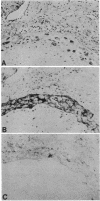
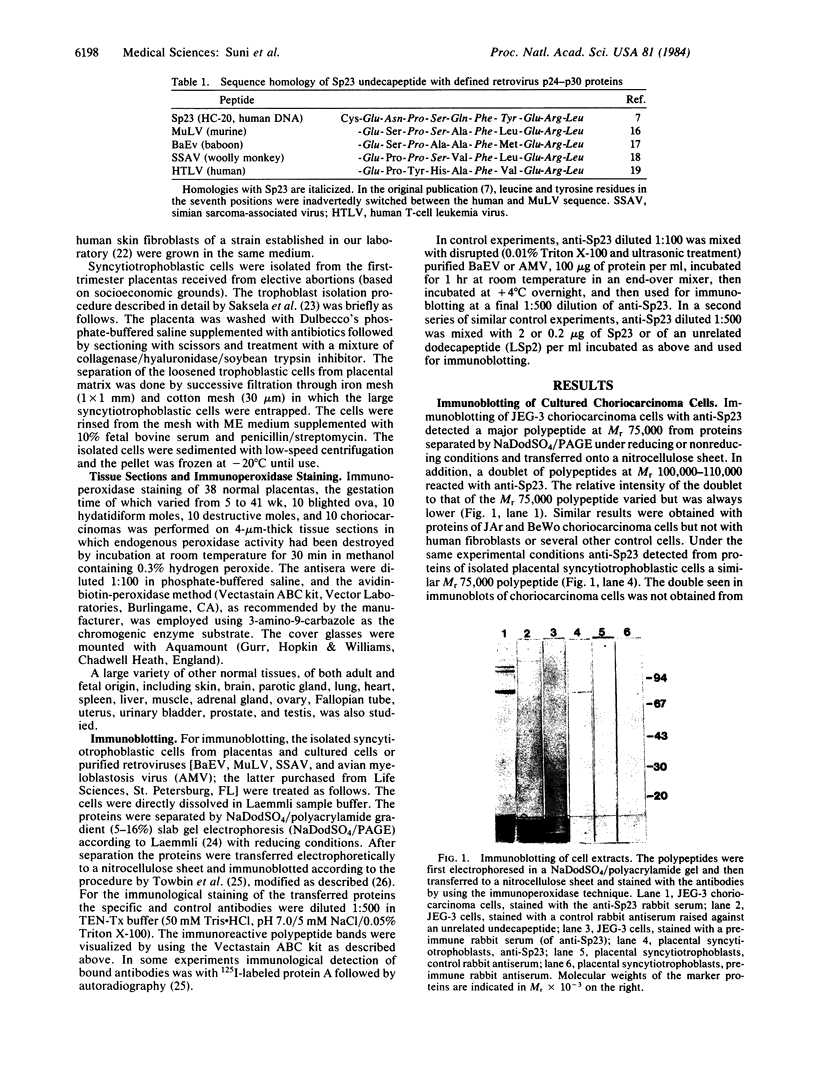
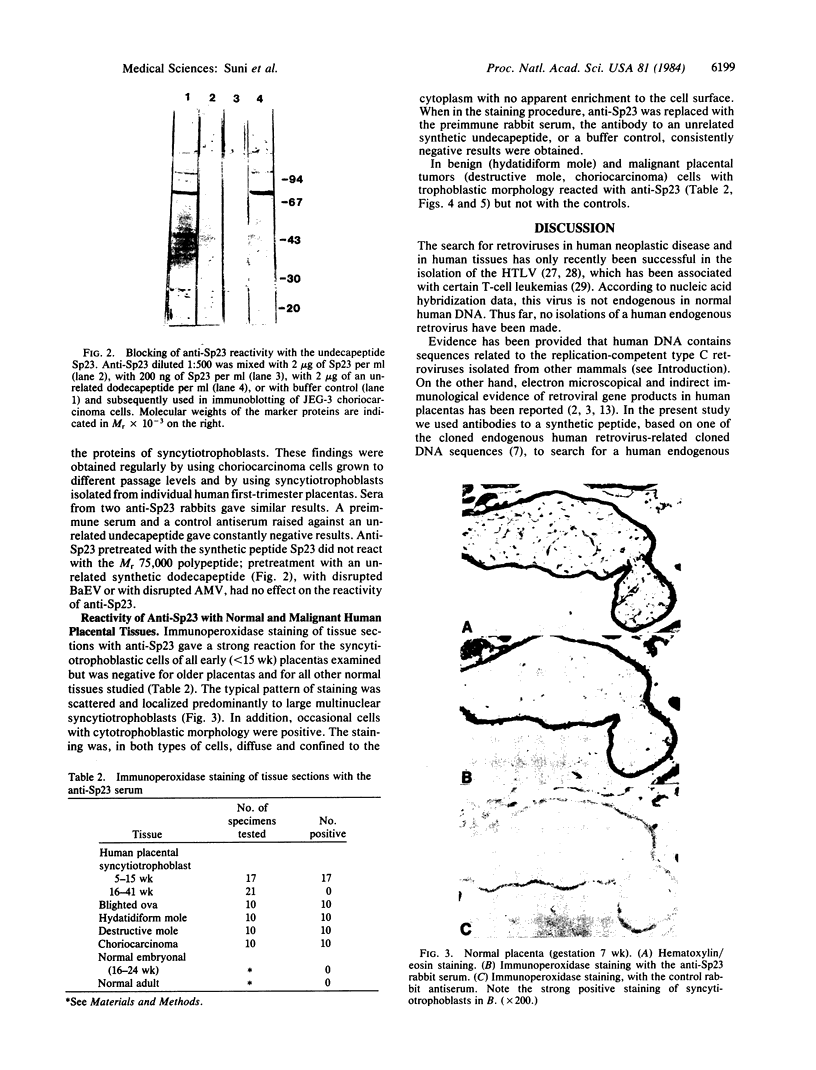
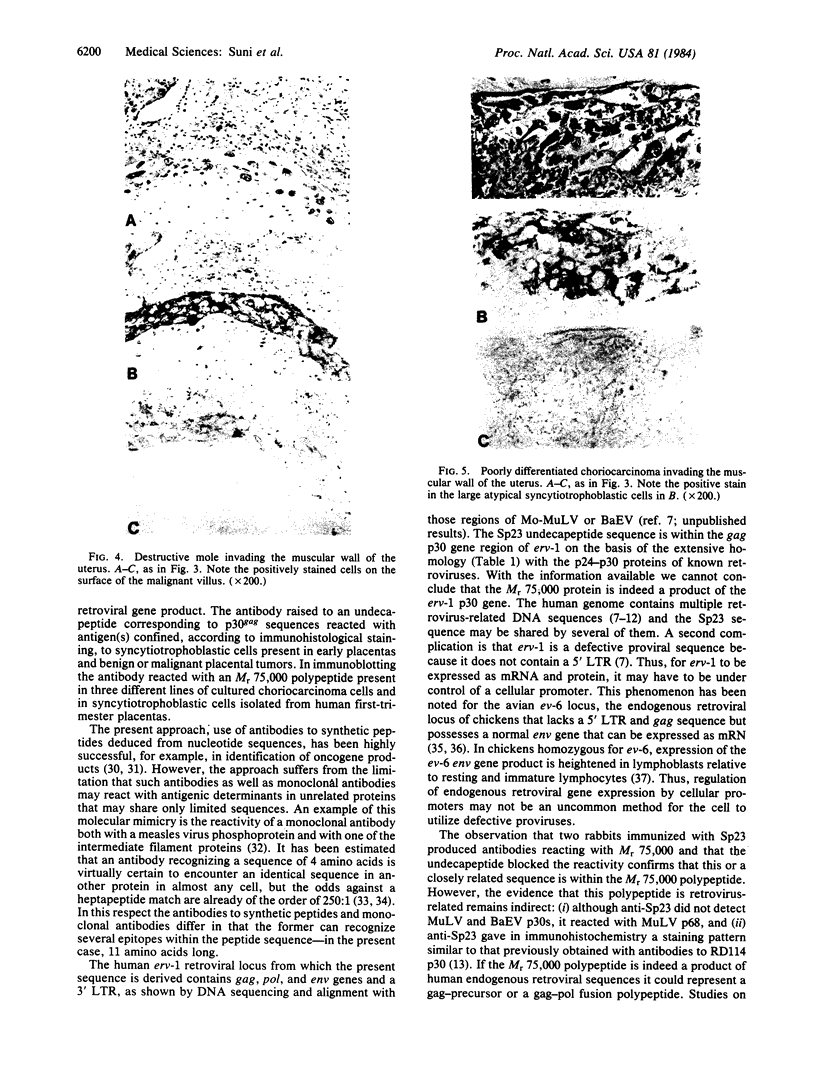
Abstract
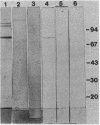
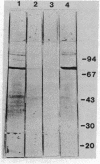
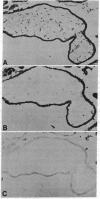
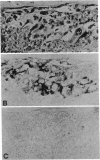
Antibodies to a synthetic undecapeptide (NH2-Cys-Glu-Asn-Pro-Ser-Gln-Phe-Tyr-Glu-Arg-Leu-COOH), the sequence (except cysteine) of which was deduced from a previously reported cloned human retroviral gag-gene-related DNA sequence erv-1, were raised in rabbits. In immunohistochemical staining these antibodies reacted with normal human first-trimester placentas and with blighted ova and benign and malignant trophoblastic tumors (hydatidiform and destructive moles, choriocarcinomas) but not with any other normal embryonic or adult tissues tested. In all tissues the reactivity was mainly confined to cells with trophoblastic morphology. In immunoblotting the antibody detected an Mr 75,000 polypeptide in syncytiotrophoblasts isolated from first-trimester placentas and in three different lines of cultured choriocarcinoma cells. The undecapeptide blocked the reactivity of the antibody.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: I. Nucleic acid from baboon type C virus as a measure of divergence among primate species. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4513–4518. doi: 10.1073/pnas.71.11.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., O'Connell C., Cohen M. Cloned endogenous retroviral sequences from human DNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4709–4713. doi: 10.1073/pnas.79.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Drohan W., Tronick S., Schlom J. Detection and cloning of human DNA sequences related to the mouse mammary tumor virus genome. