Abstract
Purpose
We determined whether basic patterns of muscle activation for speech were similar in preschool children who stutter and their fluent peers.
Method
We recorded right and left lower lip muscle activity during conversational speech and sentence repetition in 64 preschool children (CWS) diagnosed as stuttering and in 40 children who do not stutter (CWNS). Measures of EMG amplitude, right/left asymmetry, and bilateral coordination were computed for fluent speech. The potential presence of tremor-like oscillations during disfluencies of CWS was assessed, and EMG amplitudes of fluent and disfluent speech were compared in CWS.
Results
Across both speaking tasks lip muscle activation was similar in CWS and CWNS in overall amplitude, bilateral synchrony, and degree of right/left asymmetry. EMG amplitude was reduced during disfluent compared to fluent conversational speech of CWS, and there was no evidence of tremor in the disfluencies of CWS.
Conclusion
These results support the assertion that stuttering in young children arises not from basic features of muscle contraction, but rather from the command signals that control the timing and amplitude of muscle activity. Our results indicate that no frank abnormality is present in muscle activation patterns in preschoolers who stutter.
In both children and adults who stutter, disfluencies occur when the muscles involved in speech production do not produce the precisely timed and coordinated levels of activation necessary for the fluent, forward flow of speech. Given this observation, it is not surprising that considerable experimental effort has focused on the amplitude, timing, and coordination of the speech musculature in individuals who stutter. However, most of this work has focused exclusively on adults who stutter (AWS) (Kleinow & Smith, 2000; Max, Caruso, & Gracco, 2003; McClean & Runyan, 2000; McClean, Tasko, & Runyan, 2004; Smith, Sadagopan, Walsh & Weber-Fox, 2010; Zimmermann, 1980).
There is a widespread clinical impression that AWS have high levels of muscle tension during stuttering; thus the hypothesis that excessive or high amplitude muscle activity occurs during disfluent speech has been investigated. Early studies of very few adult subjects supported this claim (Freeman & Ushijima,1978; Shapiro, 1980), however, subsequent studies with larger numbers of AWS did not find higher electromyographic (EMG) amplitudes in orofacial, jaw, laryngeal, and respiratory muscles during stuttering (Denny & Smith, 1992; McClean, Goldsmith, & Cerf, 1984; McLean & Cooper 1978; Smith, Denny, Shaffer, Kelly & Hirano, 1996). Disfluent speech was characterized by EMG amplitude that was equal to or smaller than amplitudes obtained from surrounding intervals of fluent speech. A preliminary study from our group, (Kelly, Smith, & Goffman, 1995), of 9 children who stutter (CWS) aged 3–14 years suggested that the CWS did not have higher levels of EMG activity in orofacial muscles compared to children who do not stutter (CWNS). One other account of EMG activity in 10 CWS aged 10–18 years revealed that older CWS and CWNS had comparable levels of lower lip muscle activity (de Felício, Freitas, Vitti, &Regalo, 2007).
Concerning the timing of muscle activity, it has been suggested that co-activation of antagonistic muscles is an abnormal pattern of activation characteristic of stuttering (Fibiger, 1971; Freeman &Ushijima, 1978; Shapiro, 1980). Many subsequent studies of speech production have revealed, however, that co-activation of antagonistic muscles is a normal pattern of activation for speech and therefore not an abnormal feature of stuttering (Moore, 1993; Moore, Smith, & Ringel, 1988; Smith, 1989). One study of AWS revealed slight differences in the timing of lip muscle activation during fluent speech (van Lieshout, Peters, Starkweather, &Hulstijn, 1993). This report is consistent with other findings showing differences in speech motor coordination for the fluent speech of AWS compared to adults who do not stutter (AWNS) (Kleinow & Smith, 2000; McClean & Runyan, 2000; McClean et al., 2004).
The only frankly abnormal aspect of muscle activation that has been observed in AWS is tremor-like oscillations in the EMG during stuttering. These are involuntary, repetitive short bursts of muscle activity in the 5- 15 Hz range, which have been observed in orofacial, jaw, laryngeal, and strap muscles of the neck. Within an individual who stutters, they tend to occur at a consistent frequency across multiple intervals containing disfluencies (Denny & Smith, 1992; Fibiger, 1971; McClean, Goldsmith, & Cerf, 1984; Smith et al., 1996; Smith et al., 1993). Generally, these investigations suggest that tremor-like oscillations are present in about one third of AWS and are more likely to occur during longer disfluencies and in individuals with moderate to severe stuttering. Our earlier study, (Kelly et al., 1995), examined the spectral properties of EMG for the presence of tremor during stuttering. We found that only the 3 oldest CWS in the group showed tremor during disfluencies.
Various EMG measures have been employed to describe fundamental patterns of muscle activation for speech production and other motor tasks. We elected to use indices of bilateral synchrony and relative amplitude of activation. Past research in AWS provides a clear rationale for using these parameters to characterize basic lip muscle activation patterns in CWS. We targeted lower lip activity, as the activity of these muscles may be reliably recorded using surface electrodes, and because we found tremor in lower lip muscles in our preliminary study (Kelly et al., 1995). Although our primary targets were right and left orbicularis oris inferior, a lip rounding muscle, it should be noted that even with intramuscular wire electrodes, it is not possible to isolate the activity of a single lip muscle as the fibers of different lip muscles are intermingled (Blair, 1986; Blair & Smith, 1986).
