal and Treg-stimulated trophoblast invasiveness. TGF-β1 neutralizing Ab enhanced basal invasiveness and magnified the Treg-stimulated trophoblast invasiveness. (C) The invasive index was even higher in the coculture system with direct cell contact compared with the indirect cell contact coculture system. Results are shown from three independent experiments with three different samples with 3 deciduas and 22 villi. Data are shown as mean ± SE. A representative graph is shown. **p < 0.01 and ***p < 0.001 compared with the control (trophoblasts alone). △p < 0.05 and △△p < 0.01 compared with Treg–trophoblast direct cell contact coculture system. The t test one-way ANOVA with Bonferroni posttests was used for (A), and two-way ANOVA with Bonferroni posttests was used in (B) and (C).
Cell contact between Tregs and responder cells appears to be necessary for Treg-mediated suppression. So we further investigated whether enhanced invasiveness of human trophoblasts by dTregs occurs by the same mechanism. We used a well-established system for direct and indirect cell cocultures. Coculture with Tregs increased the invasive index of trophoblasts with both indirect and direct cell contact (Fig. 6C; p < 0.01, p < 0.001). The invasive index was higher in the coculture that allowed direct cell contact in comparison with that observed in the indirect cell contact coculture (p < 0.05). These data suggest that both cell surface molecules and soluble cytokines are involved in Treg-mediated trophoblast invasion.
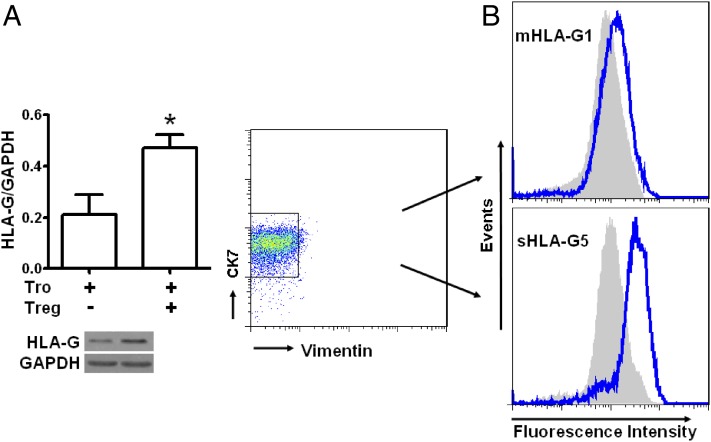
Decidual CD4+CD25+ Tregs upregulate HLA-G expression in human trophoblasts
HLA-G expression in trophoblasts is believed to be a fundamental prerequisite for promoting trophoblast invasion and maintaining local immune tolerance during pregnancy. We investigated then whether Tregs at the maternal–fetal interface could regulate HLA-G expression in trophoblasts. Trophoblasts were isolated and cocultured with Tregs at a 4:1 ratio. Western blots with the 87G Ab, which recognizes HLA-G1 and HLA-G5, showed that HLA-G expression in trophoblasts was significantly increased by coculture with Tregs (Fig. 7A). These data suggest that CD4+CD25+ Tregs may upregulate HLA-G expression in trophoblasts. Expression levels of membrane-bound HLA-G1 and intracellular HLA-G5 in trophoblasts were confirmed by FCM with MEM-G9 and 5A6G7 Abs. Although the percentage of trophoblast cells positive for either HLA-G1 or HLA-G5 was not affected by dTreg treatment (data not shown), dTregs slightly upregulated the mean fluorescence intensity (MFI) of membrane-bound HLA-G1 and markedly increased the MFI of intracellular HLA-G5 in trophoblasts (Fig. 7B).
FIGURE 7.
dTregs upregulate HLA-G expression in human primary trophoblasts. Primary trophoblasts were cultured in Matrigel-precoated six-well plates in the presence or absence of dTregs for 24 h. Trophoblasts were washed with PBS twice after discarding floating T cells. Expression levels of HLA-G by trophoblast cells were determined by Western blot with 87G (A) and FCM analysis with MEM-G/9 (B, upper panel) and 5A6G7 (B, lower panel). The blue and gray histograms represent HLA-G fluorescence intensities of trophoblasts cocultured with or without Tregs, respectively, as determined by FCM analysis. Results are shown from three independent experiments with 3 different deciduas and 13 villi. Data are shown as mean ± SE. The representative graph is shown. *p < 0.05 compared with NK culture alone. Tro, trophoblasts; Tro–Tregs, trophoblasts instructed by Tregs for 24 h.
