Abstract
B cell activation is regulated by a variety of signals. CD19 positively regulates B cell activation, augmenting signals delivered through the BCR complex. In contrast, CD32b contains an ITIM and negatively regulates BCR signaling. Importantly, there are drugs currently in clinical trials and preclinical development that cross-link CD32b to molecules within the BCR complex. We wanted to address how single engagement versus cotargeting these molecules affects human B cell function. When B cells from healthy individuals were activated by signals that mimic a T cell response (IL-21 costimulation), ligation of CD32b, but not CD19, inhibited B cell expansion and plasma cell (PC) differentiation. In contrast, when B cells were activated through TLR, anti-CD19, but not anti-CD32b, blunted the response. However, when both CD19 and CD32b were coengaged by a bispecific anti-CD19×CD32b Ab, both types of stimuli were potently inhibited. Cross-linking CD19 with CD32b also inhibited Ab-independent functions of B cells, such as HLA upregulation, cytokine production, and the ability of B cells to prime CD4+ T cells. Finally, although cross-linking CD19 and CD32b inhibited PC differentiation of primary B cells, it did not alter Ig production from pre-established PCs. These data elucidate the mechanism by which a complex set of signals determines the fate of B cell responsiveness. Although signals through CD19 influence TLR-driven activation, CD32b impacts the magnitude of the response following IL-21 costimulation. Therefore, simultaneous targeting of multiple surface molecules may be a necessary approach to comprehensively modulate B cell activation in vivo.
Introduction
A variety of autoimmune diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), are associated with chronic, polyclonal B cell hyperactivation. Understanding the signals that regulate the qualitative and quantitative response of these cells is critical for identifying efficacious treatments for patients with B cell–mediated autoimmunity.
B cell expansion and differentiation are regulated by the balance of signals delivered through activating and inhibitory receptors expressed on the surface of the cell. A number of molecules have been identified that have the potential to dampen B cell responses. CD32b, or FcγRIIb, is the only know inhibitory FcR and is expressed on a variety of immune cells, including dendritic cells, macrophages, neutrophils, and B cells (1). The inhibitory capacity of CD32b is largely dependent on expression of an intracellular ITIM, which, when phosphorylated, is responsible for recruitment of the phosphatase SHIP1 (2, 3). In the context of B cells, SHIP1 recruitment results in decreased signaling downstream of the BCR and ultimately leads to diminished BCR-dependent cell activation and Ab production (4). Mice deficient in CD32b have increased Ab responses to T cell–dependent Ags, supporting the critical role for CD32b in regulating humoral immune responses (5). Similarly, deficiency in CD32b in mice leads to chronic B cell activation and autoimmunity (6), whereas B cell–specific overexpression of CD32b reduces the incidence and severity of lupus in MRL lpr/lpr mice (7). In humans, polymorphisms in CD32b are associated with an increased prevalence of SLE (8, 9). These results suggest that Ab-mediated engagement of CD32b could provide therapeutic benefit in settings of autoimmunity by dampening the response of chronically activated B cells.
CD19 is a B cell–specific molecule that controls B cell activation by complexing with the BCR (10). CD19 is a member of the Ig superfamily and is the dominant component for the signaling complex on B cells that includes CD21, CD81, and CD225 (11). The cytoplasmic domain of CD19 contains nine tyrosine residues, three of which appear critical for mediating its biologic functions (12–14). More specifically, when phosphorylated, the tyrosine residues on CD19 can recruit Src-family kinases and amplify signals through the BCR and other surface molecules (15). B cells from CD19 gene–targeted mice have a diminished proliferative response to mitogens and have decreased serum Ig production (16, 17). Humans with CD19 deficiency have reduced proliferative responses to BCR stimulation in vitro and mount impaired Ab responses to vaccination (18). Further, CD19 expression can be dysregulated in autoimmunity. CD19 expression is significantly increased on both naive and memory B cells from patients with systemic sclerosis and correlates with increased serum IgG and IgM levels in these patients, suggesting that CD19 may be functionally linked with Ab production in human disease (19, 20). In lupus, one study (21) reported that, although CD19 was similarly expressed on naive and memory B cells, CD19 expression was decreased on plasmablasts compared with that of normal donors. Other investigators reported that CD19 expression was decreased in naive and memory B cells isolated from lupus blood (19). Importantly, studies demonstrated that B cell responses can either be enhanced or inhibited through CD19, depending on the stimulation milieu and the degree of cross-linking (22, 23).
Currently, there is an Ab-based drug in a phase 1b/2a clinical trial that cross-links the inhibitory receptor, CD32b, to the activation receptor, CD19, in patients with moderate to severe RA arthritis (Xencor, EudraCT Number: 2012-003057-29). Moreover, Macrogenics reported that they are developing a Dual-Affinity Re-Targeting (DART) drug that cross-links CD32b with CD79, a signaling molecule for the BCR (24). Discerning how humoral immune responses can be modulated by cotargeting these receptors is critically important for delineating the effect of this approach in settings of autoimmunity.
To further elucidate the mechanisms by which CD32b and CD19 regulate human B cell responses, we compared the effect of individual engagement versus cotargeting of these cell surface molecules on human B cell expansion and plasma cell (PC) differentiation in response to various activating cues. The data indicate that CD19 and CD32b regulate B cell responses differentially, depending on the nature of the B cell stimulus. Although CD32b signals appear critical for modulating activation in response to ligation of BCR, IL-21R, and CD40, CD19 plays a role in regulating TLR-mediated B cell activation. Importantly, cross-linking CD19 and CD32b with either a bispecific or anti-Fc Ab resulted in potent inhibition of B cell responses under a variety of stimulating conditions with cells from both healthy individuals and patients with RA. Cotargeting CD19 and CD32b may be an attractive way to broadly modulate B cell responses, such as Ab production, cytokine production, and APC functionality, in autoimmune diseases, such as RA and SLE, in which chronic B cell activation is driven by a variety of cues and can contribute to disease pathogenesis.
Materials and Methods
Cells from healthy donors
Human blood was collected from healthy donors following informed consent. PBMCs were isolated from CPT tubes (BD Biosciences) following centrifugation. Total B cells were negatively selected using MACS cell-separation technology (Miltenyi Biotec), which routinely yielded purity > 95%. The degree of purity and initial cell phenotype were determined by flow cytometry for all experiments on day 0. Of note, B cell preparations isolated using this method contain populations of both naive and memory B cells, but PCs were excluded because of their expression of CD43, which is targeted by the Ab-selection mixture of the MACS kit (Miltenyi Biotec). B cell subsets were isolated by magnetic separation using naive, IgM+ memory, and IgG+ memory B cell kits, according to the manufacturer’s instructions (Miltenyi Biotec). Additionally, total CD4+ T cells were isolated by negative selection using MACS technology (Miltenyi Biotec).
Cells from RA donors
RA blood samples were obtained from the Warren G. Magnuson Clinical Center Blood Bank (Bethesda, MD), as approved by the National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health, and isolated as described above. The demographics and clinical characteristics of these donors are shown in Table I.
Table I. RA cohort demographics and clinical characteristics.
| N | 14 |
| Age (y; mean ± SD) | 51.2 ± 13.3 |
| Gender | 86% female |
| CRP (μg/ml) | 14.27 ± 19.7 |
| CCP titer (IgG) | 76.9% |
| DMARD | |
| Methotrexate | 78.6% |
| Antimalarial | 71.4% |
| Anti-TNF | 42.9% |
Number of patients on other drugs: mycophenolate mofetil (1), corticosteroids (6), azathioprine (1), and leflunomide (1).
CRP, C-reactive protein; CCP, cyclic citrullinated peptide; DMARD, disease-modifying antirheumatic drug.
Ab expression, purification, and characterization
All recombinant proteins used in this study were prepared at MedImmune. Cloning, expression, and preparation of recombinant proteins were carried out as described previously by Herbst et al. (25). The anti-CD19 Ab 6C11 was engineered at MedImmune (25). The anti-CD32b Ab hu2B6-3.5 was described previously (26). To generate the anti-CD32b scFv, the VH domain was linked to the VL domain via a 15-aa linker (GGGGS)3. This scFv was genetically linked to the C terminus of the CH3 domain of the anti-CD19 Ab using an AAAGGGGS sequence to generate the bispecific Bs3Ab (Supplemental Fig. 2). All Abs used in this study carry three mutations, identified as TM, in the Fc region to ablate FcγR binding, as described by Oganesyan et al. (27). The selection and characterization of the anti-TM Ab (used to cross-link anti-CD19 and CD32b Abs) were carried out at MedImmune, and its characterization will be published elsewhere. The monomer content of the final purified constructs was >99%, as assessed by SEC-MALLS (data not shown). Endotoxin levels in rAbs were assessed using the Endo Chrome-K LAL Reagent Endosafe (Charles River) chromomeric assay kit with a disposable Limulus amebocyte lysate test cartridge. The endotoxin levels were consistently low (<0.1 EU/mg).
