Abstract
The aim of this study is to reveal the timing and growth pattern of central octavolateral projection development in the Mexican axolotl, Ambystoma mexicanum. In this amphibian species the development of the inner ear occurs first, followed by mechanosensory lateral line organs, and finally by ampullary electroreceptors. Several hypotheses have been proposed about how the development of peripheral organs, including differential projections of the ear, might relate to the development of central projections. Our data suggest that the sequence of maturation of the ear, mechanosensory lateral line, and ampullary electroreceptive organs is closely accompanied by the timed development of the trigeminal, inner ear, mechanosensory lateral line organs, and the ampullary electroreceptor afferent projections in the axolotl. Our data suggest that segregation of central termination within the alar plate is a function of time and space: later forming organs are likely innervated by later forming ganglia that project centrally later and to more dorsal areas of the alar plate that have not yet received any other afferents. Later forming ganglia of the same type may grow along existing pathways of earlier formed neurons.
Keywords: Electroreception, Ambystoma mexicanum, Afferent, Ampullary organ, Central projection
Introduction
Among tetrapods, many salamanders and some caecilians (Fig. 1) have the full complement of octavo-lateral organs (inner ear, mechanosensory lateral line, ampullary electroreceptors; Fritzsch, 1981; Fritzsch and Wahnschaffe, 1983). Such organs are also found in other aquatic vertebrates such as lampreys, elasmobranchs and other non-teleosts (Bullock and Heiligenberg, 1986; Northcutt, 1997). In contrast, frogs (Fig. 1) never develop ampullary electroreceptors (Fritzsch, 1988a; Schlosser, 2002a) and many frogs lose the lateral line system completely during metamorphosis (Fritzsch, 1990; Schlosser, 2002b). Amniotes develop only an inner ear (Fritzsch et al., 1998) and the tympanic organ in birds (Neeser and von Bartheld, 2002; von Bartheld, 1990). The central projections of these different organs are well stratified in adults and show no overlap (Fritzsch, 1988a, b; Northcutt, 1980) despite an earlier contention that they do so (Larsell, 1967). In all vertebrates possessing those organs, the ampullary electroreceptors project to the most dorsal part, and the inner ear to the most ventral part of the octavolateral area of the alar plate of the hindbrain (Fritzsch, 1981, 1988b). More ventral areas of the alar plate appear to be molecularly specified to receive trigeminal and taste bud fibers (Qian et al., 2001). Among urodele amphibians, a developmental sequence has been described that shows that the inner ear develops first followed by the mechanosensory lateral line organs and the ampullary electroreceptors (Fritzsch and Bolz, 1986; Northcutt and Brandle, 1995; Northcutt et al., 1994, 1995). However, except for generalized nerve staining of salamanders (Northcutt and Brandle, 1995) and experimental tracing studies on zebrafish, a teleost without electroreception (Gompel et al., 2001a), there are no data as yet on the timing of arrival of vestibular and cochlear afferents or on their mode of segregation within the alar plate (Rubel and Fritzsch, 2002). For the ear, experimental data in mammals suggest that specific projections of the vestibular epithelia as well as differential projections of the cochlea develop early (Fig. 1) and may be specific from the onset (Maklad and Fritzsch, 2003a). Differences in central and peripheral development have been noted for chicken (von Bartheld et al., 1991), but later more extensive work showed this to be otherwise (Fritzsch et al., 1993).
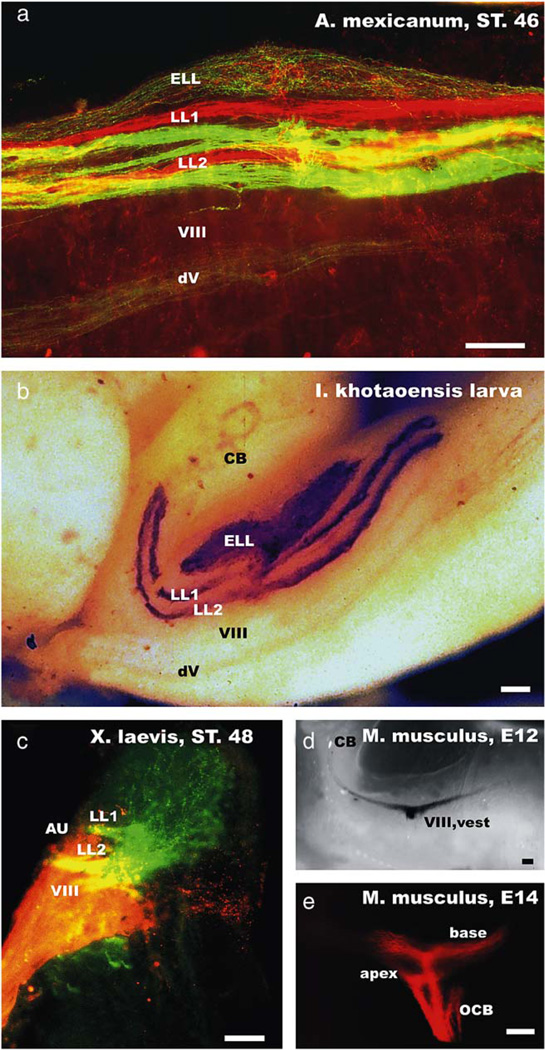
Fig. 1.