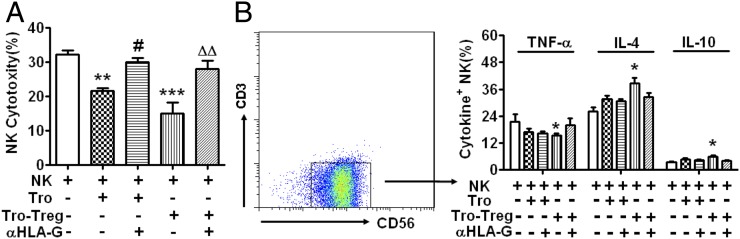
Decidual CD4+CD25+ Treg–upregulated HLA-G in trophoblasts is involved in modulating dNK cell function during early pregnancy
HLA-G expressed by human trophoblasts plays a key role in regulating dNK functions (16, 17). We investigated whether CD4+CD25+ dTregs regulate dNK cell function via upregulating HLA-G expression in trophoblasts. The cytotoxicity of dNK cells significantly decreased when cocultured with trophoblasts (p < 0.01) (Fig. 8A). This effect was particularly robust in dTreg-instructed trophoblasts (p < 0.001). Treatment with a neutralizing Ab against HLA-G (clone 87G, which recognizes both HLA-G1 and HLA-G5 isoforms) abrogated this decrease in cytotoxicity (p < 0.05, p < 0.01). Furthermore, Treg-instructed trophoblasts increased expression of Th2 cytokines IL-4 and IL-10 but inhibited expression of the Th1 cytokine TNF-α in dNK cells (Fig. 8B). However, human trophoblasts in the absence of Tregs had no significant influence on cytokine expression in NK cells. Of interest, the HLA-G neutralizing Ab did not significantly reverse CD4+CD25+ dTreg–mediated cytokine regulation of trophoblasts. Therefore, other molecules may modulate production of cytokines by dNK cells in response to Treg-instructed trophoblasts (Fig. 8B).
FIGURE 8.
HLA-G is involved in the modulation of dNK cells by dTreg-instructed primary human trophoblasts. Primary trophoblasts were cultured in Matrigel-precoated six-well plates in the presence or absence of dTregs for 24 h. Trophoblasts were washed with PBS twice after removing floating T cells. The coculture included dNKs and trophoblasts at a 1:1 ratio. dNK cells were harvested after 48 h for dNK cell cytotoxicity assays or to measure intracellular cytokines by FCM. (A) The cytotoxicity of dNK cells was significantly decreased when cocultured with trophoblasts, especially with dTreg-instructed trophoblasts. Neutralizing Abs against HLA-G abrogated this decrease. (B) Treg-instructed trophoblasts increased expression of IL-4 and IL-10 and inhibited TNF-α in dNK cells. Neutralizing Abs against HLA-G slightly reversed this upregulation. The FCM plot shows cytokine expression analysis. Results are shown from three independent experiments with 3 different deciduas and 17 villi. Data are shown as mean ± SE. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with NK culture alone. ##p < 0.01 compared with NK cells cultured with Tro. △△p < 0.01 compared with NK cells cultured with Tro–Tregs. αHLA-G, neutralizing Ab for HLA-G; Tro, trophoblasts; Tro–Tregs, trophoblasts instructed by Tregs for 24 h.