Right and left lip and jaw muscle pairs involved in speech production typically show bilateral synchrony in their activation patterns (Goffman & Smith, 1994; Moore et al., 1988; Wohlert & Goffman, 1994). The zero-lag cross-correlation coefficient computed between EMG envelopes recorded from muscle pairs has been used to assess the synchrony of muscle activation patterns both for typical adult speakers (Moore, 1993; Wohlert & Goffman, 1994) and as an indicator of developmental change in infants and toddlers (Moore, & Ruark, 1996; Ruark & Moore, 1997). To our knowledge, cross-correlational indices have not been examined in CWS; therefore, we assess whether bilateral coordination of muscle activity is in CWS is comparable to CWNS.
A potentially significant preliminary finding was reported in a recent EMG study of lip muscle activation for speech in five AWS and controls (Choo, Robb, Dalrymple-Alford, Huckabee, & O’Beirne, 2010). These investigators reported that lower lip muscle activation was asymmetric in both groups, with controls showing higher levels of right side activation and AWS showing the reverse, greater left activation. They interpreted this finding as evidence of reversed cerebral dominance for speech in AWS. This result is consistent with neuroimaging studies showing that AWS often show over activation of right hemisphere premotor and motor networks during fluent speech (Braun et al., 1997; De Nil, Kroll, Kapur, & Houle, 2000; Fox et al., 1996; Preibisch et al., 2003b). We assess the symmetry of right/left lip muscle activation as well as at the overall amplitude of each quadrant in both groups of preschool-aged children.
In addition to our measures of basic EMG activation patterns in CWS and CWNS, we wanted to characterize lip EMG activity during disfluent speech in CWS. In this case, we employ established analyses from earlier studies of disfluent speech in AWS, including measures of EMG amplitude during fluent and disfluent intervals and of the spectra of EMG amplitude envelopes during disfluencies (Denny & Smith, 1992; Fibiger, 1971; McClean et al., 1984; McLean & Cooper 1978; Smith et al., 1996; Smith et al., 1993). The spectral analysis can reveal the presence of tremor-like oscillations in the EMG signal.
Given that stuttering-like disfluencies are fundamentally a disturbance in the pattern of activation of muscles involved in speech production, it is critical to describe the patterns of muscle activation that characterize early stuttering. There has been little work devoted to EMG correlates of speech in CWS. The two EMG studies in CWS we cite did not examine the timing or symmetry of lip muscle activation and included relatively few numbers of CWS, most of who were older and had a chronic stuttering problem. The goal of the present, cross-sectional experiment is to determine if patterns of muscle activation for fluent speech differ between preschool CWS and CWNS, and to determine, if within the group of CWS, disfluent intervals of speech are characterized by an atypical pattern of muscle activity compared to their fluent speech. Given that speech motor control processes are much more variable in young children and that adult-like speech rates and inter-articulator coordination are not achieved until 16–18 years (Smith & Zelaznik, 2004; Walsh & Smith, 2002), studies of adults who stutter cannot serve as a basis to form conclusions about patterns of muscle activation in stuttering in childhood. Finally, the present findings will shed light on the neuromuscular bases of stuttering near its onset and will have important implications for the treatment of preschool CWS.
In summary, many investigations of EMG activity recorded during the fluent and disfluent speech in AWS have been completed. In general these studies suggest that in AWS, the amplitude of muscle activation is normal, but that the timing of activity may be disturbed, tremor may be present, and patterns of R/L asymmetry may be reversed. On the bases of earlier findings, we test the hypothesis that adult-like characteristics of EMG activity are present in preschool CWS. If this is the case, we expect to observe (1) similar amplitudes of lower lip muscle activation for speech between CWS and CWNS; (2) less bilateral synchrony of activity in CWS compared to CWNS; and (3) atypical R/L laterality indices in CWS.
Method
Participants
Sixty-four preschool CWS (47 males) and 40 preschool CWNS (28 males) participated in the study. The CWS ages ranged from 3;5 to 5;11 (M = 4;7 SD = 7 months); the age range for the CWNS was 3;6 to 5;10 (M = 4;7 SD = 7 months). Data from these children were collected as a part of a longitudinal research project on developmental stuttering from two sites: the Department of Speech, Language, and Hearing Sciences at Purdue University, and the Department of Communication Sciences and Disorders at the University of Iowa. All participants were native speakers of North American English and passed a pure-tone hearing screening at 20 dB at 500, 1000, 2000, 4000, and 6000 Hz. Participants had no history of neurological disorders and were not taking medications that might affect the experimental results (e.g., for depression, seizures, ADHD) per parent report. All participants scored within normative limits on assessments of nonverbal intelligence, Columbia Mental Maturity Scale (Burgemeister, Blum, & Lorge, 1972), and social development, Childhood Autism Rating Scale (Schopler, Reichler, & Renner, 1988). Additionally, the two groups of preschoolers had comparable socioeconomic status (SES) determined by their mothers’ level of education (Hollingshead, 1975). SES was evaluated by a 7-point scale (1 = less than 7th grade education to 7 = completion of a graduate or professional degree). The average score for each group was 6 (SD = 1).
Stuttering Diagnosis
Children were classified as CWS according to three criteria established by Ambrose and Yairi (1999) and Yairi and Ambrose (1999): (1) The child was diagnosed as stuttering by a certified speech-language pathologist (SLP) with extensive experience in fluency disorders by taking into account the type, duration, and frequency of disfluencies and presence/severity of secondary characteristics; (2) The child received a severity rating of 2 or higher on an 8-point rating scale (0 = normal to 7 = very severe) by either the parents and/or the SLP; (3) The child exhibited three or more stuttering-like disfluencies (SLDs) per 100 syllables of spontaneous speech. SLDs comprise part-word (sound or syllable) repetitions, single syllable word repetitions,1 and dysrhythmic phonations (i.e., sound distortions or prolongations, blocks, or broken words (Ambrose &Yairi, 1999). See Figures 1 and 2 for examples of part-word repetition and a sound prolongation, respectively from two CWS. The spontaneous speech samples were collected over two experimental sessions with the goal of eliciting a combined 750–1000 word sample. One speech sample was obtained during a parent-child play-based interaction; the other was collected during a play-based session with the SLP. For details regarding the EMG speech sample, see the section titled, “Conversational speech” below. Characteristics of the 64 CWS are provided in Table 1.