Culture conditions: purified B cells
Purified peripheral blood B cells were cultured at a density of 0.5–1.0 × 105 B cells/well in 96-well round-bottom plates in a final volume of 150–200 μl complete medium. Culture medium for all experiments was RPMI 1640 (Invitrogen) supplemented with 10% FCS, penicillin-streptomycin (100 U/ml penicillin, 100 μg/ml streptomycin), 2-ME (55 μM), l-glutamine (2 mM), and HEPES (5 mM). At initiation of culture, B cells were stimulated with either CpG-B (1 μg/ml; InvivoGen) or a combination of IL-21 (33 ng/ml; PeproTech), anti-CD40 (0.1 μg/ml; goat IgG; R&D Systems), and anti-IgM F(ab′)2 (5.0 μg/ml; Jackson ImmunoResearch Laboratories). The concentration of Abs used in these experiments was determined based on maximal inhibition achieved in extensive titration studies (Supplemental Fig. 1). B cells were cultured for up to 21 d. For some experiments, B cells were labeled with CFSE prior to culture. Briefly, B cells were incubated with CFSE (10 μM; Invitrogen) diluted in serum-free medium for 4 min with gentle rocking at room temperature. Cells were quenched with FBS and washed extensively before culture. In most experiments, control, parental, or bispecific Abs (0.1 μg/ml) were added at the initiation of culture. Anti-TM Ab was added in some experiments (0.1 μg/ml) to compare the effect using a bispecific format versus cross-linking parental Abs. Bortezomib was used at a concentration of 100 ng/ml. To determine the effect of the parental or bispecific Abs on pre-established PCs, Abs were added after 7 d of B cell stimulation.
B cell expansion
B cell expansion was determined in cultures of purified B cells stimulated as described above. B cell expansion was quantified by measuring ATP on day 3 or 4 of culture using the CellTiter-Glo Luminescent Assay (Promega), according to the manufacturer’s instructions, as we described previously (28). Briefly, the CellTiter-Glo Assay (referred to as the ATP Assay) is used to determine the number of viable cells in culture based on quantitation of ATP levels, which are indicative of the number of metabolically active cells. The CellTiter-Glo reagent leads to cell lysis and generation of a luminescent signal that is proportional to the amount of ATP present. As previously reported, the amount of ATP is directly proportional to the number of cells (29).
Flow cytometry
Cells were cultured as described above. At a variety of time points, cells were harvested and stained in round-bottom plates for 30 min at 4°C. The following combinations of Abs were used for flow cytometry: anti-CD19 (PerCP-Cy5.5 conjugated; clone SJ25C1 or clone HD37), anti-IgD (FITC or PE conjugated; clone IA6-2), anti-CD38 (PE or allophycocyanin conjugated; clone HB7), anti-CD32 (PE conjugated; clone AT10), anti–HLA-DR (V500 conjugated; clone G46-6), anti–HLA-A/B/C (PE Cy7 conjugated; clone W6/32), and anti-CD86 (allophycocyanin Cy7 conjugated; clone GL1). Anti-CD27 was often added to the mixture to confirm that the majority of the memory B cells and PCs expressed the memory B cell Ag, CD27. Cells were analyzed on an LSR II flow cytometer (BD Biosciences) using FACSDiva software. All conditions were collected for the same amount of time; thus, the number of events displayed is reflective of the relative cell number. However, cell numbers were determined using AccuCount Particles (Spherotech), according to the manufacturer’s recommendations. Quantification of the number of CD19 and CD32 antigenic sites on human B cells was performed using a QIFIKIT (Dako), according to the manufacturer’s instructions.
Ig production
Secreted Ig was quantified by ELISA following stimulation of B cells for the indicated number of days. Ninety-six–well flat-bottom plates were coated overnight at 4°C with either 5 μg/ml goat anti-human IgM or goat anti-human IgG diluted in PBS. Plates were washed and blocked with 0.2% BSA in PBS. Supernatants were diluted and incubated in plates overnight. Bound Ig was detected with goat anti-human IgM–alkaline phosphatase or goat anti-human IgG–alkaline phosphatase (0.2 μg/ml; Bethyl Laboratories) diluted in blocking buffer. Plates were developed with SIGMAFAST p-Nitrophenyl Phosphate Tablets (Sigma Aldrich), and specific absorbance was measured at 405 nm using a SpectraMax microplate reader (Molecular Devices).
Apoptosis assay
Apoptosis of B cells stimulated either with CpG-B or a combination of IL-21, anti-CD40, and anti-IgM (culture conditions as described above) was assessed after 2 d of culture using the Caspase Glo 3/7 Assay (Promega), according to the manufacturer’s instructions.
Cytokine production
Supernatants from B cell cultures, activated as described above, were harvested after 2 d, and cytokine production was quantified using a multiplex cytokine assay (Meso Scale Discovery), as per the manufacturer’s instructions.
MLR assay
B cells and CD4+ T cells were isolated from human peripheral blood, as detailed above. CD4+ T cells were labeled with CFSE prior to culture. Briefly, T cells were incubated with CFSE (10 μM; Invitrogen) diluted in prewarmed serum-free media for 4 min with gentle rocking at room temperature. The reaction was quenched with FBS, and the cells were washed before culture. Responder CD4+ T cells were cultured with allogeneic stimulator B cells in 96-well round-bottom plates at B:T cell ratios of 0:1, 1:1, 1:3, 1:10, and 1:30. Dilution of CFSE was quantified by flow cytometry on day 4.
Statistics
Mean and SD of triplicate wells for each condition are shown. Statistical analyses were performed using a one-tailed, two-sample t test. Significant differences relative to controls are noted in the figures.
Results
CD19 and CD32b are expressed equally on human B cells
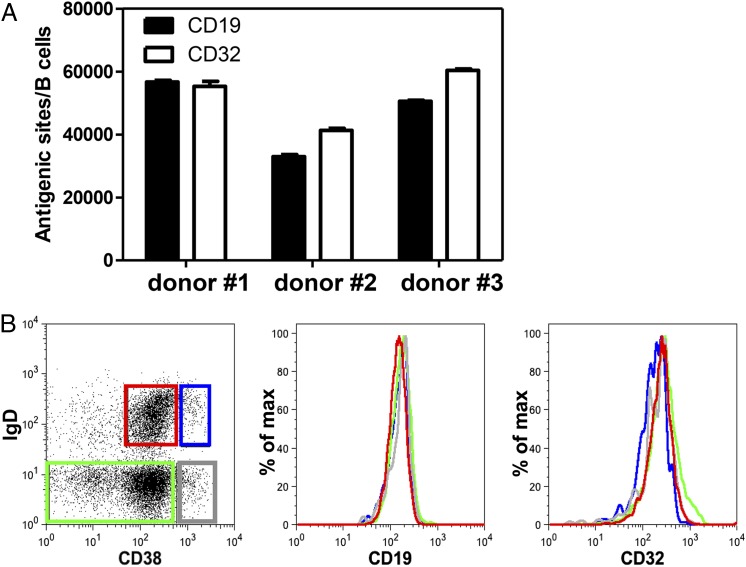
Densities of CD19 and CD32 on total peripheral blood B cells were examined. Quantification of the number of antigenic sites revealed that roughly equivalent numbers of CD19 and CD32 molecules were expressed on the surface of B cells, with some donor-to-donor variability, as expected (Fig. 1A). Approximately 3–6 × 104 CD19 and CD32 antigenic sites/B cell were calculated from each individual donor.
FIGURE 1.
CD19 and CD32 expression on B cells. (A) The number of CD19 and CD32 antigenic sites on the surface of blood B cells was quantified, as described in Materials and Methods, from three healthy donors. (B) Surface expression of CD19 and CD32 on human peripheral blood B cell subsets was measured by flow cytometry. Red graph: IgD+ CD38int (naive); blue graph: IgD+ CD38hi (transitional); green graph: IgD− CD38int/lo (postswitched memory); gray graph: IgD− CD38hi (PCs). One of three representative donors is shown.
Further, separating B cells based on surface expression of IgD and CD38 allowed for determination of CD19 and CD32 expression on various B cell subpopulations from blood (Fig. 1B). Importantly, both CD19 and CD32 were expressed at similar levels on transitional B cells (IgD+ CD38hi), naive B cells (IgD+ CD38int), postswitch memory B cells (IgD− CD38int), and PCs (IgD− CD38hi). We confirmed that the majority of the transitional and naive B cells did not express the memory B cell Ag CD27, whereas >90% of the memory B cells and PCs expressed a high density of CD27 (data not shown). These data suggest that the human B cell subsets examined have the potential to be regulated by these cell-signaling Ags.