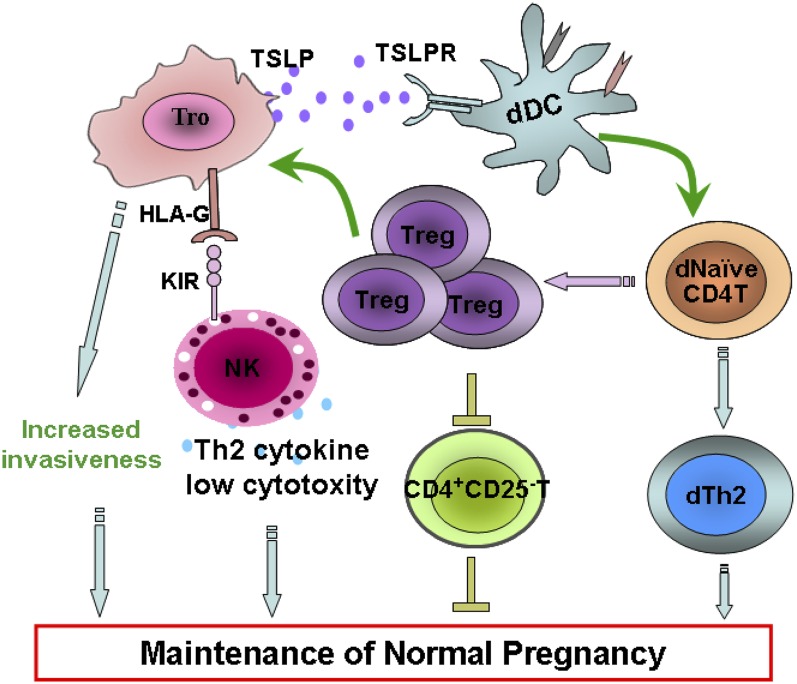
The cross-talk between embryonic trophoblasts and decidual leukocytes in human first-trimester pregnancy
In summary, our data described an interactive cross-talk and feedback between embryo-derived trophoblasts and maternal leukocyte subsets at the maternal–fetal interface, contributing to maternal–fetal immunotolerance and placental growth and development (Fig. 9). TSLP secreted by human trophoblasts instructs dDCs and then induces generation of decidual CD4+CD25+FOXP3+ Tregs in early pregnancy. These cells in turn inhibit proliferation and IFN-γ secretion from CD4+CD25−T cells and increase invasiveness and HLA-G expression in trophoblasts. These alterations decrease cytotoxicity of decidual CD56brightCD16− NK cells and increase expression of inhibitory cytokines. All these are beneficial to maintenance of normal pregnancy.
FIGURE 9.
Proposed model for crosstalk between embryo trophoblasts and decidual leukocyte subsets at the maternal–fetal interface in human first-trimester pregnancy. TSLP secreted by human trophoblasts instructs dDCs and then induces generation of decidual CD4+CD25+FOXP3+ Tregs in early pregnancy. These cells in turn inhibit proliferation and IFN-γ secretion from CD4+CD25− T cells and increase invasiveness and HLA-G expression in trophoblasts. These alterations decrease cytotoxicity of decidual CD56brightCD16− NK cells and increase expression of inhibitory cytokines. This interactive crosstalk between trophoblasts and decidual leukocytes contributes to maternal immunotolerance toward fetal Ags and placentation.
Discussion
In the current study, we have demonstrated that trophoblast-secreted TSLP instructs dDCs, which then induce generation of CD4+CD25+FOXP3+ dTregs during early pregnancy. Tregs inhibit proliferation of CD4+CD25− effector T cells and IFN-γ release and enhance invasiveness and HLA-G expression of trophoblasts. Treg-instructed trophoblasts decrease cytotoxicity of CD56brightCD16− dNKs and stimulate expression of inhibitory cytokines. The interactive crosstalk and feedback between embryo-derived trophoblasts and maternal leukocyte subsets contribute to maternal–fetal immunotolerance and placental growth and development (Fig. 9). TSLP-instructed dDCs induce the conversion of CD4+CD25− T cells into functional Tregs via TGF-β1. However, IL-10 and TGF-β1 play opposite roles in Treg-promoted trophoblast invasiveness. To our knowledge, these data are the first to show that CD4+CD25+FOXP3+ dTregs originate from the unique decidual microenvironment, and a positive regulatory loop exists between trophoblasts and decidual immune cell subsets to induce maternal–fetal immune tolerance during pregnancy. Of interest, TSLP-induced Tregs also produce Th2 cytokines, such as IL-5 and IL-4. The Th2-associated transcription factor GATA3 was also expressed by CD4+FOXP3+ T cells in our experimental system. This finding is consistent with several reports that ablation of a conditional Gata3 allele in Tregs leads to Treg dysfunction, and induced Tregs control Th2 inflammation, suggesting an essential role for GATA3 in the fate of FOXP3+ Tregs (19–21).