The organization of the lateral line and inner ear afferent projections are shown for the axolotl (a), a gymnophionan (b), a frog (c) and the vestibular and cochlear projections of a mouse embryo (d, e). Note that older salamander and gymnophionan larvae show a clear segregation of mechanosensory afferents of a given peripheral lateral line nerve into two distinct fascicles (LL1, LL2). Labeling two peripheral nerve branches (in this case the anterodorsal and anteroventral lateral line nerve) with differently colored dextran amines results in labeling of two discrete fascicles for each nerve (a). In contrast, no such fasciculation is apparent in the electrosensory afferent projections (ELL; a, b). Frog lateral line projections show two entering fascicles, but widespread ramification throughout the entire lateral line nucleus (c). The frog inner ear projection shows the vestibular component (VIII) ventral to the lateral line projection, but also the formation of the auditory projection (AU) lateral to the lateral line projection. In mice, the vestibular (d) and cochlear (e) fibers are forming distinct projections from the earliest time they can be labeled and do so even for subdivisions derived from different parts of the cochlea (e). Labeling of all cochlear afferent fibers would result in a continuous projection with no distinct fascicles being apparent. CB, cerebellum; dV, descending tract of the trigeminal nerve; LL1, LL2, lateral line afferent fascicles; ELL, electroreceptive ampullary organ projection; VIII, vest, vestibular component; OCB, olivocochlear bundle. Bars indicate 100 µm in all images.
In order to determine whether the sequential development of afferents of the three sensory systems of a salamander reflects the sequence of maturation of the ear, the mechanosensory lateral line organs, and the ampullary electroreceptors and whether the projections initially segregate or overlap, we examined the projection of the ear, mechanosensory lateral line organs, and the ampullary electroreceptors in the developing axolotl. Our study investigates the temporal and spatial organization of the octavolateral and trigeminal afferents with three hypotheses in mind:
Afferents from placodal ganglion cells may grow randomly into the brain and become assigned to specific pathways only after they are connected with a specific peripheral organ, i.e. trigeminal, inner ear, lateral line, and ampullary organs. This will be referred to here as the retrograde determination hypothesis and would suggest that central projection specification occurs as a consequence of a naïve sensory neuron coming into contact with a specific, projection determining peripheral organ. Some limited data suggest that lateral line afferents, after being diverted to innervate a transplanted ear, may indeed reorganize their central projections to project to vestibular rather than lateral line nuclei in a frog (Fritzsch, 1999). Support for this notion may be seen in the towing of lateral line nerve fibers by migrating neuromasts (Gilmour et al., 2004; Metcalfe, 1985) and the alleged and recently demonstrated clonal relationship of hair cells and sensory neurons of the inner ear (Maklad and Fritzsch, 2003b; Satoh and Fekete, 2005).
Afferents grow into the brainstem first and select their peripheral organs according to their central projection. It has been proposed that this might be a mechanism by which not only different organ systems might be selected but also a mechanism through which specific differential projection within an organ system might be achieved. This idea will be referred to as the anterograde determination hypothesis (Gompel et al., 2001b; Northcutt et al., 1994).
Placode-derived ganglion cells are specified at the onset of differentiation to select the appropriate peripheral and central termination, possibly related to their origin in a specific placodal area with an overlappingly expressed set of genes (Fritzsch et al., 2002). This idea was originally proposed in the context of the blueprint hypothesis (Carney and Silver, 1983) and was recently revived following the discovery of delamination of sensory neurons only from discrete areas of the inner ear (Fritzsch, 2003). This idea for the establishment of connections will be referred to as the predetermination hypothesis.
Above and beyond such selection of projections to organs and central termination that may be achieved through biochemical means, a temporal gradient may be superimposed on neuron formation as well as central and peripheral projection development (Rubel and Fritzsch, 2002). Thus, later forming organs may be innervated by later forming neurons that project to the more dorsal area of the alar plate that has not yet received afferents (the latest postmitotic cells).
We have studied the development of the trigeminal, inner ear, lateral line and ampullary organ projection into the medulla of the axolotl hindbrain to determine whether the sequential development of afferents reflects the sequence of maturation of organs and whether there is an initial segregation or an initial overlap of the projection and how this correlates with the arrival of fibers in the brain and the sensory organs. Our data strongly suggest that afferents project centrally and peripherally in a matching temporal sequence, progressing from ventral to dorsal in the alar plate and from the inner ear to the electroreceptive ampullary organs, with no exuberant projections into adjacent more dorsal areas forming at any time. The apparent simultaneous specification of peripheral and central projections suggests that the predetermination hypothesis most likely applies for salamander cranial sensory development. The nature of the molecular cues specifying this projection is unknown.
Materials and methods
Housing and care
Axolotl larvae, stage 9 (late blastula), were obtained from the Indiana axolotl colony and were reared to hatching in aged tap water. Larvae from stage 32 to stage 43 (Bordzilovskaya et al., 1989) were utilized in this project. Approximately 10 larvae at each stage were needed to ensure quality data. All specimens were contained in 12 × 8 × 4 in plastic boxes filled with filtered tap water at room temperature. Larvae, nourished by their yolk sacs did not require supplemental feeding. Procedures were approved by a Creighton University IACUC protocol.
Labeling with dextran amines
Axolotl larvae of stages 32–40 were freed from their egg capsules using watchmaker forceps. Axolotls were staged utilizing a dissection microscope. Larvae were placed in frog Ringer/0.2% benzocaine solution in a Petri dish for anesthesia. The surgery was conducted in straight frog Ringer after all responsiveness had ceased in the larvae. A shallow, vertical incision was made dorsal to the eye (to label the trigeminal nerve, including anterodorsal lateral line fibers) or posterior to the eye (to label the ophthalmic and maxillary division of the trigeminal nerve including all anterodorsal lateral line fibers) by a tungsten microneedle. Biotinylated dextran amine (BDA; Molecular Probes Inc., Eugene, OR) was inserted into the incision with another tungsten microneedle as previously described (Fritzsch, 1993; Glover, 1995). The larvae were then transferred into straight frog Ringer for 2 h to allow the diffusion of the BDA. After 2 h, larvae were euthanized in 0.2% benzocaine and placed in 2.5% glutaraldehyde for 4–8 days.