CD19 and CD32b differentially regulate B cell responses, depending on the context of activation
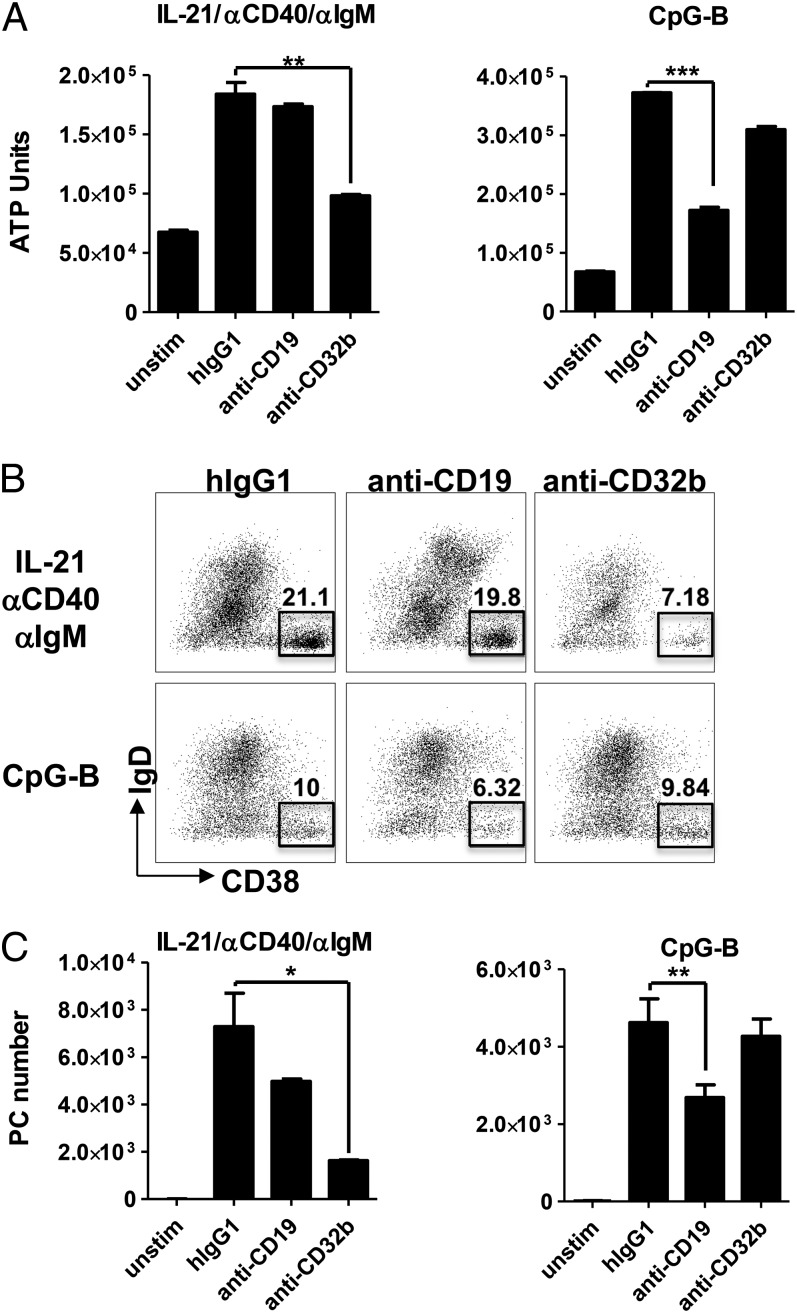
To determine the effect of individually targeting CD19 or CD32b on human B cell activation, blood B cells were purified and stimulated under two conditions. To induce BCR-dependent activation that mimics signals that might be received in the context of a germinal center response, including activation by T cells, B cells were stimulated with a combination of IL-21, anti-CD40, and anti-IgM. Alternatively, B cells were stimulated with CpG-B, mimicking an extrafollicular B cell response in which B cell activation can be achieved through TLR-dependent signals. Quantification of B cell activation on day 4 revealed that both stimuli induced significant B cell expansion, as determined by a 4–5-fold increase in ATP units at this time point (Fig. 2A). Interestingly, targeting CD32b inhibited IL-21/αCD40/αIgM-induced B cell expansion by 74%, but it had no effect on CpG-B–induced cell growth. Conversely, CD19 engagement inhibited CpG-B growth by 66% without affecting expansion driven in the context of BCR ligation and IL-21 costimulation (Fig. 2A). Similar results were observed using CFSE as a measure of B cell proliferation (data not shown).
FIGURE 2.
Individually, engagement of CD19 or CD32b differentially regulates B cell responses depending on the context of the stimulation. Purified peripheral blood B cells were isolated from healthy donors and stimulated with IL-21/anti-CD40/anti-IgM or CpG-B in the presence of isotype control, anti-CD19, or anti-CD32b Abs. (A) Expansion was quantified on day 4. (B and C) PC differentiation was quantified on day 7. Frequency (B) and absolute number (C) of IgD− CD38hi PCs are indicated. One of five representative donors is shown. *p < 0.05, **p < 0.01, ***p < 0.005.
In addition to promoting B cell division, both stimulating conditions induced differentiation of purified B cells into PCs by day 7 of culture, as evidenced by the emergence of a significant population of CD19+ IgD− CD38hi cells (Fig. 2B). Similar to the trends observed on B cell expansion, engagement of CD32b (but not CD19) specifically inhibited IL-21/anti-CD40/anti-IgM–induced PC differentiation (78% inhibition), whereas cross-linking of CD19, but not CD32b, inhibited PC differentiation induced by CpG (42% inhibition) (Fig. 2B, 2C). These data suggest that signals delivered through CD32b play an important role in regulating BCR- and IL-21–induced B cell activation and differentiation, whereas CD19 can influence BCR-independent responses, such as those mediated by TLRs.
Cotargeting CD19 and CD32b results in broad-spectrum inhibition of B cell responses
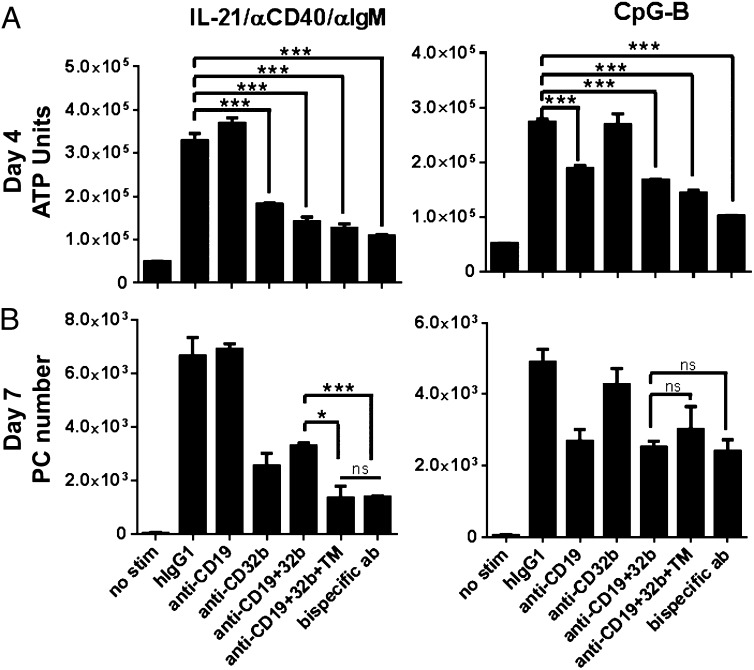
We next tested the effect of coengagement of CD19 and CD32b on B cell expansion and PC differentiation. Cross-linking of CD19 and CD32b was achieved using different approaches. The molecules were cotargeted using either the monospecific Abs in the presence of an anti-TM Ab, which binds to the Fc portion of the molecules, or with a bispecific-format Ab, which has the capacity to bind both CD19 and CD32b (Bs3AB) (Supplemental Fig. 2). As shown above, anti-CD32b alone significantly inhibited IL-21/anti-CD40/anti-IgM–induced B cell expansion at day 4, whereas anti-CD19 alone blunted CpG-B–driven responses (Fig. 3A). Coaddition of anti-CD19 and anti-CD32b monospecific Abs did not further reduce B cell expansion under either stimulation condition. Additionally, cross-linking CD19 and CD32b either with an anti-TM Ab or with Bs3Ab also did not significantly enhance the inhibitory capacity over each monospecific Ab alone with regard to B cell expansion at day 4 (Fig. 3A). Importantly, however, although each monospecific Ab had a narrow inhibitory capacity that is dependent on the context of stimulation, coengagement of CD19 and CD32b using Bs3Ab inhibited B cell expansion under both IL-21–induced and TLR-driven activation conditions (Fig. 3A).
FIGURE 3.
Cross-linking CD19 and CD32b broadly inhibits B cell responses. B cells were stimulated in the presence of parental Abs alone (anti-CD19 or anti-CD32b), parental Abs together without cross-linking (anti-CD19+32b) or with cross-linking (anti-CD19+3b+TM), or with Bs3Ab bispecific Ab. (A) B cell expansion was quantified on day 4 (ATP Units). (B) PC number was assessed on day 7 by flow cytometry. One of four representative donors is shown. *p < 0.05, ***p < 0.005. ns, Not significant.