TSLP was first identified in conditioned media from mouse thymic stromal cell cultures (22). As a member of the IL-7 cytokine family, TSLP is expressed primarily by barrier epithelial cells. TSLP expression by thymic epithelial cells of Hassall’s corpuscles potently activates human myeloid DCs, which can potently induce homeostatic expansion of naive CD4+ T cells (12). TSLP produced by medullary thymic epithelial cells contributes to FOXP3 expression and Treg maturation (13). Increasing numbers of studies have shown that TSLP plays roles in the Th2 response and progression of allergic diseases (23–25).
Pregnancy is a unique situation in which the allogeneic fetus is actively tolerated by the maternal immune system. Trophoblasts can influence the immune system during pregnancy by expressing soluble and cell surface molecules, such as HLA-G (16, 17), IDO (26), and noninflammatory cytokines (27, 15). These molecules can limit proliferation and activation of T cells, APCs, and NK cells in deciduas. Pregnancy induces T cell anergy by selectively stimulating accumulation of maternal CD4+FOXP3+ T cells with fetal specificity (28). CD4+CD25+ Tregs play a major role in preventing autoimmunity and tolerating allogeneic organ grafts (29). Increasingly, data indicate that Tregs play key roles in mediating intrauterine survival of the fetus (5–8). Indeed, many studies have shown that Tregs are critical for normal pregnancy. For example, an absence of Tregs impairs murine pregnancy, whereas adoptive transfer of Tregs prevents fetal rejection (30). CD4+CD25+ T cells are expanded at the maternal–fetal interface and in the peripheral blood. The percentage of CD4+CD25bright T cells was higher in decidual tissue than in peripheral blood. In humans, pregnancy wastage has been associated with reduced Treg levels, and therapy to increase Tregs improves pregnancy outcomes (6, 8). Fetal-specific Tregs maintain tolerance to pre-existing fetal Ags after delivery and rapidly reaccumulate during subsequent pregnancy. Thus, pregnancy imprints CD4+FOXP3+ T cells, which sustain protective regulatory memory to fetal Ags (28). Functional studies have shown that Tregs can regulate immune responses directly at the maternal–fetal interface (8). Tregs also create a tolerant microenvironment by interacting with DCs and NK cells and inducing expression of immune regulatory molecules, such as TGF-β, LIF, or HO-1 directly at the maternal–fetal interface (31). The present study demonstrates that decidual CD4+CD25+ Tregs that are produced in decidua modulate dNK cell tolerance via trophoblasts.
The human maternal–fetal interface is characterized by intimate contact between the maternal decidua and extravillous cytotrophoblasts that invade the decidua. Trophoblasts play central parts in the development and maintenance of a successful pregnancy, as they modulate maternal immune cells to tolerate the fetus (32–37). Invasive trophoblasts encounter maternal immune cells, particularly NK cells, which aid in uterine tissue invasion and remodeling of uterine spiral arteries (36). During these processes, HLA-G is thought to play a vital role in the maintenance of maternal–fetal tolerance. The HLA-G gene encodes two isoforms, which are membrane-bound HLA-G (HLA-G1, HLA-G2, HLA-G3, and HLA-G4) and soluble HLA-G (HLA-G5, HLA-G6, and HLA-G7). Soluble HLA-G1 is produced by metalloproteinase-dependent shedding. Both isoforms are present at the placental interface on trophoblasts except for HLA-G7, and the HLA-G1 and HLA-G5 are the most frequently observed. HLA-G was described as a key physiological mediator of maternal–fetal tolerance (38–41). HLA-G inhibits multiple immune cell functions, including cytolytic functions of uterine and peripheral blood NK cells, Ag-specific cytolytic functions of cytotoxic T lymphocytes, allo-responsiveness of CD4+ T cells, proliferation of T and peripheral blood NK cells, and maturation and function of DCs. HLA-G expression on invasive extravillous trophoblasts might provide vulnerability to NK cells (42, 43). Interaction of HLA-G with DCs can also indirectly influence induction of DC tolerance (44). HLA-G–expressing DC-10 and CD4+ T cells accumulate in human deciduas during pregnancy (45). In addition to direct inhibition of these immune functions, HLA-G promotes long-term tolerance by inducing Tregs (46–48). Decreased HLA-G expression may induce immune destruction of extravillous trophoblasts. Abnormal HLA-G expression may also play a pathogenetic role during pregnancy-related complications (49). Our study revealed a novel role for dTregs in HLA-G upregulation and improved invasiveness of trophoblasts. Furthermore, dTreg-instructed trophoblasts can modulate NK cells toward a tolerant phenotype with reduced cytotoxicity, and increased inhibitory Th2 cytokines.