Larvae were then placed in phosphate buffer saline (PBS) pH 7.4 while their facial skin and brains were dissected. The skins and brains were immersed in 70% ethyl alcohol. Three ethyl alcohol washes of 10 min each in 70%, 90%, and 100% ethyl alcohol followed. The skins and brains were then placed in methanol for 10 min. A bath of 1 h in xylene followed. After the xylene, the samples were washed in methanol for 10 min. Three 10 min washes in 100%, 90%, and 70% ethyl alcohol followed. The samples were then placed in distilled water until they sank. They were then placed in freshly prepared ABC solution made from a Vector kit overnight. The next morning the samples were washed for 5 h with changes every 30 min in pH 5.1 phosphate buffer. The tissue was then soaked in 0.001% diaminobenzidine (DAB) in 0.1M phosphate buffer (pH 5.1) for 2 h in a refrigerator. The tissues in the DAB were subsequently reacted with 0.3% hydrogen peroxide until the brown reaction product staining the BDA filled fibers was revealed. The reaction was stopped by adding 100% ethyl alcohol. The tissue was then placed in 70% ethyl alcohol and later 2.5% glutaraldehyde for preservation.
At least two animals of each stage were processed differently. The heads were taken from the 2.5% glutaraldehyde and placed into a glutaraldehyde/sucrose mixture to change the specific density of the specimens. The heads were then dabbed dry and oriented in warm gelatin. The gelatin was allowed to harden and they were placed in 2.5% glutaraldehyde in the refrigerator overnight. Sections were then cut at 200 mm using a vibratome and reacted for BDA detection as described above. Some reacted sections were mounted and imaged directly. Other sections were washed in PBS for 15 min, placed in a 1:1 mixture of 0.2M PB and osmium tetroxide 2% for 1 h and dehydrated in 50%, 70%, and 90% ethanol for 10 min each. Following 100% ethanol and propylene oxide washes, the tissue was placed in an epoxy resin and propylene oxide mixture, 1:1, overnight. After evaporation of propylene oxide, the tissue was oriented flat on the bottom of rubber molds in fresh epoxy resin. The hardened epoxy was sectioned at 1 mm thickness, counterstained with toluidine blue and imaged.
In addition, selective albino axolotls of older stages received application of Texas red dextran amine (TDA) and fluoresceine dextran amine (FDA) to either the anterodorsal or the anteroventral lateral line nerves to show the degree of overlap and segregation of mechanosensory and electrosensory lateral line afferents.
Labeling with DiI
We also used the diffusion of lipophilic dyes in fixed tissue to assess the inner ear projections. As dissection of the inner ear is possible only after removal of the skin, we always had some unwanted labeling of lateral line fibers in dextran amine applications. However, in fixed animals a large flap of skin could be removed and thus the inner ear exposed directly to allow insertion of a dye soaked filter strip directly into the inner ear or, at later stages, into selective endorgans (Maklad and Fritzsch, 2003a). After appropriate diffusion time at 36 °C, the brains were dissected, cleared, mounted and viewed in a compound microscope (Nikon Eclipse 800). Images were digitized and plates were composed using Corel Draw.
Development of neuromasts and ampullary electroreceptors
Axolotl skin prepared from the above outlined experiments to study central projections was utilized to determine at what stage neuromasts and ampullary organs are first innervated. The skin was flattened and mounted on a glass slide. The distribution of TDA or FDA labeled nerve fibers was analyzed in combination with DIC imaging of the skin to identify organs using criteria previously described (Fritzsch and Bolz, 1986; Northcutt et al., 1994). We also used the diffusion of DiI to image progression of organ innervation. This approach offered additional information as it allowed investigating direct contacts of fibers and sensory cells through the transfer of dye.
We also investigated very crudely the response properties to mechanical and electrical stimulation. For mechanical stimulation we used pipettes and let drops fall on the water surface. These drops provide for mechanical stimulation that can be perceived through either the trigeminal, inner ear or lateral line sensory system. We also used two carbon electrodes to generate electric fields that were measured to be around 600 µV/cm at 10 Hz near the head of individuals tested. Only one test per animal was applied to ensure that habituation did not result in false negative responses.
Results
Organization of the octavolateral projections in amphibians and mammals
Central projections of the lateral line nerves show distinct differences in the detailed organization of the lateral line afferents. We want to present those data first so as to provide a basis for our developmental analysis. Previous work on European salamanders had shown that the three main divisions of the lateral line project each into exactly two fascicles for a total of six fascicles (Fritzsch, 1981). We show here that neuromast projections for a given peripheral nerve branch form two segregated longitudinal bands, with other fascicles from other nerve branches being interspersed (Fig. 1a). Each of these bundles enters the medulla as a distinct fascicle with very little fiber exchange between these fascicles. In contrast to this well-ordered organization is the organization of the ampullary electroreceptor afferents. Those fibers are profusely intermingled with no apparent anatomical organization. Similar organizations are even more obvious in the gymnophionan larvae, with two fascicles for each peripheral lateral line nerve and an entangled mass of electroreceptive afferents (Fig. 1b). In contrast to salamanders and gymnophionans, frogs have no electroreceptive afferents (Fig. 1c). Interestingly, while lateral line afferents of a given peripheral nerve tend to enter the medulla as two more or less discrete fascicles, the central organization shows little overall distinction and profound overlap of fibers from different peripheral nerves (Fig. 1c). Fascicular projection is not only a feature of lateral line organs but also of parts of the mammalian ear. Different parts of the cochlea each project as discrete fascicles virtually from the earliest formation of such projection, resembling in its organization the different projection fascicles of the mechanosensory lateral line (Fig. 1e).