PC differentiation was quantified following 7 d of activation. CpG-induced PC differentiation was inhibited to a similar degree when CD19 was targeted alone or in combination with CD32b (with or without cross-linking). Notably, however, cross-linking CD19 and CD32b on B cells with a bispecific Ab in the context of IL-21, anti-CD40, and anti-IgM stimulation led to a more significant reduction in PC differentiation than did targeting both the CD19 or CD32b Ags in the absence of cross-linking. Although coaddition of both anti-CD19 and anti-CD32b resulted in 50.4% inhibition, addition of the bispecific Ab had the capacity to blunt the PC differentiation by 80.1% (Fig. 3B). Importantly, these data demonstrate that, unlike targeting CD19 or CD32b alone, cotargeting CD19 and CD32b results in broad-spectrum inhibition of B cell expansion and differentiation in response to various activating cues.
The effect of Bs3Ab on cell death does not account for its immunomodulatory activity
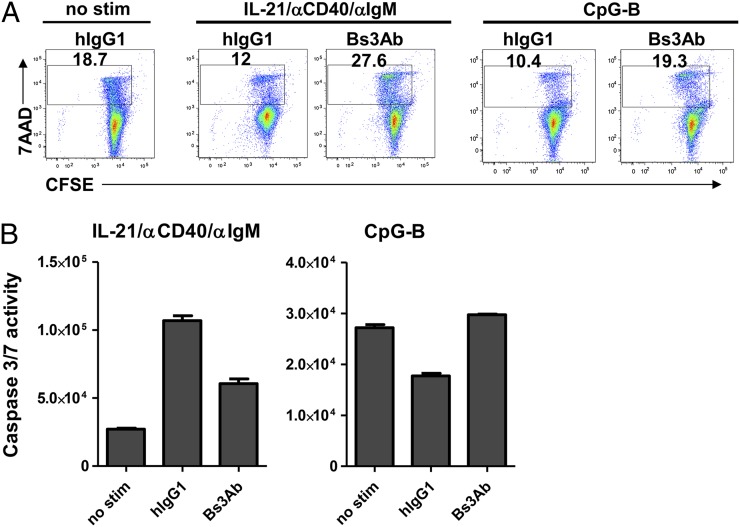
We next sought to determine whether the ability of Bs3Ab to inhibit B cell responses was due to the induction of cell death. Compared with unstimulated cells, there was a reduced percentage of 7-aminoactinomycin D (7AAD)+ dead cells following stimulation with either CpG-B or IL-21/anti-CD40/anti-IgM (18.7% 7AAD+ versus 10.4 and 12%, respectively) (Fig. 4A). Following stimulation with IL-21/anti-CD40/anti-IgM, Bs3Ab had a modest impact on cell survival, as evidenced by an increase in the percentage of 7AAD+ dead B cells in the presence of Bs3Ab compared with unstimulated cells (27.6% versus 18.7%, respectively) (Fig. 4A). In a readout of cell death using caspase 3/7 activity, cells stimulated with IL-21, anti-CD40, and anti-IgM had increased activity. Addition of Bs3Ab reduced caspase levels compared with that found in stimulated cells. In the context of CpG-B activation, the percentage of 7AAD+ cells in the presence of Bs3Ab was comparable to unstimulated cells (19.3% versus 18.7%) (Fig. 4A). Further, caspase 3/7 levels were equivalent in unstimulated cells and CpG-B–stimulated cells in the presence of the bispecific Ab, suggesting that Bs3Ab was not inducing cell death in CpG-B–stimulated B cells (Fig. 4B). Overall, these data show that coengagement of CD19 and CD32b can impact B cell survival, depending on the nature of the activating milieu. Importantly, however, our data show that, upon stimulation with CpG-B, Bs3Ab can suppress B cell responses independently of a direct effect on cell death.
FIGURE 4.
Effect of Bs3Ab on B cell survival is dependent on the activating milieu. B cells were isolated from healthy donors and stimulated with IL-21/anti-CD40/anti-IgM or CpG-B for 2 d in the presence of isotype control (hIgG1) or bispecific (Bs3Ab) Abs. (A) Percentage of 7AAD+ cells (dead cells) is indicated. (B) Caspase 3/7 activity was quantified on day 2. Data from one of two independent studies are shown.
CD19 and CD32b differentially regulate naive and memory B cells
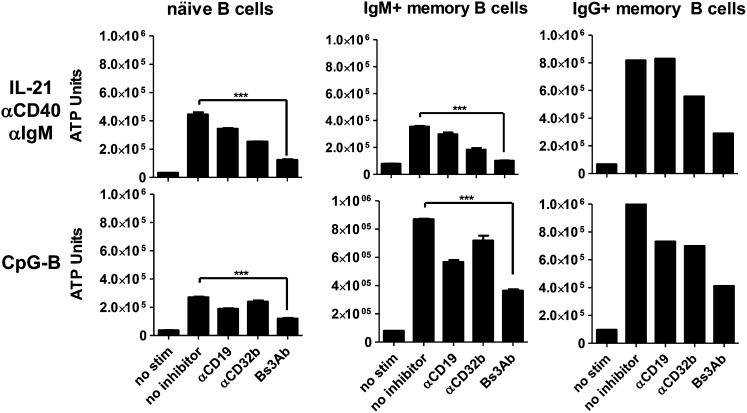
Next, the ability of CD19 and/or CD32b to regulate the expansion of specific B cell subsets was evaluated (Fig. 5). B cell subsets from peripheral blood were isolated into naive, IgM+ memory, or IgG+ memory fractions. IgM+ CD27+ preswitched memory B cells expanded ∼5-fold in response to stimulation with IL-21, anti-CD40, and anti-IgM following 4 d of culture, whereas naive CD27− B cells and IgG+ CD27+ memory B cells expanded up to 12-fold over unstimulated cells (Fig. 5, top panels). Expansion of B cells in response to CpG-B stimulation also varied between subsets. Although naive B cells expanded 6–7-fold, IgM+ and IgG+ memory B cells expanded 10–12-fold (Fig. 5, bottom panels). As observed with bulk B cell populations, engagement of CD32b on naive, IgM+ memory, or IgG+ memory B cells resulted in blunted B cell expansion in response to stimulation with IL-21, anti-CD40, and anti-IgM. However, CD32b was not able to modulate responses to CpG-B in all B cell subsets examined. Conversely to CD32b, engagement of CD19 impaired the response of all purified B cell fractions in response to CpG-B but did not affect responsiveness to IL-21 costimulation (Fig. 5). Importantly, cross-linking CD19 with CD32b with Bs3Ab inhibited cell growth to a similar degree in all B cell subsets in response to both IL-21 coactivation (∼78–91% inhibition achieved) and CpG-B stimulation (∼65% inhibition achieved) (Fig. 5). These results suggest that circulating naive and memory B cell subsets are equally susceptible to regulation when CD19 and CD32b are cotargeted.
FIGURE 5.
Coengagement of CD19 and CD32b inhibits both naive and memory B cell subsets. Naive, IgM+ memory, and IgG+ memory blood B cell subsets were stimulated with IL-21/anti-CD40/anti-IgM (upper panels) or CpG-B (lower panels). B cell expansion was quantified on day 4. ***p < 0.005.
CD19 and CD32b modulate the responsiveness of B cells from donors with RA
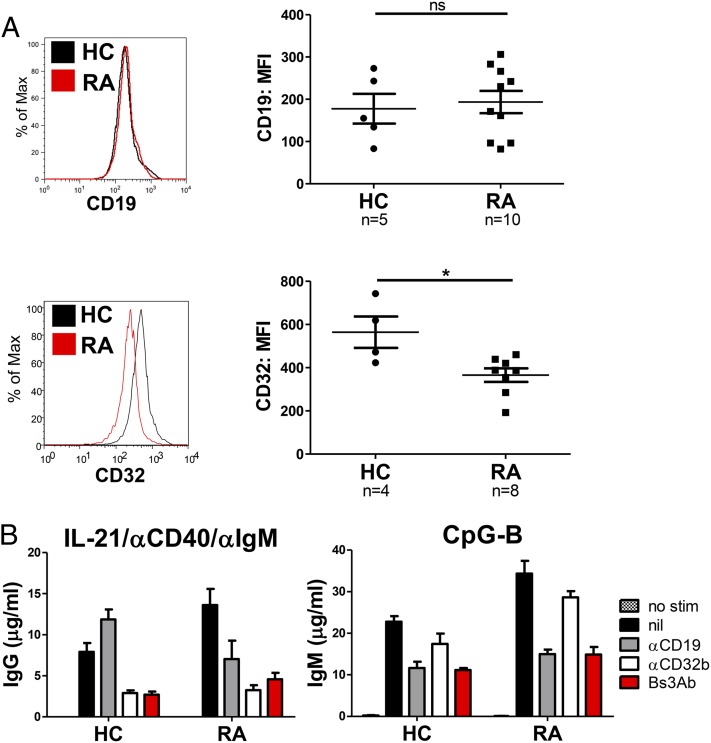
B cells from patients with autoimmune disease often display altered phenotypes and respond abnormally to stimulation. To determine the ability of Bs3Ab to modulate the activity of B cells from autoimmune individuals, a cohort of RA patients was studied. Demographics and clinical characteristics for the RA cohort examined are provided in Table I. First, purified B cells from RA patients were compared with that of healthy controls (HCs) with regard to cell surface expression of CD19 and CD32b Ags (Fig. 6A). Although expression of CD19 was observed to be equivalent on B cells isolated from HC and RA donors, there was a significant reduction in CD32b Ag expressed on RA B cells (Fig. 6A). Because rheumatoid factor (RF) is common in RA, it is possible that RF binding of IgG bound to CD32b (FcγR) could interfere with staining of CD32b. However, we noted no correlation between soluble RF blood levels in these patients and downregulation of CD32b (data not shown), suggesting that the downregulation of CD32b noted in B cells isolated from RA patients was not due to interference of RF.