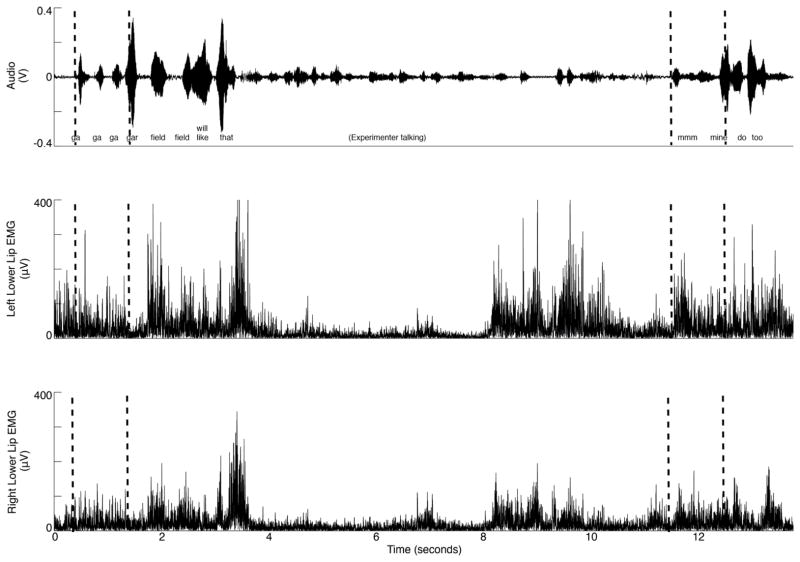
Figure 1.
Example of the typical pattern of LL EMG activity associated with part word repetitions from a CWS. The top trace is the audio signal, while the lower two traces show left and right LL EMG after rectification and smoothing, respectively. Vertical dashed lines through the audio trace indicate 1-sec segments extracted for analysis.
Figure 2.
Example of the typical pattern of LL EMG muscle activity associated with a distortion from a CWS. Vertical dashed lines through the audio trace indicate a 1-sec segment extracted for analysis.
Table 1.
General characteristics of the CWS
| Participant | Sex | Age (months) | Time Since Onset (months) | Reported Age of Onset (months) | Socioeconomic Status | Total SLDs (per 100 syllables) | Part Word Repetition (per 100 syllables) | Whole Word Repetition (per 100 syllables) | Dysrhythmic Phonations (per 100 syllables) | Clinician Severity Rating |
|---|---|---|---|---|---|---|---|---|---|---|
| CWS 1 | M | 48 | 18 | 30 | 5 | 2.86 | 1.20 | 1.37 | 0.29 | 3 |
| CWS 2 | M | 66 | 36 | 30 | 7 | 2.84 | 0.69 | 1.46 | 0.69 | 3 |
| CWS 3 | M | 67 | 37 | 30 | 4 | 7.83 | 3.52 | 3.73 | 0.58 | 5 |
| CWS 4 | F | 49 | 25 | 24 | 6 | 4.94 | 1.16 | 2.47 | 1.31 | 4 |
| CWS 5 | M | 49 | 13 | 36 | 6 | 6.63 | 2.85 | 1.77 | 2.01 | 3.5 |
| CWS 6 | M | 49 | 13 | 36 | 6 | 5.37 | 1.40 | 3.22 | 0.75 | 4 |
| CWS 7 | M | 48 | 12 | 36 | 7 | 4.77 | 1.47 | 2.20 | 1.10 | 4 |
| CWS 8 | M | 50 | 14 | 36 | 4 | 6.10 | 3.05 | 2.09 | 0.96 | 4.5 |
| CWS 9 | M | 55 | 13 | 42 | 6 | 5.06 | 1.25 | 2.89 | 0.92 | 2.5 |
| CWS 10 | M | 48 | 12 | 36 | 4 | 2.25 | 0.94 | 0.47 | 0.84 | 3 |
| CWS 11 | M | 48 | 24 | 24 | 6 | 6.29 | 2.57 | 1.33 | 2.39 | 4 |
| CWS 12 | M | 61 | 13 | 48 | 6 | 9.73 | 4.12 | 1.69 | 3.92 | 4.5 |
| CWS 13 | F | 49 | 7 | 42 | 4 | 14.46 | 5.04 | 1.12 | 8.30 | 5 |
| CWS 14 | M | 45 | 15 | 30 | 6 | 4.06 | 1.58 | 0.96 | 1.52 | 3 |
| CWS 15 | M | 58 | 22 | 36 | 4 | 11.76 | 4.40 | 2.58 | 4.78 | 5.5 |
| CWS 16 | F | 49 | 19 | 30 | 4 | 19.97 | 7.33 | 7.84 | 4.80 | 6 |
| CWS 17 | M | 50 | 8 | 42 | 5 | 4.32 | 1.73 | 1.62 | 0.97 | 4 |
| CWS 18 | M | 49 | 12 | 37 | 6 | 7.12 | 1.70 | 3.12 | 2.30 | 4 |
| CWS 19 | M | 54 | 30 | 24 | 6 | 4.18 | 1.85 | 1.81 | 0.52 | 3 |
| CWS 20 | M | 60 | 12 | 48 | 6 | 4.77 | 1.84 | 2.26 | 0.67 | 3 |
| CWS 21 | M | 57 | 21 | 36 | 6 | 1.68 | 0.93 | 0.75 | 0.00 | 2 |
| CWS 22 | M | 71 | 47 | 24 | 3 | 4.75 | 2.05 | 2.21 | 0.49 | 5 |
| CWS 23 | M | 57 | 33 | 24 | 6 | 6.76 | 2.88 | 2.94 | 0.94 | 4 |
| CWS 24 | F | 50 | 26 | 24 | 7 | 3.61 | 1.35 | 1.58 | 0.68 | 3.5 |
| CWS 25 | M | 53 | 29 | 24 | 5 | 3.40 | 1.22 | 1.70 | 0.48 | 3 |
| CWS 26 | F | 55 | 13 | 42 | 4 | 3.14 | 0.60 | 1.54 | 1.00 | 3.5 |