Developmental analysis
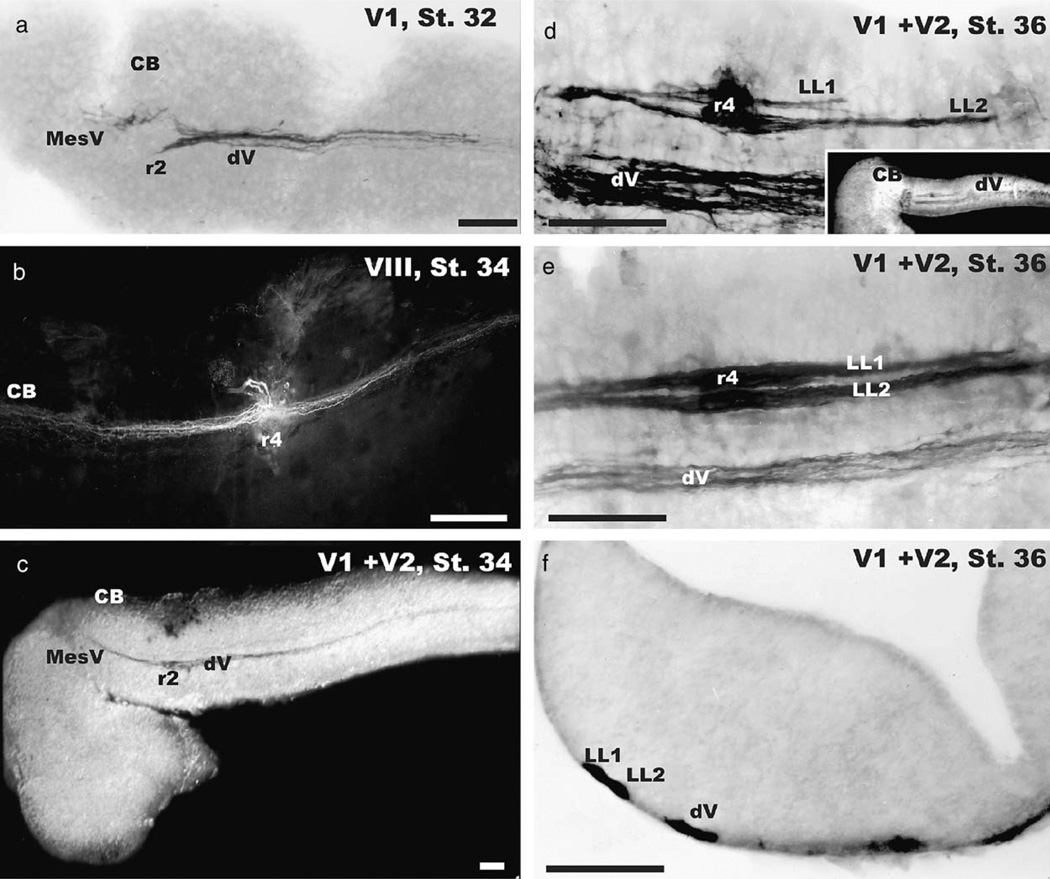
The first fibers that we could label formed a short fascicle that entered the medulla at rhombomere 2 and projected to approximately rhombomere 6 where fibers terminated in growth cones (Fig. 2a). Neither inner ear nor lateral line fibers could be labeled at this stage. We presume that these fibers belong to the profundus division of the trigeminus (abbreviated as V1) as this division is the first cranial nerve that matures (Northcutt and Brandle, 1995). The further development of the trigeminal projection in stage 33 coincides with observations of mechanosensory responses. Water droplets elicit full-body anterior to posterior curling responses in stage 33 axolotls but not in axolotls prior to this stage. This indicates the functionality of some sensory input as early as stage 33.
Fig. 2.
The progressive development of central afferent projections of cranial nerves in the axolotl is shown. At stage 32 (a) there is only labeling of the trigeminal portion of the profundal nerve (designated as V1) in the descending trigeminal tract (dV). Fibers enter at rhombomere 2 (r2) and extend to r6 where they end in growth cones. There is also some labeling of fibers of the mesencephalic root of the trigeminal nerve (MesV) in r1. At stage 34 the inner ear projection can be labeled (b). Fibers ascend and descend along the medulla for equal distances from their entry at r4 (b). Afferent trigeminal fibers (dV) labeled from both profundal (V1) and maxillary nerve (V2) fibers at this stage (c) extend from the entry at r2 further caudally and now have the MesV fibers extending toward the midbrain as well. In contrast, stage 36 larvae have additional dorsal fascicles (inset in d). These dorsal fascicles are the lateral line afferent fibers (LL1, LL2) which enter the medulla at rhombomere 4 (r4) and project for variable distances along the hindbrain (d, e). In smaller animals the fibers are shorter (compare d, e). The more dorsal lateral line afferent fascicle (LL1) tends to be shorter than the ventral fascicle (LL2). Coronal sections through rhombomere 4 (f) show the distinct segregation of the lateral line bundles from the descending trigeminal tract as well as a partial segregation of the two afferent bundles. V1, profundal nerve with associated superficial ophthalmic lateral line branch; V2, maxillary nerve with associated buccal lateral line branch. All bars indicate 100 µm.
The next major projection we could label was that of the inner ear. At stage 34 we labeled fibers that entered at rhombomere 4 and extended for considerable distances along the alar plate of the hindbrain (Fig. 2b), both toward the cerebellum and toward the spinal cord. The trigeminal projection had lengthened at this stage, reaching the spinal cord. At this stage we could also label the mesencephalic component of the trigeminal system that extended toward the trochlear nerve decussation, as previously described (Fritzsch and Sonntag, 1987). No changes other than continuous growth of fibers were found throughout stage 35.
Stage 36 Ambystoma mexicanum brains revealed two additional fascicles, dorsal to the trigeminal projections. These fibers entered in rhombomere 4 and formed two fascicles of different lengths (Figs. 2d, e). In smaller animals of this stage, the more dorsal projection was considerably shorter than the more ventral fascicle. In larger animals of this stage we found both fascicles to be much longer but still of different lengths, with the more dorsal fascicle being the shorter. Growth cones were present on the lateral line afferents but not on the trigeminal projection. The fasciculation of lateral line fibers into bundles was also obvious in the 200 mm coronal sections through rhombomere 4 (Fig. 2f).