FIGURE 6.
Despite reduced surface expression of CD32, B cells from donors with RA are susceptible to inhibition through CD32b. (A) CD19 (upper panel) or CD32 (lower panel) surface expression was measured on blood B cells from HCs and patients with RA. (B) Purified B cells from HC and RA donors were stimulated with IL-21/anti-CD40/anti-IgM (left panel) or CpG-B (right panel) in the presence of parental or bispecific Abs, and Ig production was quantified on day 7. Representative data from one of seven RA donors tested is shown in (B). *p < 0.05. ns, Not significant.
To investigate the potential consequences of reduced CD32b expression and possible CD19 dysfunction of B cells from RA donors, purified blood B cells from RA patients were compared with those from HCs with regard to the ability of CD19 and/or CD32b to modulate B cell responsiveness. Purified B cells were stimulated as above, and Ab production was assessed in cultures on day 7 as a measurement of PC differentiation. Stimulation of HC and RA B cells with IL-21, anti-CD40, and anti-IgM elicited differentiation of PCs and significant production of IgG by day 7, whereas stimulation with CpG-B led to a predominance of IgM production (data not shown, Fig. 6B). Addition of anti-CD32b, but not anti-CD19, resulted in inhibition of IgG production following IL-21 costimulation in RA patients similar to HCs, regardless of reduced CD32b expression (Fig. 6B). Addition of anti-CD19, but not anti-CD32b, inhibited responses to CpG in both HC and RA cultures, suggesting that CD19 on RA B cells is functioning normally. Importantly, under both stimulation conditions, cross-linking of CD19 and CD32b with the bispecific Bs3Ab comparably inhibited Ig production from HC and RA B cells (inhibition of IgG production: HC, 66% versus 66.5%, respectively; inhibition of IgM production: 51% versus 57%, respectively). These data imply that, despite altered expression of CD32b, therapeutics that cross-link CD19 and CD32b may be beneficial for regulating B cell responses in settings of autoimmunity.
CD19 and CD32b do not regulate Ig production from pre-established PCs
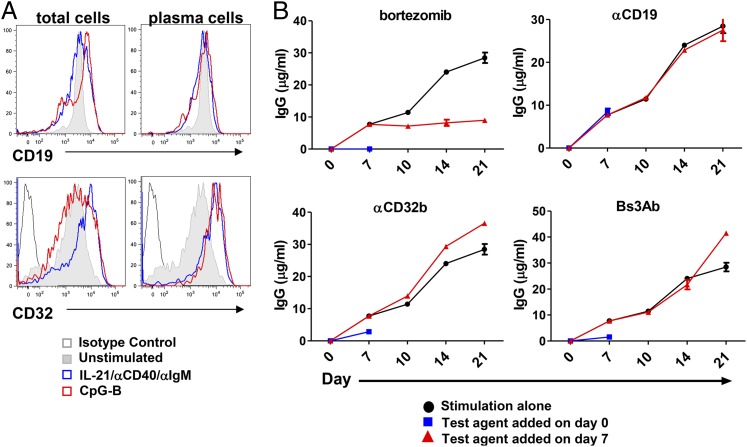
Both newly differentiated and long-lived PCs are a source of protective immunity against pathogens but can also contribute to pathogenic autoantibody production in settings of autoimmunity. We next wanted to understand the role of CD19 versus CD32b in the control and regulation of established PCs. Importantly, a substantial fraction of PCs that produce Ig express both CD19 and CD32, and we reported that in vitro–differentiated PCs also express measurable densities of CD19 (21, 28, 30). PCs were generated by stimulating purified human B cells with either CpG-B or with a combination of IL-21, anti-CD40, and anti-IgM. By day seven, a population of terminally differentiated, CD19+ IgD− CD38hi PCs was present, as we reported previously (28, 31). All B cell populations in the culture, including the PCs, maintained high expression of CD19, comparable to levels expressed on unstimulated B cells (Fig. 7A, top panels). CD32b expression was also maintained on all B cell subsets examined following 7 d of stimulation, with PCs expressing higher levels of CD32b than unstimulated B cells (Fig. 7A, bottom panels). To assess the role of CD19 and/or CD32b on Ig production of these pre-established PCs, these molecules were engaged with both mono- and bispecific Abs. As was shown previously in this system (28), addition of bortezomib, a proteasome inhibitor, completely blocked PC differentiation under these culture conditions and, thus, IgG production when added at the initiation of culture (Fig. 7B, upper left panel, blue line). Moreover, bortezomib also inhibited pre-established PCs from continuing to produce IgG when added after 7 d in culture (Fig. 7B, upper left panel, red line). Consistent with our earlier results, when anti-CD32b or Bs3Ab was added at the initiation of B cell culture, IgG production was inhibited by 63 or 80%, respectively, following activation with IL-21, anti-CD40, and anti-IgM (Fig. 7B, lower panels, blue line), whereas anti-CD19 was unable to inhibit IgG production (Fig. 7B, upper right panel, blue line). Interestingly, however, none of the Abs was able to blunt IgG production when added to cultures containing pre-established PCs at day 7, as noted by a continued increase in IgG concentration (Fig. 7B, lower panels, red line). These results suggest that, unlike primary B cells, neither CD19 nor CD32b influences Ig production by terminally differentiated PCs.
FIGURE 7.
Cross-linking CD19 and CD32 on pre-established PCs does not inhibit Ig secretion. (A) CD19 or CD32 surface expression was measured on cultures of purified blood B cells on day 7. Total cells in culture (left panels). Gated on IgD− CD38hi PCs (right panels). (B) Purified B cells were stimulated with IL-21/anti-CD40/anti-IgM, and IgG production was measured on the days indicated. Black line: IgG levels over 21 d in the absence of inhibitor; blue line: test agent indicated was added at the initiation of culture, and IgG levels were assessed on day 7; red line: test agent indicated was added after ∼25% of B cells had differentiated into PCs on day 7, and IgG levels were assessed on days 10, 14, and 21. One of four independent studies is shown.
Targeting CD19 and CD32b inhibits cytokine production, as well as T cell priming, by B cells
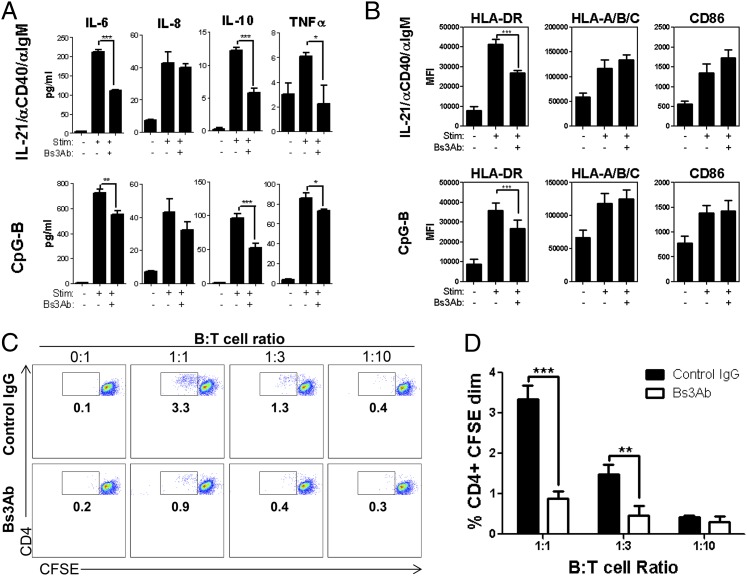
In addition to the production of Abs, B cells have diverse functions that contribute to the development and maintenance of autoimmune disease progression. More specifically, B cells produce immunomodulatory cytokines and act as APCs capable of promoting T cell priming and expansion (32). We next determined the effect of cotargeting CD19 and CD32b on cytokine production by human B cells. Stimulation with either CpG-B or a combination of IL-21, anti-CD40, and anti-IgM induced B cells to secrete IL-6, IL-8, IL-10, and TNF-α (Fig. 8A). Interestingly, addition of Bs3Ab to these cultures selectively inhibited the production of IL-6, IL-10, and TNF-α but had no effect on IL-8 production (Fig. 8A).