| CWS 27 | F | 54 | 18 | 36 | 5 | 3.83 | 0.62 | 1.79 | 1.42 | 4.5 |
| CWS 28 | M | 48 | 18 | 30 | 6 | 3.67 | 0.97 | 1.56 | 1.14 | 3 |
| CWS 29 | M | 70 | 28 | 42 | 5 | 2.6 | 1.07 | 0.99 | 0.54 | 3 |
| CWS 30 | M | 68 | 38 | 30 | 7 | 3.19 | 1.49 | 1.21 | 0.49 | 2.5 |
| CWS 31 | M | 56 | 32 | 24 | 7 | 10.74 | 4.44 | 4.39 | 1.91 | 5 |
| CWS 32 | M | 61 | 13 | 48 | 6 | 9.15 | 4.71 | 3.17 | 1.27 | 4.5 |
| CWS 33 | M | 54 | 18 | 36 | 7 | 2.64 | 1.57 | 0.99 | 0.08 | 3.5 |
| CWS 34 | F | 59 | 23 | 36 | 6 | 1.86 | 0.60 | 0.60 | 0.66 | 3 |
| CWS 35 | F | 52 | 28 | 24 | 6 | 4.00 | 1.52 | 1.60 | 0.88 | 4.5 |
| CWS 36 | F | 47 | 11 | 36 | 7 | 7.92 | 2.45 | 4.98 | 0.49 | 4 |
| CWS 37 | M | 60 | 24 | 36 | 7 | 14.77 | 1.14 | 2.27 | 11.36 | 6 |
| CWS 38 | M | 55 | 7 | 48 | 6 | 3.56 | 1.59 | 1.22 | 0.75 | 3.5 |
| CWS 39 | M | 68 | 26 | 42 | 5 | 14.16 | 9.07 | 3.50 | 1.59 | 5.5 |
| CWS 40 | F | 48 | 15 | 33 | 7 | 2.73 | 0.50 | 1.82 | 0.41 | 3 |
| CWS 41 | M | 57 | 11 | 46 | 6 | 7.05 | 1.80 | 3.90 | 1.35 | 3 |
| CWS 42 | M | 59 | 29 | 30 | 4 | 9.33 | 3.25 | 4.19 | 1.89 | 5 |
| CWS 43 | F | 58 | 12 | 46 | 7 | 1.95 | 0.51 | 0.93 | 0.51 | 2 |
| CWS 44 | F | 58 | 10 | 48 | 4 | 4.40 | 2.06 | 2.00 | 0.34 | N/A |
| CWS 45 | F | 49 | 13 | 36 | 6 | 5.70 | 1.69 | 2.11 | 1.90 | 2 |
| CWS 46 | M | 55 | 13 | 42 | 6 | 8.09 | 1.46 | 6.63 | 0.00 | 6 |
| CWS 47 | M | 41 | 5 | 36 | 7 | 3.36 | 0.67 | 2.15 | 0.54 | 2 |
| CWS 48 | F | 59 | 23 | 36 | 7 | 5.01 | 2.34 | 1.36 | 1.31 | 3 |
| CWS 49 | M | 55 | 33 | 22 | 6 | 2.47 | 0.63 | 1.39 | 0.45 | 2 |
| CWS 50 | M | 48 | 12 | 36 | 7 | 2.12 | 0.29 | 1.29 | 0.54 | 2 |
| CWS 51 | M | 48 | 24 | 24 | 6 | 10.34 | 4.74 | 4.63 | 0.97 | 5 |
| CWS 52 | M | 47 | 23 | 24 | 7 | 5.85 | 1.67 | 4.18 | 0.00 | 2 |
| CWS 53 | M | 68 | 32 | 36 | 5 | 12.73 | 2.38 | 8.71 | 1.64 | 7 |
| CWS 54 | M | 61 | 19 | 42 | 5 | 2.83 | 0.21 | 2.30 | 0.32 | 4 |
| CWS 55 | M | 60 | 6 | 54 | 6 | N/A | N/A | N/A | N/A | 6 |
| CWS 56 | M | 63 | 15 | 48 | 6 | 1.66 | 0.29 | 0.80 | 0.57 | 3 |
| CWS 57 | F | 58 | 22 | 36 | 4 | 4.37 | 0.95 | 3.29 | 0.13 | 3 |
| CWS 58 | M | 56 | N/A | N/A | 6 | 1 | 0.33 | 0.56 | 0.11 | 2 |
| CWS 59 | M | 57 | 27 | 30 | 5 | 4.54 | 3.14 | 1.05 | 0.35 | 2 |
| CWS 60 | M | 48 | 12 | 36 | 5 | 4.89 | 1.02 | 2.50 | 1.37 | 3 |
| CWS 61 | M | 60 | 6 | 54 | 6 | 7.21 | 3.49 | 2.25 | 1.47 | 2 |
| CWS 62 | F | 45 | 15 | 30 | 7 | N/A | N/A | N/A | N/A | 3 |
| CWS 63 | F | 52 | 16 | 36 | 6 | 2.07 | 0.81 | 0.90 | 0.36 | 2 |
| CWS 64 | M | 65 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 2 |
CWS 64 withdrew from the study before completing the full protocol; therefore his data were excluded from correlation analyses involving SLDs.