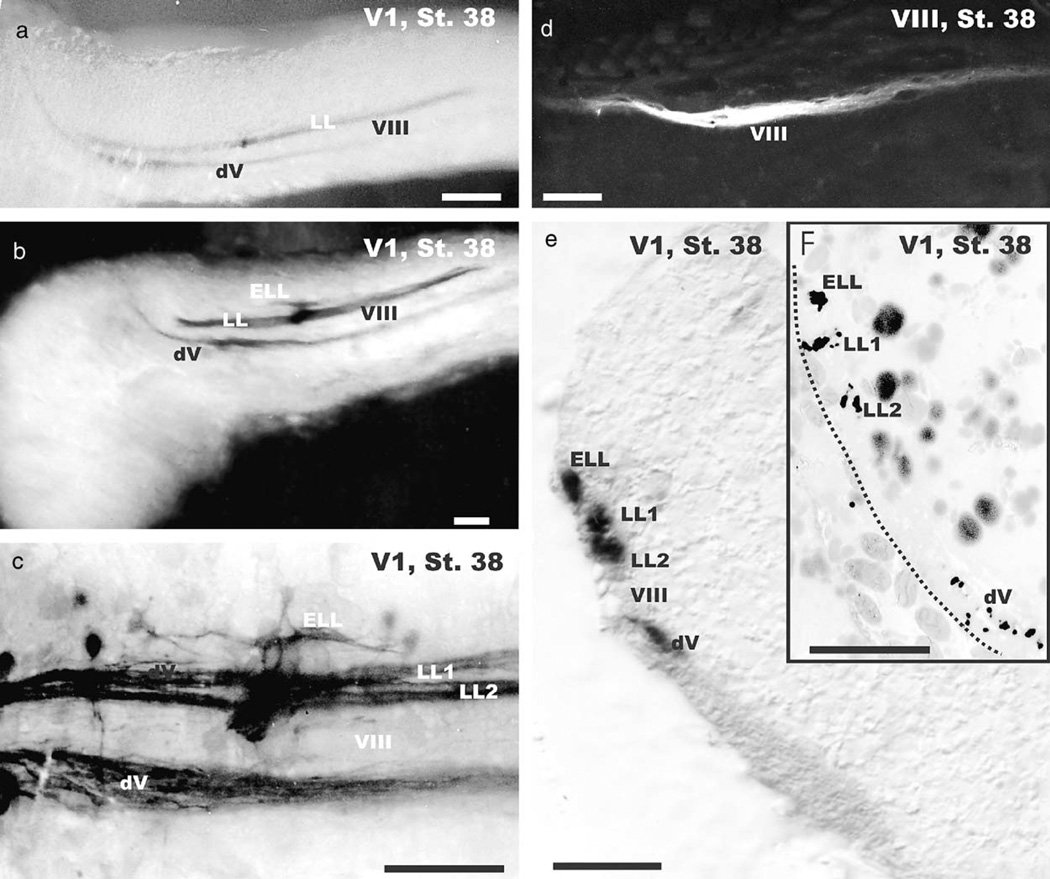
None of the stage 37 or younger embryos showed any response to a short electric pulse whereas older embryos and newly hatched larvae responded to such a stimulus with a lateral flexion that resulted in a head to tail curl. However, stage 38 embryos could be divided into two groups based on differences in preliminary electroreception behavior experiments. Animals that did not respond to such stimulation were considered early stage 38 axolotls whereas those that responded were considered late stage 38 axolotls. Early stage 38 larvae had a fully developed mechanosensory lateral line projection and trigeminal projections but showed no additional electroreceptive projections (Fig. 3a). In contrast, late stage 38 A. mexicanum showed not only the trigeminal and mechanosensory lateral line projection, but also had a few fibers labeled which were slightly more dorsal and parallel to the two bundles of mechanosensory lateral line fibers (Figs. 3b, c). Based on the adult organization of the lateral line projection (Fig. 1a) we interpret these fibers to be the central ampullary organ projection.
Fig. 3.
The appearance of ampullary organ afferents in the medulla is delayed until stage 38. Only animals of this stage or older show electroreceptive ampullary organ afferents (ELL) dorsal to the two mechanosensory lateral line fascicles (a–c). These fibers enter through a distinct dorsal root in rhombomere 4 and extend only some 100 µm along the hindbrain (c). The inner ear projection has extended further along the alar plate (d). Coronal sections (e, f) through rhombomere 4 show the organization of the four fascicles visible in the lateral view (c). Higher magnification shows that each of these fibers forms a dense fascicle in the lateral line electrosensory projection area (f) whereas the trigeminal tract shows more diffuse organization of fibers. Bars indicate 100 µm in (a–e), 50 µm in (f).
At this stage we could also label projections of individual inner ear sensory organs (Fig. 3d). Analysis of thick or thin cross sections at rhombomere 4 near the entry of the lateral line fibers showed the existence of at least three labeled fascicles; the most ventral one presented the descending trigeminal tract, followed by the VIII tract (not labeled) followed by the two mechanosensory afferent fascicles and the single electrosensory afferent fascicle.