Secreted suppressor cytokines, such as IL-10 and TGF-β, and cell contact–mediated suppression by an uncharacterized membrane molecule are involved in the regulation of Tregs (50, 51). Our data showed that CD4+CD25+ Tregs significantly increased the numbers of cells that invaded the lower well in both direct and indirect cell contact conditions. Of note, trophoblast invasiveness in the direct cell contact coculture was much higher than in the indirect cell contact Transwell. Treg cell surface molecules and soluble cytokines contribute to enhanced trophoblast invasion. Further, treatment with IL-10 neutralizing Ab significantly inhibited basal and Treg-induced trophoblast invasiveness. Surprisingly, the TGF-β1 neutralizing Ab dramatically augmented trophoblast invasiveness with or without Treg coculture, which was completely opposite to the effect of TGF-β1 on tumor cells. Embryonic trophoblasts are physiological cells that invade maternal decidua temporally and spatially; it is not difficult to understand the discrepancy when considering the context. Our study suggests that trophoblasts promote invasion by secreting IL-10 and simultaneously inhibit excessive invasion by secreting TGF-β1. dTregs may enhance this effect to ensure fetal nutrition and protection of the uterine wall against trophoblast overinvasion. In fact, Fig. 6 showed that blockade of TGF-β1 in trophoblasts dramatically increased migration with a minor additional effect when dTregs were added. Our unpublished data (S.-C. Wang, H.-L. Piao, Y. Tao, M.-R. Du, and D.-J. Li) also show that > 90% of primary trophoblasts are TGF-β+, and ∼ 70% of dTregs express TGF-β. TGF-β secretion from primary trophoblasts was slightly higher than from dTregs. Thus, TGF-β secretion from trophoblasts might be the major player. However, the interplay between trophoblasts and Tregs is complex. Future studies will clarify the cellular source of TGF-β important for limiting trophoblast invasiveness by inhibiting TGF-β specifically in dTregs or trophoblasts.
In conclusion, our results show that trophoblasts activate dDCs through secretion of TSLP, resulting in differentiation and expansion of CD4+CD25+FOXP3+ Tregs. These expanded CD4+FOXP3+ Tregs suppress the maternal immune response and boost trophoblast performance, both of which are crucial for maintaining a successful pregnancy (Fig. 9). Combined with the previous reports of decreased levels of TSLP/TSLPR at the maternal–fetal interface during miscarriage and lower Treg numbers and Th2 cytokine production in the deciduas from miscarriage (15, 50), our study might be helpful in understanding the mechanism of maternal–fetal immunotolerance and may supply a potential immunotherapy for miscarriage in human beings. However, the trophoblast cell number from one villus is far less than we need for one experiment. We pooled trophoblast cells isolated from several different pregnancies and used them to coculture with immune cells, which does not resemble the situation in a single pregnancy in vivo. The theoretical limitations existing might, in a way, affect the interpretation of our results that needs optimization of the experimental settings in future. The interaction between decidual immune cells and embryo trophoblasts needs further investigation by establishing cell coculture systems using cells from the same pregnancy.