FIGURE 8.
Coengagement of CD19 and CD32b inhibits cytokine production and APC functionality of human B cells. Purified B cells were stimulated with IL-21/anti-CD40/anti-IgM or CpG-B. Cytokine production (A) and expression of costimulatory markers (B) were measured on day 2. (C) B cells were cocultured with CFSE-labeled CD4+ T cells in the indicated ratios in the presence of control Ab or Bs3Ab. On day 4, dilution of CFSE was quantified by flow cytometry. Plots shown are gated on CD4+ T lymphocytes. (D) Quantification of CFSEdim CD4+ T cells from (C). Average and SD of triplicate wells are shown for one of three representative donors tested. *p < 0.05, **p < 0.01, ***p < 0.005.
The ability of B cells to serve as APCs relies on the expression of HLA molecules, as well as costimulatory ligands, such as CD40 and CD80/CD86. We next determined the effect of Bs3Ab on the expression of these molecules. Following stimulation with either CpG-B or IL-21, anti-CD40, and anti-IgM, there was a significant increase in the expression of HLA-DR and HLA-A/B/C (Fig. 8B). Expression of CD86 was also increased under these conditions (Fig. 8B). Targeting CD19 and CD32b with Bs3Ab significantly blunted the upregulation of HLA-DR but did not impact the expression of HLA-A/B/C or CD86 (Fig. 8B). To determine the effect of diminished HLA-DR expression on the ability of human B cells to prime CD4+ T cell expansion, an allogeneic MLR assay was performed. As expected, ∼3% of CD4+ T cells responded to allogeneic HLA, as noted by CFSE dilution of the T cells after 4 d in culture with B cells at a 1:1 ratio (Fig. 8C). The percentage of responding T cells was reduced in a dose-dependent manner with diminishing B cell numbers (Fig. 8C, 8D). Importantly, Bs3Ab significantly reduced the ability of CD4+ T cells to respond to allogeneic B cells (Fig. 8C, 8D). These results provide critical evidence that cotargeting CD19 and CD32b can affect both humoral and Ab-independent aspects of the B cell response.
Discussion
The activation of human B cells is a complex process in which a number of cell surface receptors regulate the activation threshold of the cell. B cells can be activated in the context of T-dependent responses, which largely take place in the germinal center and are associated with Ag stimulation through the BCR in the context of T cell–derived costimulatory signals, such as IL-21 and CD40L (33). Alternatively, B cells can be activated by T-independent Ags: for example, by signals delivered through TLRs. Chronic activation of self-reactive B cells may be initiated through several pathways; understanding the underlying mechanisms that control and regulate B cell responsiveness may prove beneficial in settings of autoimmunity.
In this study, we further elucidated the role of CD19 and CD32b in B cell activation and differentiation by using Abs that engage CD19 or CD32b. The ability of these Ags to coordinately modulate B cell responses was also determined when they were coengaged with cross-linking or with a bispecific Ab. Interestingly, our data demonstrate that CD19 and CD32b differentially inhibit B cell expansion and PC differentiation, depending on the nature of the activating stimuli, when engaged with monospecific Abs. Abs directed against CD32b inhibited B cells activated in the context of BCR signals and costimulation through IL-21R and CD40. These results are in alignment with several publications (2–4) that showed that ligation of CD32b leads to dephosphorylation of BCR-associated ITAM and decreased downstream calcium flux, expansion, and differentiation. Additionally, anti-CD32b inhibited IgG secretion from B cells stimulated with IL-21 and anti-CD40 in the absence of BCR engagement (data not shown), suggesting that CD32b can regulate signals delivered through IL-21R and CD40, independently of effects on the BCR. In contrast to IL-21 and BCR signals, we show that engagement of CD19, but not CD32b, with a mAb inhibited CpG-B–induced B cell activation and PC differentiation. Previous studies in mice identified a link between CD19 and TLR signaling in B cells. LPS signaling induces phosphorylation of CD19 (34), and mice deficient in CD19 have decreased proliferative responses to LPS in vitro (19). Further, CD19−/− mice have reduced fibrosis and autoantibody titers in a bleomycin model, which is driven largely by hyaluronan-induced TLR responses (35). Indeed, our study provides novel data that demonstrate a role for CD19 in the regulation of TLR9 signaling in human B cells.
Importantly, although engagement of either CD19 or CD32b has the ability to regulate B cell activation under select conditions, cross-linking these molecules with a bispecific Ab potently inhibited the expansion and differentiation of human B cells activated under conditions that mimic both T-dependent and T-independent responses. In addition to the conditions described above, we found that cross-linking CD19 and CD32b inhibited B cell activation and differentiation in response to various combinations of activating cues, including IL-21/anti-CD40, anti-IgM/anti-CD40, and anti-CD79 alone (data not shown). Further, we demonstrate that naive, IgM+ memory, and IgG+ memory B cells from circulation are all susceptible to inhibition by CD19 and CD32b. These data cumulatively suggest that cotargeting CD19 and CD32b broadly inhibits B cell responses.
The ability to regulate B cell responses in vivo by targeting CD32b has been studied in several animal models. Using a model of collagen-induced arthritis, Veri et al. (36) demonstrated that prophylactic dosing of a bispecific “DART” molecule that targets CD32b and CD79 delayed disease onset. Importantly, therapeutic dosing of this “DART” Ab following disease onset also reduced disease severity (36). Additionally, studies were carried out in rodent models using an Fc-engineered anti-CD19 Ab with increased affinity for CD32b (37). In an immunization setting, cotargeting CD19 and CD32b suppressed the humoral immune response, including reduced production of IgM, IgG, and IgE isotypes following challenge with a T-dependent Ag (37).
The effect of cotargeting CD19 and CD32b has also been studied in humans. Chu et al. (38) generated an Fc-engineered anti-CD19 Ab with increased affinity for CD32b (referred to as IIbE), which has recently begun a Ph1b/2a clinical trial in patients with RA. Unlike the approach described in this study in which CD32b is specifically targeted with the scFv of an Ab, this Fc-engineered construct has greatly increased affinity for CD32b but also retains its natural binding to CD32a and to FcγRI. Consistent with our results, the investigators demonstrates that IIbE inhibits BCR-induced calcium flux and B cell proliferation. Importantly, these studies were conducted by stimulating human B cells with anti-CD79b to induce BCR activation, and the investigators report that coengagement of BCR and CD32b by IIbE under these conditions induces enhanced apoptosis of B cells (38). Our studies extend these findings and characterize the effect of cross-linking CD19 and CD32b in the context of both BCR-dependent and -independent signals. Interestingly, data reported in this article demonstrate that engagement of CD19 and CD32b, using a bispecific or cross-linking Ab, can impact cell survival, but this effect is influenced by the nature of the activating milieu (Fig. 4). Although previous studies indicated (38) that coengagement of CD32b and BCR induces robust cell death, we show that inhibition of TLR-driven responses occurs independently of effects on cell death. Although cotargeting CD19 and CD32b can modestly impact B cell survival following stimulation with IL-21, anti-CD40, and anti-IgM, the impact on death does not account for the robust ability of Bs3Ab to inhibit B cell expansion and differentiation. Differences in these two studies may reflect a different mode of inhibition when CD32b is engaged by a mutated Fc versus the scFv of an Ab. Alternatively, the disparity may be a result of the context of signals in which the BCR was engaged. Chu et al. (38) engaged CD32b in the context of BCR signals alone. In contrast, our studies deliver a CD32b signal with BCR ligation and costimulation by IL-21 and CD40, an array of stimuli that more closely mimics the signals that a B cell is likely to receive in vivo during an immune response. In this context, although BCR signaling is likely attenuated by the bispecific Ab, IL-21 and CD40 signals may partially rescue cells from death (31). Importantly, the data presented in this article demonstrate that it may be possible to tune cellular activation without directly killing B cells in vivo. Further, we show that targeting CD19 and CD32b with a bispecific or cross-linking Ab potently inhibited downstream readouts, such as B cell expansion, PC differentiation, and Ig production.