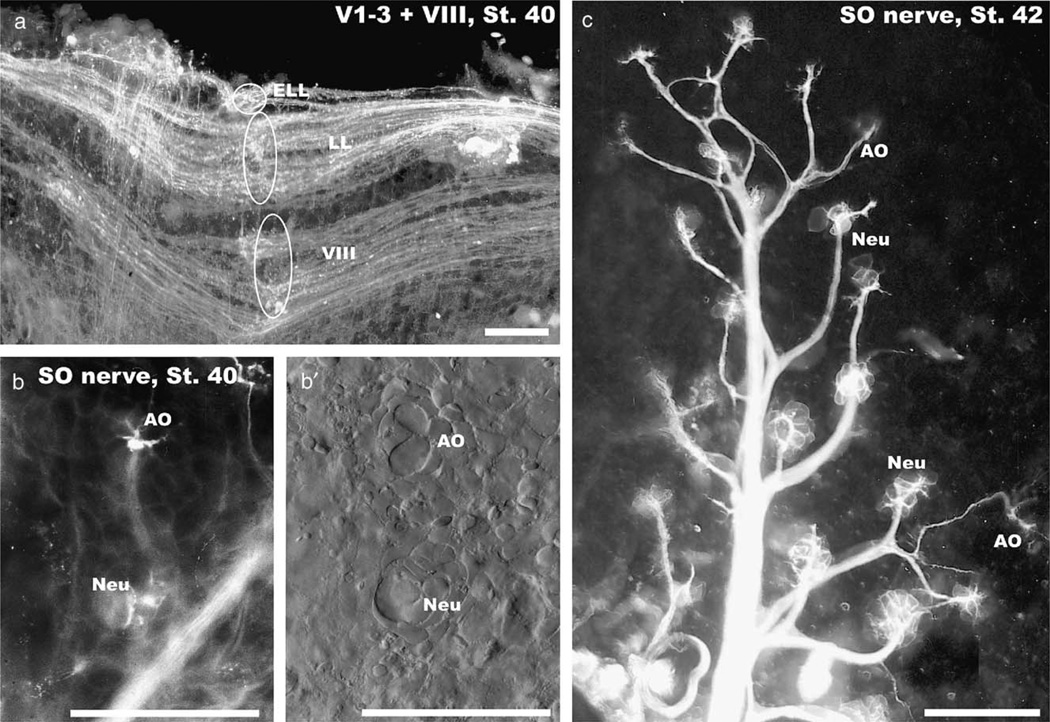
Stage 40 embryos showed a more adult-like pattern of lateral line and inner ear afferents (Fig. 4a). Filling of afferent fibers to the superficial ophthalmic ramus showed multiple afferents to the larger neuromasts that were closely spaced near the nerve. In contrast, ampullary organs received only a single nerve fiber that branched to reach all sensory cells in such an organ (Figs. 4b, c).
Fig. 4.
At stage 40 all octavolateral afferents extend in an adult pattern along the alar plate (a) and different organs can be identified in the skin (b, c). Separate roots for inner ear (VIII), lateral line (LL) and electroreceptive ampullary organ afferents (ELL) at rhombomere 4 are indicated (ovals and circle in a). Ampullary organs (AO) and neuromasts (Neu) show differences in the pattern of innervation (b, c) and the distribution of cells as revealed with differential interference microscopy (b′). Note that ampullary organs (AO) are always further away from the main trunk of the superficial ophthalmic nerve (SO) than the neuromasts (Neu). Bars indicate 100 µm in all images.
Discussion
Previous work has shown that neuromasts and ampullary organs of salamanders develop from single placodes in a sequential way, with the neuromasts forming before the ampullary organs (Fritzsch and Bolz, 1986; Northcutt et al., 1994, 1995). Subsequent work has revealed that each of these placodes gives rise to ganglia that reach the brain with their central axons around stage 34 (Northcutt and Brandle, 1995). However, owing to the limited resolution of the generalized nerve fiber stain employed in this study, details of termination of each of the different sensory systems have not yet been revealed. We show here that there is a simple progression of fiber invasion of the different sensory systems into the medulla that essentially follows the progression of placode formation and peripheral organ formation as previously described (Northcutt et al., 1994). However, while these easily delineable events seem to follow with a delay of about two stage differences (Fig. 5) there is no clarity as yet with respect to terminal mitosis and topographical origin of the various sensory ganglia or the target in the skin or the brain. Thus, no information exists about the delay between terminal mitosis of these neurons and the formation of the proximal and distal processes. Clonal analysis combined with experimental birthdating and fiber tracing is required to further analyze this issue.
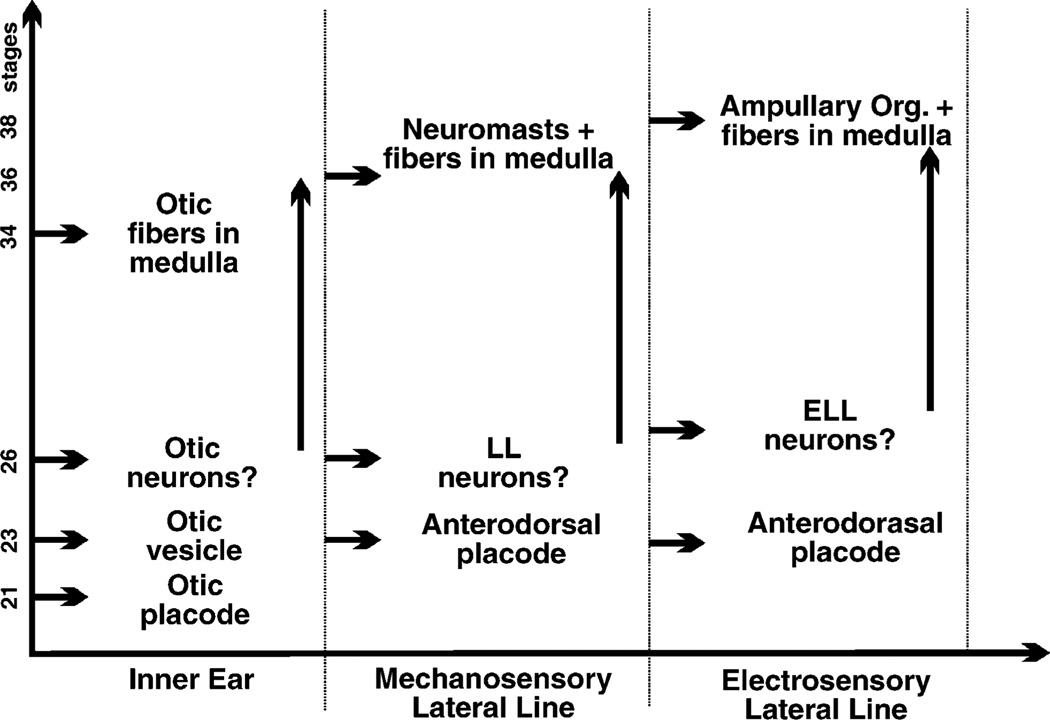
Fig. 5.