Data Collection and Experimental Tasks
EMG and audio recordings were collected during the clinician-child play session with a Biopac MP 150 (Goleta, CA) data acquisition system. After lightly cleaning the lower lip skin surface with an alcohol pad, five silver/silver chloride Ag/AgCl 10 mm pediatric prewired disposable “Kitty Cat” disc electrodes (Kendall) were affixed to the child’s skin surface with adhesive collars. A ground electrode was placed on the center of the forehead, and a pair of electrodes were placed horizontally just below the vermillion border of the LL between the facial midline and the right corner of the mouth. Another pair of electrodes was placed between the facial midline and the left corner of the mouth (Lapatki, Oostenveld, Van Dijk, Jonas, Zwarts, & Stegeman, 2010). The acoustic signal was recorded with a microphone positioned near the child that was synchronized to the EMG signal via the Biopac unit. The experimental sessions were videotaped to assist with transcription and classifying disfluencies.
For EMG data collection, 60-sec successive trials were collected as each child engaged in conversation with the SLP. Body movement was minimized during data collection by having the child seated at a small table while playing with age-appropriate toys (e.g., games, crayons). The SLP elicited longer speech samples by asking the child questions about his/her special interests (identified on the parent questionnaire). Data collection continued until the child produced 300–500 words or approximately half of the target sample (combined with parent-child interaction) of 750–1000 words. Most children produced the requisite number of words in 30 minutes or less. During this time, another experimenter monitored the EMG recording on a screen and noted instances of fluent and disfluent conversation segments within each 60-sec record. Next we recorded successive repetitions of the phrases, “Buy Bobby a puppy” and “Mommy bakes potpies.” These short sentences were included in the protocol so that EMG activation patterns could be compared across participants producing the same utterance. The SLP modeled each sentence for the child then cued him/her to produce it independently. Each time the child repeated the sentence, s/he earned a toy monkey to add to a chain until at least 10 productions were obtained.
After the experiment, each speech sample was transcribed and analyzed by research assistants trained by the SLP to code the type (typical or SLD) and frequency of disfluencies. Examples of typical disfluencies include whole phrase repetitions, fillers (e.g., uh, um), interjections, and revisions. For reliability, the SLP reanalyzed each transcript. On the occasion where agreement accuracy levels between the SLP and the research assistant were less than 85%, the SLP met with the assistant to review specific examples until a consensus was reached.
Data Analysis
EMG processing
During data collection, EMG signals were amplified, band-passed filtered at 10–500 Hz, and sampled at 5000 samples/sec. These files were then exported into MATLAB. Using a custom-written MATLAB script, the data were down sampled to yield a sampling rate of 1000 samples/sec per channel. EMG signals were then high-pass filtered at 20 Hz and low-passed at 300 Hz, notch filtered at 60 Hz to remove line noise, demeaned, and full-wave rectified.
Conversational speech
A separate MATLAB script allowed the experimenter to view any portion of a 60 sec record of right and left LL EMG and acoustic signals and to play back the audio from selected portions. The transcripts created from the clinician-child interactions along with online judgments of fluency were used to guide the location of fluent and disfluent conversation segments. The experimenter carefully reviewed each of the 60-sec trials containing disfluencies (in case of CWS) and extracted up to 20 1-sec disfluent EMG segments from the record with a cursor. An algorithm determined each 1-sec segment from where the experimenter initially indicated with a cursor. Video was used in instances of uncertainty; for example, to ascertain whether the child was in a silent block versus pausing. The 1-sec disfluent segments could be part of a longer, or contain a shorter (< 1 sec) SLD. If the child produced more than the requisite 20 disfluencies, we attempted to take an approximately equal number of samples from the beginning, middle and end of the session. Twenty 1-sec fluent conversational EMG segments were also extracted for all subjects using the same algorithm. As with the disfluent samples, attempts were made to extract approximately equal numbers of fluent segments from the beginning, middle and end of the session. Fluent segments from CWS were not selected if they were immediately adjacent (within .5 sec) to disfluent segments (Smith et al., 1993). The 1-sec fluent/disfluent conversation segments were then smoothed with a 61 pt. window to produce an EMG envelope for each extracted sample. See Figures 1 and 2 for examples of EMG and acoustic records for SLDs in the context of conversational speech.
Sentence repetition
Up to ten fluent productions of “Buy Bobby a puppy” and ten fluent productions of “Mommy bakes potpies” were extracted using the acoustic signal for each participant (Figure 3). A sentence was judged to be error-free when it did not contain substitutions, omissions, additions, any disfluency, aberrant prosody, or inappropriate pauses. The extracted sentence files were also smoothed with a 61 pt. window to produce the EMG envelope for each sentence repetition.
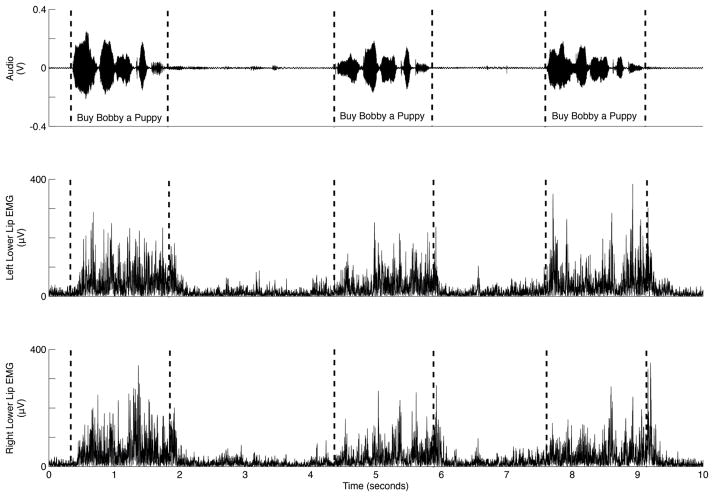
Figure 3.