The most likely benefit of a bispecific Ab that modulates B cell activation by targeting CD19 and CD32b would be observed in autoimmune patients in whom chronically activated B cells contribute to disease pathogenesis. Importantly, a number of studies demonstrated that regulation of FcγR expression and function could be a determining factor in autoimmunity. CD32 expression is reduced on monocytes in SLE and RA patients (39), whereas NK cells from RA patients have reduced expression of both CD16 (FcγRIII) and CD32a (FcγRIIa) and increased expression of CD32b (40). Shifts in expression patterns of CD32a and CD32b were also noted on macrophages from RA patients (41). Importantly, surface expression of CD32b is reduced on SLE B cells (42) and is associated with increased calcium flux and activation in response to BCR ligation (42, 43). Further, polymorphisms in CD32b are associated with SLE (44–46). In this study, we show that CD32b expression is also reduced on circulating B cells from RA patients. This reduced expression of CD32 could contribute to the hyperactivation of RA B cells in vivo. Importantly, however, despite reduced expression of CD32b, engagement of CD32b blunted PC differentiation and Ig production from primary RA B cells, similar to what we observed with B cells from healthy donors.
Although single or coengagement of CD32b with CD19 blocked the differentiation of PCs in vitro, it did not impact Ig production by pre-established PCs. These results were somewhat surprising given a previous study (30) that demonstrated that ligation of CD32b induces apoptosis of murine PCs and human plasmablasts. Several possibilities could account for these differences. First, unique stimulation conditions were used to generate PCs in these studies. Although Xiang et al. (30) used a combination of soluble CD40L and IL-4 to stimulate B cells, studies presented in this article used CD40 signals with IL-21 and BCR ligation, which were shown previously to induce the differentiation of terminally differentiated, noncycling PCs (31). Moreover, IL-21 was shown to increase the survival of some subsets of PCs, including those isolated from secondary lymphoid tissues (47). Therefore, the addition of IL-21 may have been sufficient to prevent apoptosis. Additionally, these earlier studies demonstrated a modest increase in cell death among plasmablasts using annexin V staining following 8 h of ligation with CD32b. In contrast, our studies measure Ig production by PCs over the course of 3 wk. CD32b engagement may induce apoptosis of a subset of plasmablasts in culture, but our data suggest that it does not induce cell death of the terminally differentiated PCs capable of sustained Ab production.
B cells have diverse functions that contribute to disease progression in settings of autoimmunity. In addition to the production of pathogenic autoantibodies, B cells play an immunomodulatory role through the production of proinflammatory cytokines and by the presentation of self-Ags via HLA molecules (32). Notably, in a human clinical trial using B cell–depletion therapy in patients with lupus nephritis, remission following B cell depletion was associated with a significant decrease in the expression of CD40L on CD4+ T cells (48). These results suggest that B cells play a critical Ab-independent role in autoimmune disease by regulating the activation of CD4+ T cells. Importantly, we demonstrate that cotargeting CD19 and CD32b with a bispecific approach has the ability to dampen both the cytokine response of B cells, as well as their ability to prime CD4+ T cells in allogeneic MLR cultures, presumably as the result of the downregulation of HLA and costimulatory Ags.
Cumulatively, the data presented in this article elucidate the differential regulation of adaptive immune responses by CD19 and CD32b. These data suggest that cross-linking CD19 and CD32b regulates primary human B cells from both healthy donors and patients with RA in response to a variety of activating cues. The mechanism of action of a bispecific Ab that cross-links CD19 and CD32b likely mimics that of immune complexes. Importantly, the data presented in this article suggest that coengagement of these molecules does not result in a synergistic signal or a dramatic enhancement of inhibition. Rather, coengagement of CD19 and CD32b promotes simultaneous blockade of two pathways that are triggered by distinct signals. In vivo, particularly in autoimmune disease in which autoantibodies, such as anti-DNA, are present, it is most likely that BCR- and TLR-driven pathways are activated concurrently. Therefore, only coinhibition of both signals is likely to have a significant effect on B cell activation in this setting. Importantly, modulation of B cell activity using a bispecific approach also could provide a safer alternative to B cell–depletion therapy for autoimmune patients who are likely to require long-term treatment. Future studies are required to identify targets that have the ability to specifically inhibit long-lived PCs and could be used in a complementary approach to provide additional benefit to patients with autoantibody-mediated disorders.
Acknowledgments
We thank Anna Hansen and Chris Hostage for technical assistance.
The online version of this article contains supplemental material.
- 7AAD
- 7-aminoactinomycin D
- DART
- Dual-Affinity Re-Targeting
- HC
- healthy control
- PC
- plasma cell
- RA
- rheumatoid arthritis
- RF
- rheumatoid factor
- SLE
- systemic lupus erythematosus
- TM
- three mutations.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Nimmerjahn F., Ravetch J. V. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8: 34–47. [DOI] [PubMed] [Google Scholar]
- 2.Amigorena S., Bonnerot C., Drake J. R., Choquet D., Hunziker W., Guillet J. G., Webster P., Sautes C., Mellman I., Fridman W. H. 1992. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science 256: 1808–1812. [DOI] [PubMed] [Google Scholar]
- 3.Muta T., Kurosaki T., Misulovin Z., Sanchez M., Nussenzweig M. C., Ravetch J. V. 1994. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature 369: 340. [DOI] [PubMed] [Google Scholar]
- 4.Fong D. C., Brauweiler A., Minskoff S. A., Bruhns P., Tamir I., Mellman I., Daeron M., Cambier J. C. 2000. Mutational analysis reveals multiple distinct sites within Fc gamma receptor IIB that function in inhibitory signaling. J. Immunol. 165: 4453–4462. [DOI] [PubMed] [Google Scholar]
- 5.Takai T., Ono M., Hikida M., Ohmori H., Ravetch J. V. 1996. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature 379: 346–349. [DOI] [PubMed] [Google Scholar]
- 6.McGaha T. L., Karlsson M. C., Ravetch J. V. 2008. FcgammaRIIB deficiency leads to autoimmunity and a defective response to apoptosis in Mrl-MpJ mice. J. Immunol. 180: 5670–5679. [DOI] [PubMed] [Google Scholar]
- 7.Brownlie R. J., Lawlor K. E., Niederer H. A., Cutler A. J., Xiang Z., Clatworthy M. R., Floto R. A., Greaves D. R., Lyons P. A., Smith K. G. 2008. Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb. J. Exp. Med. 205: 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J. Y., Wang C. M., Ma C. C., Luo S. F., Edberg J. C., Kimberly R. P., Wu J. 2006. Association of a transmembrane polymorphism of Fcgamma receptor IIb (FCGR2B) with systemic lupus erythematosus in Taiwanese patients. Arthritis Rheum. 54: 3908–3917. [DOI] [PubMed] [Google Scholar]
- 9.Floto R. A., Clatworthy M. R., Heilbronn K. R., Rosner D. R., MacAry P. A., Rankin A., Lehner P. J., Ouwehand W. H., Allen J. M., Watkins N. A., Smith K. G. 2005. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat. Med. 11: 1056–1058. [DOI] [PubMed] [Google Scholar]
- 10.Haas K. M., Tedder T. F. 2005. Role of the CD19 and CD21/35 receptor complex in innate immunity, host defense and autoimmunity. Adv. Exp. Med. Biol. 560: 125–139. [DOI] [PubMed] [Google Scholar]
- 11.Tedder T. F., Inaoki M., Sato S. 1997. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity 6: 107–118. [DOI] [PubMed] [Google Scholar]
- 12.Buhl A. M., Cambier J. C. 1999. Phosphorylation of CD19 Y484 and Y515, and linked activation of phosphatidylinositol 3-kinase, are required for B cell antigen receptor-mediated activation of Bruton’s tyrosine kinase. J. Immunol. 162: 4438–4446. [PubMed] [Google Scholar]
- 13.Li X., Sandoval D., Freeberg L., Carter R. H. 1997. Role of CD19 tyrosine 391 in synergistic activation of B lymphocytes by coligation of CD19 and membrane Ig. J. Immunol. 158: 5649–5657. [PubMed] [Google Scholar]
- 14.Wang Y., Brooks S. R., Li X., Anzelon A. N., Rickert R. C., Carter R. H. 2002. The physiologic role of CD19 cytoplasmic tyrosines. Immunity 17: 501–514. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto M., Fujimoto Y., Poe J. C., Jansen P. J., Lowell C. A., DeFranco A. L., Tedder T. F. 2000. CD19 regulates Src family protein tyrosine kinase activation in B lymphocytes through processive amplification. Immunity 13: 47–57. [DOI] [PubMed] [Google Scholar]