Acknowledgments
We thank Prof. Yongjun Liu from M. D. Anderson Cancer Center (Houston, TX) for kind suggestions regarding the design of this study and Prof. Bejamin K Tsang from Ottawa University (Ottawa, ON, Canada) for language editing of the manuscript.
This work was supported by the Major International Joint Research Project of the National Natural Science Foundation of China Grant 30910103909, National Natural Science Foundation of China Grant 31270969, Key Project of Shanghai Basic Research Grant 12JC1401600, Shenkang Fund Grant SHDC12010122, and the Program for Outstanding Medical Academic Leader (all to D.-J.L.); and National Natural Science Foundation of China Grant 81070537, National Natural Science Foundation of China Grant 31171437, and the Shanghai PuJiang Talent Program Grant 10PJ1401600 (all to M.-R.D.).
The online version of this article contains supplemental material.
- dDC
- decidual dendritic cell
- dNK
- decidual NK cell
- dTreg
- decidual regulatory T cell
- FCM
- flow cytometry
- MFI
- mean fluorescence intensity
- rh
- recombinant human
- TCM
- trophoblast culture medium
- Treg
- regulatory T cell
- TSLP
- thymic stromal lymphopoietin
- TSLP-DC
- TSLP-activated dendritic cell
- TSLPR
- TSLP receptor.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Medawar P. B. 1953. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Soc. Exp. Biol. 7: 320–328. [Google Scholar]
- 2.Trowsdale J., Betz A. G. 2006. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol. 7: 241–246. [DOI] [PubMed] [Google Scholar]
- 3.Munoz-Suano A., Hamilton A. B., Betz A. G. 2011. Gimme shelter: the immune system during pregnancy. Immunol. Rev. 241: 20–38. [DOI] [PubMed] [Google Scholar]
- 4.Tilburgs T., Scherjon S. A., Claas F. H. 2010. Major histocompatibility complex (MHC)-mediated immune regulation of decidual leukocytes at the fetal-maternal interface. J. Reprod. Immunol. 85: 58–62. [DOI] [PubMed] [Google Scholar]
- 5.Gobert M., Lafaille J. J. 2012. Maternal-fetal immune tolerance, block by block. Cell 150: 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somerset D. A., Zheng Y., Kilby M. D., Sansom D. M., Drayson M. T. 2004. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 112: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aluvihare V. R., Kallikourdis M., Betz A. G. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5: 266–271. [DOI] [PubMed] [Google Scholar]
- 8.Leber A., Teles A., Zenclussen A. C. 2010. Regulatory T cells and their role in pregnancy. Am. J. Reprod. Immunol. 63: 445–459. [DOI] [PubMed] [Google Scholar]
- 9.Chang X., Liu F., Wang X., Lin A., Zhao H., Su B. 2011. The kinases MEKK2 and MEKK3 regulate transforming growth factor-β-mediated helper T cell differentiation. Immunity 34: 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grainger J. R., Smith K. A., Hewitson J. P., McSorley H. J., Harcus Y., Filbey K. J., Finney C. A., Greenwood E. J., Knox D. P., Wilson M. S., et al. 2010. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 207: 2331–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hübner M. P., Shi Y., Torrero M. N., Mueller E., Larson D., Soloviova K., Gondorf F., Hoerauf A., Killoran K. E., Stocker J. T., et al. 2012. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β. J. Immunol. 188: 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe N., Wang Y. H., Lee H. K., Ito T., Wang Y. H., Cao W., Liu Y. J. 2005. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 13.Hanabuchi S., Ito T., Park W. R., Watanabe N., Shaw J. L., Roman E., Arima K., Wang Y. H., Voo K. S., Cao W., Liu Y. J. 2010. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J. Immunol. 184: 2999–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samstein R. M., Josefowicz S. Z., Arvey A., Treuting P. M., Rudensky A. Y. 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo P. F., Du M. R., Wu H. X., Lin Y., Jin L. P., Li D. J. 2010. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood 116: 2061–2069. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo R., Melchiorri L., Stignani M., Baricordi O. R. 2007. HLA-G expression is a fundamental prerequisite to pregnancy. Hum. Immunol. 68: 244–250. [DOI] [PubMed] [Google Scholar]