This comparison shows the sequence of progression in octavolateral organ and sensory neuron development in the axolotl. The first placode to appear is the otic placode (St. 21) and otic afferents are the first to enter the medulla (St. 34). Mechanosensory and electrosensory lateral line organs both derive from the anterodorsal placode; it has not yet been determined how much of a delay occurs in the induction of both organ types. Uncertainty exists also with respect to sensory neuron formation from these placodes. It is conceivable that neuronal proliferation will continue for a longer time than indicated here. Comparison of the first appearance of afferents in the medulla shows a delay of approximately two stages between these three sensory systems and a ventral to dorsal progression in first appearance. These data suggest that central projection formation transforms a temporal gradient of fiber ingrowth into a spatial gradient of fiber layering, much like what has been described for the mammalian cochleotopic projection. Data are compiled from Northcutt and Brandle (1995), Northcutt et al. (1994) and this study.
Most important for our initially posed question is that the development of the inner ear, neuromasts, and ampullary organs coincides in this order with the progression of development of their respective central projections (Fig. 5). As the different organs develop at different times, so do the central projections. The timed growth of fibers into the brainstem alar plate allows for an orderly innervation of organs and formation of their respective central projections apparently at around the same stage in development. We interpret these data to indicate that the respective ganglia form both the peripheral and central projections virtually simultaneously. It appears that differentiating ganglia innervate only specific organs and form precise central projections from the start. There is, however, still the above outlined uncertainty about the final mitosis of sensory neurons. The possibly multistage gap in terminal mitosis and onset of innervation could provide substantial time for these postmitotic neurons to interact with differentiating organs as the nerve fibers accompany the differentiating placode.
Spatially, the central projection of each octavolateral subsystem shows no overlap in the hindbrain from the earliest stages onward and they develop in a ventral to dorsal progression. Such ventral to dorsal progression has been noted in the developing cochlear nuclei and ingrowing cochlear nerve fibers (Fig. 1e) of mammals (Maklad and Fritzsch, 2003b; Rubel and Fritzsch, 2002) and may suggest a generalized way by which temporal segregation is used to generate spatially distinct projections. Transformation of temporal gradients into spatial projections is a feature that appears to be particularly important for the peripheral development of inner ear projections (Fritzsch et al., 2004) and experimental alterations of the timing will result in misguided fibers (Tessarollo et al., 2004). Timed delay of fiber growth to the target also plays a role in fiber sorting in the developing visual system of arthropods (Flaster and Macagno, 1984) and may thus be a widespread mechanism. For the axolotl octavolateral system it appears that the hypothesis of predetermination of sensory neurons is more likely than either the retrograde (Fritzsch, 1999) or anterograde (Gompel et al., 2001a; Northcutt et al., 1994) specifying hypotheses outlined above. Interestingly, ‘predetermination’ appears to be a factor of timing that provides order for the projections without necessitating specifying molecules. There could still be a role for the other two proposed mechanisms in the context of further refinement of an already well-organized initial projection pattern.
Of note is that the dorsal of the two fascicles related to a given neuromast’s central projection is lagging behind the formation of the ventral fascicle (Fig. 2). Previous work has shown that each neuromast of a salamander is innervated by only two afferents and that each afferent contributes to only one of the two central fascicles present for each peripheral nerve (Fritzsch, 1981, 1989). Each neuromast of amphibians has hair cells of opposing polarity (Fritzsch, 1981). Electrophysiological work has suggested that each group of hair cells polarized in a given direction is innervated by a single afferent. It has been hypothesized that each hair cell of a given polarity within a given line is presented in a single fascicle (Fritzsch, 1989). It seems possible that the neurons giving rise to the two afferents, and possibly also the two opposingly polarized hair cell populations, are separated by different birthdates. Closer examination using BrdU labeling combined with single neuromast afferent labeling may substantiate this idea.
In summary, our data reveal for the first time the progression of central projection in trigeminal, facial, inner ear and lateral line nerves in the axolotl. Our data are compatible with suggestions that specification of central and peripheral projections of a given sensory neuron is tightly regulated from the earliest differentiation onward, presumably by molecular means.
Acknowledgments
This research was supported by a grant from the Nebraska Behavioral Biology Group (DG) and a grant from NIH (RO1 DC005590; BF) and NASA (NAG 2-1611; BF). The senior author dedicates this paper to two of his mentors, Drs. M.H. Wake and R.G. Northcutt, both of whom were influential in his early career. BF wishes to express his gratitude to K. Schwenk for his invitation to contribute to this special volume that encouraged him to compile these data into a manuscript. Special thanks to Dr. A. Ghysen and two referees for helpful comments and suggestions on an earlier draft.