Example of a typical LL EMG pattern observed for sentence production from a CWNS. Vertical dashed lines through the audio trace indicate how sentences in the record were segmented for analysis.
EMG amplitude
We calculated the area of the left and right smoothed LL EMG using trapezoidal Riemann sums to estimate the integral for each 1-sec conversation record, and then averaged across the total number of fluent and disfluent records for each child. Similarly, the area of the left and right LL EMG signals was calculated for each sentence repetition, then the average across all productions of “Buy Bobby a puppy” and “Mommy bakes potpies” was determined for each child. We use values of the integrated amplitude of the EMG envelope for all analyses. These amplitude values are highly variable across participants. In addition to the activity of muscles, the signal reflects the relative impedance at the electrode/skin interface and other variables such as the thickness of the tissue between the electrode and the active muscle fibers (for recent review, see Stepp, 2012). A common approach to minimize these potential sources of variability is to normalize EMG amplitude for the experimental tasks to a criterion value (e.g., express task EMG as a percent of the criterion EMG amplitude for a maximal gesture, such as lip pursing—see Smith, McFarland, Weber, & Moore, 1987; and Weber & Smith, 1987). We elected not to use this approach in the current study, however. We speculated that preschoolers’ “interpretation” and performance of such a criterion gesture would be unreliable. We believe it is reasonable to make across-subject and between-group comparisons using non-normalized EMG values, because we have no reason to believe that, for example, impedance values would be on average higher in one or the other group. Potential problems in amplitude comparisons are also mitigated by the large number of preschoolers in each experimental group.
Laterality index
Using the average right and left LL EMG amplitude measures for the set of 1-sec speech segments, we computed a separate index of laterality, (R−L)/(R+L)*100, for each participant for the fluent conversation and sentence production segments.
Bilateral synchrony
To provide an index of bilateral synchrony, we computed pairwise zero-lag cross-correlation coefficients for the two (left and right) EMG recording sites across each 1-sec conversational speech interval and sentence repetition for each participant. These were the same samples utilized for the EMG amplitude calculations. The correlation coefficient for each production was squared and then an average was calculated across all samples for a particular condition (i.e., fluent or disfluent sample, “Mommy” or “Bobby” sentence) for each participant.
Spectral analysis
After weighting with a hamming window, spectra of the EMG amplitude envelopes were computed using a fast Fourier transform (FFT). The total spectral energy from 1–35 Hz and the spectral energy associated with tremor (5–15 Hz) were determined. We elected to include energy out to 35 Hz only, as this region comprises most of the energy in the EMG signal after it has been rectified and smoothed (Figure 4). Finally, we divided the spectral energy in the 5–15 Hz band by the total spectral energy to determine what percentage of the total spectral energy occurred within the 5–15 Hz region that is associated with tremor in AWS (Denny & Smith, 1992; Fibiger, 1971; McClean et al., 1984; Smith et al., 1996; Smith et al., 1993). The 5–15 Hz region is marked by vertical lines in Figure 4.
Figure 4.
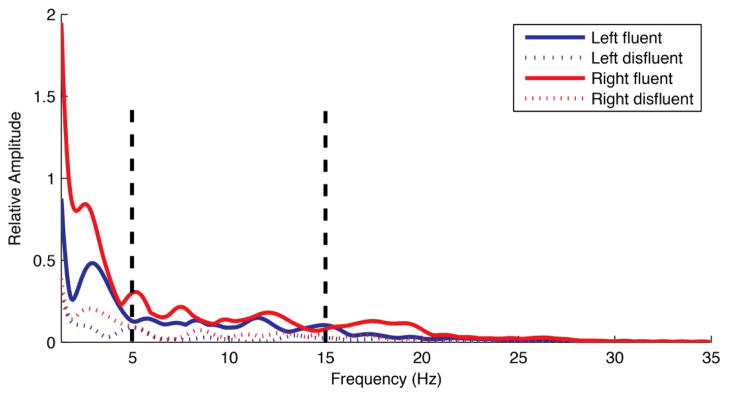
Spectra computed from the left and right EMG amplitude envelopes during fluent and disfluent conversational speech from a CWS. These spectra represent the average data across all CWS. Vertical lines in this figure mark the 5–15 Hz band associated with tremor.
Statistical Analysis
Table 2 provides the number of sentence repetitions and disfluent and fluent 1-sec speech samples produced by the preschoolers in each group. Data from participants who produced six or fewer fluent or disfluent conversational speech samples or sentence productions was omitted from the statistical analysis.
Table 2.
Number of speech samples and sentence repetitions produced by the participants
| Conversational Speech | Sentence Production Task | |||||||
|---|---|---|---|---|---|---|---|---|
| 1-sec Fluent Samples | < 6 | 7–13 | 14–19 | 20 | Bobby Tokens | < 6 | 7–9 | 10 |
| CWS n = 64 | 64 | CWS n = 64 | 11 | 7 | 46 | |||
| CWNS n = 40 | 1* | 1 | 38 | CWNS n = 40 | 5 | 5 | 30 | |
| 1-sec Disfluent Samples | Mommy Tokens | |||||||
| CWS n = 64 | 8 | 13 | 7 | 36 | CWS n = 64 | 16 | 6 | 42 |
| CWNS n = 40 | 5 | 5 | 30 | |||||
Data from this subject was not available due to technical problems.