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5503–5507. doi: 10.1073/pnas.79.18.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L., Leppard K., Lane D., Harlow E. Cellular proteins reactive with monoclonal antibodies directed against simian virus 40 T-antigen. J Virol. 1982 May;42(2):612–620. doi: 10.1128/jvi.42.2.612-620.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen E. R., Levy J. A. Virus-like particles in placentas from normal individuals and patients with systemic lupus erythematosus. J Natl Cancer Inst. 1977 Oct;59(4):1187–1192. doi: 10.1093/jnci/59.4.1187. [DOI] [PubMed] [Google Scholar]
- Essex M., McLane M. F., Lee T. H., Falk L., Howe C. W., Mullins J. I., Cabradilla C., Francis D. P. Antibodies to cell membrane antigens associated with human T-cell leukemia virus in patients with AIDS. Science. 1983 May 20;220(4599):859–862. doi: 10.1126/science.6342136. [DOI] [PubMed] [Google Scholar]
- Ewert D. L., Chalmers J. H., Jr, McBride R. A., Halpern M. S. Individual ev loci determine characteristic patterns of endogenous retroviral envelope antigen expression during lymphocyte maturation. Virology. 1984 Feb;133(1):128–136. doi: 10.1016/0042-6822(84)90431-8. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B., Wroblewska Z., Frankel M. E., Koprowski H. Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2346–2350. doi: 10.1073/pnas.80.8.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Braverman S. B., Astrin S. M. Transcriptional products and DNA structure of endogenous avian proviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1111–1121. doi: 10.1101/sqb.1980.044.01.120. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Toyoshima K., Bishop J. M., Varmus H. E. Organization of the endogenous proviruses of chickens: implications for origin and expression. Virology. 1981 Jan 15;108(1):189–207. doi: 10.1016/0042-6822(81)90538-9. [DOI] [PubMed] [Google Scholar]