References
- Bordzilovskaya NP, Dettlaff TA, Duhon ST, Malacinski GM. Developmental-stage series of axolotl embryos. In: Armstrong JB, Malacinski GM, editors. Developmental Biology of the Axolotl. New York: Oxford University Press; 1989. pp. 201–219. [Google Scholar]
- Bullock TH, Heiligenberg W. Electroreception. New York: Wiley; 1986. [Google Scholar]
- Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J. Comp. Neurol. 1983;215:359–369. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- Flaster MS, Macagno ER. Cellular interactions and pattern formation in the visual system of the branchiopod crustacean, Daphnia magna. III. The relationship between cell birthdates and cell fates in the optic lamina. J. Neurosci. 1984;4:1486–1498. doi: 10.1523/JNEUROSCI.04-06-01486.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B. The pattern of lateral-line afferents in urodeles. A horseradish-peroxidase study. Cell Tissue Res. 1981;218:581–594. doi: 10.1007/BF00210117. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The amphibian octavo-lateralis system and its regressive and progressive evolution. Acta Biol. Hung. 1988a;39:305–322. [PubMed] [Google Scholar]
- Fritzsch B. The lateral-line and inner-ear afferents in larval and adult urodeles. Brain Behav. Evol. 1988b;31:325–348. doi: 10.1159/000116599. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Diversity and regression in the amphibian lateral line system. In: Coombs S, Gorner P, Munz H, editors. The Mechanosensory Lateral Line Neurobiology and Evolution. New York: Springer; 1989. pp. 99–115. [Google Scholar]
- Fritzsch B. The evolution of metamorphosis in amphibians. J. Neurobiol. 1990;21:1011–1021. doi: 10.1002/neu.480210707. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Fast axonal diffusion of 3000 molecular weight dextran amines. J. Neurosci. Meth. 1993;50:95–103. doi: 10.1016/0165-0270(93)90060-5. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Hearing in two worlds: theoretical and realistic adaptive changes of the aquatic and terrestrial ear for sound reception. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. New York: Springer; 1999. pp. 15–42. [Google Scholar]
- Fritzsch B. Development of inner ear afferent connections: forming primary neurons and connecting them to the developing sensory epithelia. Brain Res. Bull. 2003;60:423–433. doi: 10.1016/s0361-9230(03)00048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Bolz D. On the development of electroreceptive ampullary organs of Triturus alpestris (Amphibia: Urodela) Amphibia–Reptilia. 1986;7:1–9. [Google Scholar]
- Fritzsch B, Sonntag R. The trochlear nerve of amphibians and its relation to proprioceptive fibers: a qualitative and quantitative HRP study. Anat. Embryol. 1987;177:105–114. doi: 10.1007/BF00572534. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Wahnschaffe U. The electroreceptive ampullary organs of urodeles. Cell Tissue Res. 1983;229:483–503. doi: 10.1007/BF00207693. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Christensen MA, Nichols DH. Fiber pathways and positional changes in efferent perikarya of 2.5- to 7-day chick embryos as revealed with DiI and dextran amines. J. Neurobiol. 1993;24:1481–1499. doi: 10.1002/neu.480241104. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Barald K, Lomax M. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research Vol. XII: Development of the Auditory System. New York: Springer; 1998. pp. 80–145. [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53(2):143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog. Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Gilmour D, Knaut H, Maischein HM, Nusslein-Volhard C. Towing of sensory axons by their migrating target cells in vivo. Nat. Neurosci. 2004;7:491–492. doi: 10.1038/nn1235. [DOI] [PubMed] [Google Scholar]
- Glover JC. Retrograde and anterograde axonal tracing with fluorescent dextran-amines in the embryonic nervous system. Neurosci. Protocols. 1995;30:1–13. [Google Scholar]
- Gompel N, Cubedo N, Thisse C, Thisse B, Dambly Chaudiere C, Ghysen A. Pattern formation in the lateral line of zebrafish. Mech. Dev. 2001a;105:69–77. doi: 10.1016/s0925-4773(01)00382-3. [DOI] [PubMed] [Google Scholar]
- Gompel N, Dambly Chaudiere C, Ghysen A. Neuronal differences prefigure somatotopy in the zebrafish lateral line. Development. 2001b;128:387–393. doi: 10.1242/dev.128.3.387. [DOI] [PubMed] [Google Scholar]
- Larsell O. Anura. In: Jansen J, editor. The Comparative Anatomy and Histology of the Cerebellum from Myxinoids through Birds. Minneapolis: The University of Minnesota Press; 1967. pp. 163–178. [Google Scholar]
- Maklad A, Fritzsch B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res. Bull. 2003a;60:497–510. doi: 10.1016/s0361-9230(03)00054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Develop. Brain Res. 2003b;140:223–236. doi: 10.1016/s0165-3806(02)00609-0. [DOI] [PubMed] [Google Scholar]
- Metcalfe WK. Sensory neuron growth cones comigrate with posterior lateral line primordial cells in zebrafish. J. Comp. Neurol. 1985;238:218–224. doi: 10.1002/cne.902380208. [DOI] [PubMed] [Google Scholar]
- Neeser JA, von Bartheld CS. Comparative anatomy of the paratympanic organ (vitali organ) in the middle ear of birds and non-avian vertebrates: focus on alligators, parakeets and armadillos. Brain Behav. Evol. 2002;60:65–79. doi: 10.1159/000065206. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Central auditory pathways in anamniotic vertebrates. In: Popper AN, Fay RR, editors. Comparative Studies of Hearing in Vertebrates. New York: Springer; 1980. pp. 79–118. [Google Scholar]
- Northcutt RG. Evolution of gnathostome lateral line ontogenies. Brain Behav. Evol. 1997;50:25–37. doi: 10.1159/000113319. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Brandle K. Development of branchiomeric and lateral line nerves in the axolotl. J. Comp. Neurol. 1995;355:427–454. doi: 10.1002/cne.903550309. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Catania KC, Criley BB. Development of lateral line organs in the axolotl. J. Comp. Neurol. 1994;340:480–514. doi: 10.1002/cne.903400404. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Brandle K, Fritzsch B. Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Dev. Biol. 1995;168:358–373. doi: 10.1006/dbio.1995.1086. [DOI] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen CL, Choi Y, Ma Q. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu. Rev. Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–1697. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Development and evolution of lateral line placodes in amphibians I. Dev. Zool. 2002a;105:119–146. doi: 10.1078/0944-2006-00058. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Development and evolution of lateral line placodes in amphibians. II Evolutionary diversification. Zoology. 2002b;105:177–193. doi: 10.1078/0944-2006-00062. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J. Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS. Development and innervation of the paratympanic organ (Vitali organ) in chick embryos. Brain Behav. Evol. 1990;35:1–15. doi: 10.1159/000115851. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Patterson SL, Heuer JG, Wheeler EF, Bothwell M, Rubel EW. Expression of nerve growth factor (NGF) receptors in the developing inner ear of chick and rat. Development. 1991;113:455–470. doi: 10.1242/dev.113.2.455. [DOI] [PubMed] [Google Scholar]