For the conversational speech task, a potential group difference (CWS vs. CWNS) in EMG amplitude was assessed with an ANOVA with repeated measures on side (2: right, left). A one-way ANOVA was calculated to assess a between-group difference in the squared cross-correlation coefficients between left/right LL EMG amplitude envelopes. For the sentence production task, separate ANOVAs with repeated measures on side (2) and sentence type (2: Bobby, Mommy) for EMG amplitude and sentence (2) for the squared cross-correlation coefficients were calculated. Similarly, between-group differences in laterality index values were assessed with a one-way ANOVA or, in the case of the sentence task, a repeated measures ANOVA. Finally, within group differences in EMG amplitude, tremor, and cross-correlation coefficients in the CWS were examined with separate ANOVAs with repeated measure on fluency (2 fluent, disfluent) and side (2). For all ANOVAs, alpha level was set at p < .05.
We performed correlational analyses to assess the potential relationships between (1) laterality index values and age (in months); (2) between type of SLD and EMG amplitude and presence of tremor; and (3) between severity (stuttering frequency) and EMG amplitude, tremor, and laterality during conversational speech. Scatterplots of these correlations were examined to ensure homoscedasticity and an approximately linear relationship of the data before conducting Pearson’s r correlations (p < .01).
Results
CWS and CWNS: Between Group Comparisons
Initial ANOVAs showed no significant differences between the preschool boys and girls in either subject group with respect to any EMG dependent variable. Therefore data were pooled across sex in each group for all subsequent analyses.
Conversational speech
We compared LL EMG activity during fluent conversational speech between the two groups of participants. There was no significant difference in EMG amplitude between the two groups of preschoolers F(1,101) < 1. Table 3 provides means and standard deviations for measured variables. There was, however, an effect of side. Left LL EMG was significantly larger than right LL EMG for both groups of preschoolers during conversational speech F(1,101) = 8.08, p = 0.005. The interaction between side and group was not significant. An ANOVA did not reveal a between-group difference in the squared cross-correlation coefficients between left/right LL EMG amplitude envelopes for fluent conversational speech F(1,101) < 1.
Table 3.
Means and (standard deviations) for variables of interest by group and speech task
| Left EMG Amp (μV/s) | Right EMG Amp (μV/s) | Lat Index (R−L)/(R+L)*100 | L-R Corr (r)* | |
|---|---|---|---|---|
| Fluent Speech | ||||
| CWS n = 64 | 42.5 (20.5) | 38.6 (22.1) | −6.0 (19.4) | 0.71 (0.12) |
| CWNS n = 39 | 49.6 (25.1) | 45.2 (23.6) | −5.7 (20.8) | 0.70 (0.09) |
| “Buy Bobby a puppy” | ||||
| CWS n = 53 | 50.6 (21.1) | 46.2 (24.9) | −7.8 (18.7) | 0.69 (0.14) |
| CWNS n=35 | 54.9 (28.3) | 50.9 (28.1) | −5.6 (18.9) | 0.71 (0.11) |
| “Mommy bakes potpies” | ||||
| CWS n = 48 | 52.2 (23.0) | 47.2 (25.2) | −7.3 (17.36) | 0.71 (0.14) |
| CWNS n = 35 | 54.5 (27.0) | 50.2 (25.7) | −5.6 (17.8) | 0.72 (0.10) |
Squared cross-correlation coefficients were used in statistical analyses
Sentence production task
Similarly, there was no significant between-group difference in LL EMG amplitude F(1,75) < 1 for sentence production. The effect of sentence was significant F(1,75) = 87.64, p < 0.001; “Mommy bakes potpies” elicited greater LL EMG amplitudes compared to “Buy Bobby a puppy” in both groups of preschoolers (the interaction between sentence and group was not significant). The effect of side was also significant F(1,75) = 7.55, p = 0.008. As in the conversational speech analysis, left LL EMG was significantly larger than right LL EMG in both groups. The side x group and sentence x side x group interactions were not significant. Analysis of the squared cross-correlation coefficients between left/right LL amplitude envelopes did not reveal a significant between-group difference F(1,75) < 1. Differences between the two types of sentences or in the interaction between sentence and group were not significant. The means and standard deviations for these variables are shown in Table 3.
Laterality index
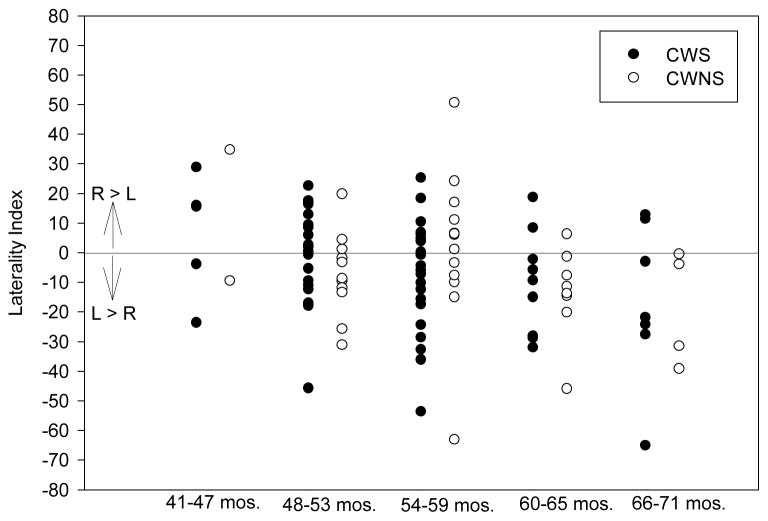
The group means and standard deviations for the laterality index are also listed in Table 3. The higher the positive index, the greater the degree of right LL EMG amplitude; a negative index denotes larger EMG amplitude in left LL. There was no significant difference in degree of laterality between the groups for the conversation F(1,101) < 1, or sentence production F(1,75) = <1. We noted a high degree of individual variation in the laterality indices; Figure 5 contains a plot of individual indices for conversational speech for each subject as a function of age. Pearson’s r correlations between age and laterality indices were obtained for fluent speech and the two sentences. Modest correlations were found between age and laterality index for fluent conversation, (r = −0.28) and the sentence, “Buy Bobby a puppy” (r = −0.25), but narrowly missed significance for the sentence, “Mommy bakes potpies” “ (r = −0.20) at p < .01 after Bonferroni correction. These negative correlations suggest that, to some degree, older children had higher frequency of L>R laterality indices.