- 17.Carosella E. D., Moreau P., Lemaoult J., Rouas-Freiss N. 2008. HLA-G: from biology to clinical benefits. Trends Immunol. 29: 125–132. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W. H., Du M. R., Dong L., Zhu X. Y., Yang J. Y., He Y. Y., Li D. J. 2007. Cyclosporin A increases expression of matrix metalloproteinase 9 and 2 and invasiveness in vitro of the first-trimester human trophoblast cells via the mitogen-activated protein kinase pathway. Hum. Reprod. 22: 2743–2750. [DOI] [PubMed] [Google Scholar]
- 19.Josefowicz S. Z., Niec R. E., Kim H. Y., Treuting P., Chinen T., Zheng Y., Umetsu D. T., Rudensky A. Y. 2012. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wohlfert E. A., Grainger J. R., Bouladoux N., Konkel J. E., Oldenhove G., Ribeiro C. H., Hall J. A., Yagi R., Naik S., Bhairavabhotla R., et al. 2011. GATA3 controls Foxp3⁺ regulatory T cell fate during inflammation in mice. J. Clin. Invest. 121: 4503–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Su M. A., Wan Y. Y. 2011. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity 35: 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friend S. L., Hosier S., Nelson A., Foxworthe D., Williams D. E., Farr A. 1994. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp. Hematol. 22: 321–328. [PubMed] [Google Scholar]
- 23.Ziegler S. F. 2012. Thymic stromal lymphopoietin and allergic disease. J. Allergy Clin. Immunol. 130: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagarkar D. R., Poposki J. A., Comeau M. R., Biyasheva A., Avila P. C., Schleimer R. P., Kato A. 2012. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 130: 225–232, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shikotra A., Choy D. F., Ohri C. M., Doran E., Butler C., Hargadon B., Shelley M., Abbas A. R., Austin C. D., Jackman J., et al. 2012. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J. Allergy Clin. Immunol. 129: 104–111, e1–e9. [DOI] [PubMed] [Google Scholar]
- 26.Munn D. H., Zhou M., Attwood J. T., Bondarev I., Conway S. J., Marshall B., Brown C., Mellor A. L. 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281: 1191–1193. [DOI] [PubMed] [Google Scholar]
- 27.Torricelli M., Voltolini C., Bloise E., Biliotti G., Giovannelli A., De Bonis M., Imperatore A., Petraglia F. 2009. Urocortin increases IL-4 and IL-10 secretion and reverses LPS-induced TNF-alpha release from human trophoblast primary cells. Am. J. Reprod. Immunol. 62: 224–231. [DOI] [PubMed] [Google Scholar]
- 28.Rowe J. H., Ertelt J. M., Xin L., Way S. S. 2012. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccirillo C. A., d’Hennezel E., Sgouroudis E., Yurchenko E. 2008. CD4+Foxp3+ regulatory T cells in the control of autoimmunity: in vivo veritas. Curr. Opin. Immunol. 20: 655–662. [DOI] [PubMed] [Google Scholar]
- 30.Yin Y., Han X., Shi Q., Zhao Y., He Y. 2012. Adoptive transfer of CD4+CD25+ regulatory T cells for prevention and treatment of spontaneous abortion. Eur. J. Obstet. Gynecol. Reprod. Biol. 161: 177–181. [DOI] [PubMed] [Google Scholar]
- 31.Vacca P., Cantoni C., Vitale M., Prato C., Canegallo F., Fenoglio D., Ragni N., Moretta L., Mingari M. C. 2010. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc. Natl. Acad. Sci. USA 107: 11918–11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parham P. 2004. NK cells and trophoblasts: partners in pregnancy. J. Exp. Med. 200: 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao L., Jacobs A. R., Johnson V. V., Mayer L. 2005. Activation of CD8+ regulatory T cells by human placental trophoblasts. J. Immunol. 174: 7539–7547. [DOI] [PubMed] [Google Scholar]
- 34.Fest S., Aldo P. B., Abrahams V. M., Visintin I., Alvero A., Chen R., Chavez S. L., Romero R., Mor G. 2007. Trophoblast-macrophage interactions: a regulatory network for the protection of pregnancy. Am. J. Reprod. Immunol. 57: 55–66. [DOI] [PubMed] [Google Scholar]