- Kalter S. S., Helmke R. J., Heberling R. L., Panigel M., Fowler A. K., Strickland J. E., Hellman A. Brief communication: C-type particles in normal human placentas. J Natl Cancer Inst. 1973 Apr;50(4):1081–1084. doi: 10.1093/jnci/50.4.1081. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsson E., Nilsson B. O., Sundström P., Widéhn S. Morphological and microbiological signs of endogenous C-virus in human oocytes. Int J Cancer. 1981 Nov 15;28(5):551–557. doi: 10.1002/ijc.2910280504. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Zinnecker M., Hamaoka T., Katz D. H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979 Feb 20;18(4):690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Bryan T., Rasheed S., Khan A. S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Kurihara M., Takano T. Retrovirus-related sequences in human DNA: detection and cloning of sequences which hybridize with the long terminal repeat of baboon endogenous virus. Nucleic Acids Res. 1982 May 11;10(9):2865–2878. doi: 10.1093/nar/10.9.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien S. J., Bonner T. I., Cohen M., O'Connell C., Nash W. G. Mapping of an endogenous retroviral sequence to human chromosome 18. Nature. 1983 May 5;303(5912):74–77. doi: 10.1038/303074a0. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Lai M. H., Hunter T., Verma I. M. Analysis of transforming gene products from Moloney murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):109–119. doi: 10.1016/0092-8674(81)90365-2. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., O'Neill R. R., Steele P. E., Martin M. A. Characterization and partial nucleotide sequence of endogenous type C retrovirus segments in human chromosomal DNA. Proc Natl Acad Sci U S A. 1983 Feb;80(3):678–682. doi: 10.1073/pnas.80.3.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins K. C., Devare S. G., Reddy E. P., Aaronson S. A. In vivo identification of the transforming gene product of simian sarcoma virus. Science. 1982 Dec 10;218(4577):1131–1133. doi: 10.1126/science.6293053. [DOI] [PubMed] [Google Scholar]
- Saksela O., Wahlström T., Lehtovirta P., Seppälä M., Vaheri A. Presence of alpha 2-macroglobulin in normal but not in malignant human syncytiotrophoblasts. Cancer Res. 1981 Jun;41(6):2507–2513. [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Suni J., Wahlström T., Vaheri A. Retrovirus p30-related antigen in human syncytiotrophoblasts and IgG antibodies in cord-blood sera. Int J Cancer. 1981 Nov 15;28(5):559–566. doi: 10.1002/ijc.2910280505. [DOI] [PubMed] [Google Scholar]
- Tamura T. A. Provirus of M7 baboon endogenous virus: nucleotide sequence of the gag-pol region. J Virol. 1983 Jul;47(1):137–145. doi: 10.1128/jvi.47.1.137-145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Kurkinen M., Lehto V. P., Linder E., Timpl R. Codistribution of pericellular matrix proteins in cultured fibroblasts and loss in transformation: fibronectin and procollagen. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4944–4948. doi: 10.1073/pnas.75.10.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartio T., Zardi L., Balza E., Towbin H., Vaheri A. Monoclonal antibodies in analysis of cathepsin G-digested proteolytic fragments of human plasma fibronectin. J Immunol Methods. 1982 Dec 30;55(3):309–318. doi: 10.1016/0022-1759(82)90090-4. [DOI] [PubMed] [Google Scholar]