- 16.Engel P., Zhou L. J., Ord D. C., Sato S., Koller B., Tedder T. F. 1995. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 3: 39–50. [DOI] [PubMed] [Google Scholar]
- 17.Rickert R. C., Rajewsky K., Roes J. 1995. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 376: 352–355. [DOI] [PubMed] [Google Scholar]
- 18.van Zelm M. C., Reisli I., van der Burg M., Castaño D., van Noesel C. J., van Tol M. J., Woellner C., Grimbacher B., Patiño P. J., van Dongen J. J., Franco J. L. 2006. An antibody-deficiency syndrome due to mutations in the CD19 gene. N. Engl. J. Med. 354: 1901–1912. [DOI] [PubMed] [Google Scholar]
- 19.Sato S., Hasegawa M., Fujimoto M., Tedder T. F., Takehara K. 2000. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J. Immunol. 165: 6635–6643. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya N., Kuroki K., Fujimoto M., Murakami Y., Tedder T. F., Tokunaga K., Takehara K., Sato S. 2004. Association of a functional CD19 polymorphism with susceptibility to systemic sclerosis. Arthritis Rheum. 50: 4002–4007. [DOI] [PubMed] [Google Scholar]
- 21.Mei H. E., Schmidt S., Dörner T. 2012. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Res. Ther. 14(Suppl. 5): S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett T. B., Shu G. L., Draves K. E., Pezzutto A., Clark E. A. 1990. Signaling through CD19, Fc receptors or transforming growth factor-beta: each inhibits the activation of resting human B cells differently. Eur. J. Immunol. 20: 1053–1059. [DOI] [PubMed] [Google Scholar]
- 23.Rigley K. P., Callard R. E. 1991. Inhibition of B cell proliferation with anti-CD19 monoclonal antibodies: anti-CD19 antibodies do not interfere with early signaling events triggered by anti-IgM or interleukin 4. Eur. J. Immunol. 21: 535–540. [DOI] [PubMed] [Google Scholar]
- 24.Johnson S., Burke S., Huang L., Gorlatov S., Li H., Wang W., Zhang W., Tuaillon N., Rainey J., Barat B., et al. 2010. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J. Mol. Biol. 399: 436–449. [DOI] [PubMed] [Google Scholar]
- 25.Herbst R., Wang Y., Gallagher S., Mittereder N., Kuta E., Damschroder M., Woods R., Rowe D. C., Cheng L., Cook K., et al. 2010. B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. [Published erratum appears in 2011 J. Pharmacol. Exp. Ther. 336: 294.] J. Pharmacol. Exp. Ther. 335: 213–222. [DOI] [PubMed] [Google Scholar]
- 26.Rankin C. T., Veri M. C., Gorlatov S., Tuaillon N., Burke S., Huang L., Inzunza H. D., Li H., Thomas S., Johnson S., et al. 2006. CD32B, the human inhibitory Fc-gamma receptor IIB, as a target for monoclonal antibody therapy of B-cell lymphoma. Blood 108: 2384–2391. [DOI] [PubMed] [Google Scholar]
- 27.Oganesyan V., Gao C., Shirinian L., Wu H., Dall’Acqua W. F. 2008. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr. D Biol. Crystallogr. 64: 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karnell J. L., Karnell F. G., III, Stephens G. L., Rajan B., Morehouse C., Li Y., Swerdlow B., Wilson M., Goldbach-Mansky R., Groves C., et al. 2011. Mycophenolic acid differentially impacts B cell function depending on the stage of differentiation. J. Immunol. 187: 3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crouch S. P., Kozlowski R., Slater K. J., Fletcher J. 1993. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 160: 81–88. [DOI] [PubMed] [Google Scholar]
- 30.Xiang Z., Cutler A. J., Brownlie R. J., Fairfax K., Lawlor K. E., Severinson E., Walker E. U., Manz R. A., Tarlinton D. M., Smith K. G. 2007. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 8: 419–429. [DOI] [PubMed] [Google Scholar]
- 31.Ettinger R., Sims G. P., Fairhurst A. M., Robbins R., da Silva Y. S., Spolski R., Leonard W. J., Lipsky P. E. 2005. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 175: 7867–7879. [DOI] [PubMed] [Google Scholar]
- 32.Yanaba K., Bouaziz J. D., Matsushita T., Magro C. M., St. Clair E. W., Tedder T. F. 2008. B-lymphocyte contributions to human autoimmune disease. Immunol. Rev. 223: 284–299. [DOI] [PubMed] [Google Scholar]
- 33.Kuchen S., Robbins R., Sims G. P., Sheng C., Phillips T. M., Lipsky P. E., Ettinger R. 2007. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J. Immunol. 179: 5886–5896. [DOI] [PubMed] [Google Scholar]
- 34.Yazawa N., Fujimoto M., Sato S., Miyake K., Asano N., Nagai Y., Takeuchi O., Takeda K., Okochi H., Akira S., et al. 2003. CD19 regulates innate immunity by the toll-like receptor RP105 signaling in B lymphocytes. Blood 102: 1374–1380. [DOI] [PubMed] [Google Scholar]
- 35.Yoshizaki A., Iwata Y., Komura K., Ogawa F., Hara T., Muroi E., Takenaka M., Shimizu K., Hasegawa M., Fujimoto M., et al. 2008. CD19 regulates skin and lung fibrosis via Toll-like receptor signaling in a model of bleomycin-induced scleroderma. Am. J. Pathol. 172: 1650–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veri M. C., Burke S., Huang L., Li H., Gorlatov S., Tuaillon N., Rainey G. J., Ciccarone V., Zhang T., Shah K., et al. 2010. Therapeutic control of B cell activation via recruitment of Fcgamma receptor IIb (CD32B) inhibitory function with a novel bispecific antibody scaffold. Arthritis Rheum. 62: 1933–1943. [DOI] [PubMed] [Google Scholar]
- 37.Horton H. M., Chu S. Y., Ortiz E. C., Pong E., Cemerski S., Leung I. W., Jacob N., Zalevsky J., Desjarlais J. R., Stohl W., Szymkowski D. E. 2011. Antibody-mediated coengagement of FcγRIIb and B cell receptor complex suppresses humoral immunity in systemic lupus erythematosus. J. Immunol. 186: 4223–4233. [DOI] [PubMed] [Google Scholar]
- 38.Chu S. Y., Vostiar I., Karki S., Moore G. L., Lazar G. A., Pong E., Joyce P. F., Szymkowski D. E., Desjarlais J. R. 2008. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcgammaRIIb with Fc-engineered antibodies. Mol. Immunol. 45: 3926–3933. [DOI] [PubMed] [Google Scholar]
- 39.Hepburn A. L., Mason J. C., Davies K. A. 2004. Expression of Fcgamma and complement receptors on peripheral blood monocytes in systemic lupus erythematosus and rheumatoid arthritis. Rheumatology (Oxford) 43: 547–554. [DOI] [PubMed] [Google Scholar]
- 40.Stewart-Akers A. M., Cunningham A., Wasko M. C., Morel P. A. 2004. Fc gamma R expression on NK cells influences disease severity in rheumatoid arthritis. Genes Immun. 5: 521–529. [DOI] [PubMed] [Google Scholar]
- 41.Wijngaarden S., van de Winkel J. G., Jacobs K. M., Bijlsma J. W., Lafeber F. P., van Roon J. A. 2004. A shift in the balance of inhibitory and activating Fcgamma receptors on monocytes toward the inhibitory Fcgamma receptor IIb is associated with prevention of monocyte activation in rheumatoid arthritis. Arthritis Rheum. 50: 3878–3887. [DOI] [PubMed] [Google Scholar]
- 42.Mackay M., Stanevsky A., Wang T., Aranow C., Li M., Koenig S., Ravetch J. V., Diamond B. 2006. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J. Exp. Med. 203: 2157–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enyedy E. J., Mitchell J. P., Nambiar M. P., Tsokos G. C. 2001. Defective FcgammaRIIb1 signaling contributes to enhanced calcium response in B cells from patients with systemic lupus erythematosus. Clin. Immunol. 101: 130–135. [DOI] [PubMed] [Google Scholar]
- 44.Kono H., Kyogoku C., Suzuki T., Tsuchiya N., Honda H., Yamamoto K., Tokunaga K., Honda Z. 2005. FcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum. Mol. Genet. 14: 2881–2892. [DOI] [PubMed] [Google Scholar]
- 45.Kyogoku C., Dijstelbloem H. M., Tsuchiya N., Hatta Y., Kato H., Yamaguchi A., Fukazawa T., Jansen M. D., Hashimoto H., van de Winkel J. G., et al. 2002. Fcgamma receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCGR2B to genetic susceptibility. Arthritis Rheum. 46: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 46.Siriboonrit U., Tsuchiya N., Sirikong M., Kyogoku C., Bejrachandra S., Suthipinittharm P., Luangtrakool K., Srinak D., Thongpradit R., Fujiwara K., et al. 2003. Association of Fcgamma receptor IIb and IIIb polymorphisms with susceptibility to systemic lupus erythematosus in Thais. Tissue Antigens 61: 374–383. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Bayona B., Ramos-Amaya A., Bernal J., Campos-Caro A., Brieva J. A. 2012. Cutting edge: IL-21 derived from human follicular helper T cells acts as a survival factor for secondary lymphoid organ, but not for bone marrow, plasma cells. J. Immunol. 188: 1578–1581. [DOI] [PubMed] [Google Scholar]
- 48.Sfikakis P. P., Boletis J. N., Lionaki S., Vigklis V., Fragiadaki K. G., Iniotaki A., Moutsopoulos H. M. 2005. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 52: 501–513. [DOI] [PubMed] [Google Scholar]