- 35.Atay S., Gercel-Taylor C., Suttles J., Mor G., Taylor D. D. 2011. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am. J. Reprod. Immunol. 65: 65–77. [DOI] [PubMed] [Google Scholar]
- 36.Hanna J., Goldman-Wohl D., Hamani Y., Avraham I., Greenfield C., Natanson-Yaron S., Prus D., Cohen-Daniel L., Arnon T. I., Manaster I., et al. 2006. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 12: 1065–1074. [DOI] [PubMed] [Google Scholar]
- 37.Hiby S. E., Apps R., Sharkey A. M., Farrell L. E., Gardner L., Mulder A., Claas F. H., Walker J. J., Redman C. W., Morgan L., et al. 2010. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 120: 4102–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMaster M. T., Librach C. L., Zhou Y., Lim K. H., Janatpour M. J., DeMars R., Kovats S., Damsky C., Fisher S. J. 1995. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J. Immunol. 154: 3771–3778. [PubMed] [Google Scholar]
- 39.Rouas-Freiss N., Gonçalves R. M., Menier C., Dausset J., Carosella E. D. 1997. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA 94: 11520–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apps R., Gardner L., Sharkey A. M., Holmes N., Moffett A. 2007. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur. J. Immunol. 37: 1924–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales P. J., Pace J. L., Platt J. S., Langat D. K., Hunt J. S. 2007. Synthesis of beta(2)-microglobulin-free, disulphide-linked HLA-G5 homodimers in human placental villous cytotrophoblast cells. Immunology 122: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonen-Gross T., Goldman-Wohl D., Huppertz B., Lankry D., Greenfield C., Natanson-Yaron S., Hamani Y., Gilad R., Yagel S., Mandelboim O. 2010. Inhibitory NK receptor recognition of HLA-G: regulation by contact residues and by cell specific expression at the fetal-maternal interface. PLoS ONE 5: e8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madeja Z., Yadi H., Apps R., Boulenouar S., Roper S. J., Gardner L., Moffett A., Colucci F., Hemberger M. 2011. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc. Natl. Acad. Sci. USA 108: 4012–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang S., Ristich V., Arase H., Dausset J., Carosella E. D., Horuzsko A. 2008. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6—STAT3 signaling pathway. Proc. Natl. Acad. Sci. USA 105: 8357–8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amodio G., Mugione A., Sanchez A. M., Viganò P., Candiani M., Somigliana E., Roncarolo M. G., Panina-Bordignon P., Gregori S. 2013. HLA-G expressing DC-10 and CD4(+) T cells accumulate in human decidua during pregnancy. Hum. Immunol. 74: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carosella E. D., Gregori S., LeMaoult J. 2011. The tolerogenic interplay(s) among HLA-G, myeloid APCs, and regulatory cells. Blood 118: 6499–6505. [DOI] [PubMed] [Google Scholar]
- 47.Selmani Z., Naji A., Zidi I., Favier B., Gaiffe E., Obert L., Borg C., Saas P., Tiberghien P., Rouas-Freiss N., et al. 2008. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 26: 212–222. [DOI] [PubMed] [Google Scholar]
- 48.Feger U., Tolosa E., Huang Y. H., Waschbisch A., Biedermann T., Melms A., Wiendl H. 2007. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood 110: 568–577. [DOI] [PubMed] [Google Scholar]
- 49.Cecati M., Giannubilo S. R., Emanuelli M., Tranquilli A. L., Saccucci F. 2011. HLA-G and pregnancy adverse outcomes. Med. Hypotheses 76: 782–784. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura K., Kitani A., Strober W. 2001. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 194: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakaguchi S., Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T. 2009. Regulatory T cells: how do they suppress immune responses? Int. Immunol. 21: 1105–1111. [DOI] [PubMed] [Google Scholar]