Figure 5.
Laterality index scores, computed from left and right mean EMG amplitude values for fluent conversation, are plotted as a function of age group for the CWS and CWNS. The age groups, on the x-axis, were organized into 6 month intervals beginning with the youngest child participant and ending with the oldest. Higher positive index values indicate greater right asymmetry, while negative values indicate left asymmetry.
CWS: Fluent and Disfluent Speech
Types of stuttering-like disfluencies
Table 1 provides a breakdown of the types of SLDs produced by each CWS per 100 syllables of conversational speech. Single syllable word repetitions (SS) repetitions (45%) and part word repetitions (PW) (34%) were the most frequent type of SLD manifested in CWS. Dysrhythmic phonations (DP) accounted for approximately 21% of the total number of SLDs. To determine if one particular type of SLD was associated with a specific pattern of EMG activation (i.e. greater amplitude of LL muscle activity or presence of tremor), Pearson’s r correlations were computed to summarize the strength of the relationship between EMG amplitude and spectral characteristics, and frequency scores for the three categories of SLDs (SS, PW, DP). No correlation reached statistical significance at p< 0.01; (all r between −.19 and .01). Moreover, earlier EMG reports in AWS have not shown one type of SLD to be associated with appreciably different EMG activity in terms of tremor oscillations or amplitude of activation (Denny & Smith, 1992; McClean et al., 1984; Smith et al., 1996). Therefore, disfluent segments from each CWS were combined for all subsequent analyses of disfluent speech.
Another issue that arises relating to combining all SLDs from one participant for analysis is whether EMG patterns for SLDs would systematically differ depending on the phonetic construct of the target word. For example, intuitively “ma ma mommy” would show a different lip EMG pattern compared to “ca ca cake.” In continuous speech, however, EMG activation patterns do not map onto single phonetic segments, rather present results and previous results for lip, jaw, and laryngeal muscles (e.g., Moore et al., 1988; Smith et al., 1996) suggest that EMG activity is continuous and graded, both prior to and during speech. The EMG records in our figures illustrate this graded activation.2
Conversational speech
For the CWS, there was a significant difference between the fluent and disfluent conversation samples in the overall amplitude of EMG activity F(1,54) = 61.65, p< 0.001. Disfluent conversational segments (M =28.5 μV/s, SD = 15.8 μV/s), were associated with reduced activity compared to fluent segments (M= 38.7 μV/s, SD = 21.8 μV/s). There was also an effect of side F(1,54) = 4.88, p = 0.03. Left LL (M = 35.3 μV/s, SD = 18.5 μV/s) showed greater activation compared to right LL (M = 31.9 μV/s, SD = 19.1 μV/s) for fluent and disfluent speech combined. The interaction between side and speech fluency was not significant.
We found no evidence of tremor (seen as higher energy in the 5–15 Hz band in disfluent compared to fluent segments) during moments of stuttering in the CWS; F(1,54) < 1. Disfluent speech (M = 24%, SD = 9%), had the same percentage of spectral energy within the 5–15 Hz band as fluent speech (M = 24%, SD = 12%). In addition visual inspection of all disfluent speech samples suggested no evidence of the presence of tremor-like oscillations, which, in adults who stutter are often clearly visible in the unprocessed EMG recordings (see Figure 3A, Smith et al., 1993). Finally, there was no difference in the synchrony of right and left LL muscle activity as indexed by zero-lag squared cross-correlations between left/right LL EMG amplitude envelopes for disfluent and fluent speech, F(1,54) < 1.
Severity correlations
Lastly, we computed correlations between our measure of stuttering frequency (SLDs/100 syllables) and measures of left and right LL EMG amplitude, laterality, and tremor during disfluent conversational speech. None of the correlations reached statistical significance at p< 0.01; r values fell between −.07 and .19.
Discussion
We assessed fundamental properties of perioral muscle activation in a large group of preschool CWS and in a matched group of CWNS. Our results clearly demonstrate that basic patterns of lip muscle activation are similar between groups of four- and five-year-old CWS and CWNS in terms of overall amplitude, bilateral synchrony, and degree of right/left amplitude asymmetry. These EMG characteristics were consistent across two speaking tasks: fluent conversational speech and repetitions of two short sentences that included many bilabial sounds. We found that, on average, EMG amplitude was reduced during disfluent compared to fluent conversational speech intervals in the CWS. Finally, we found no indication that the EMG activity of LL muscles in CWS was entrained in tremor-like oscillations during their disfluencies.
Basic Muscle Activation Patterns: CWS vs. CWNS
Bilateral synchrony
The surface electrode pairs we placed on the right and left LL would be expected to record the combined activity of orbicularis oris inferior and activity of other perioral muscles whose fibers are found in this region (Blair & Smith 1986; Zemlin, 1998). Earlier investigations in which these electrode placements were used to detect LL muscle activity demonstrated robust muscle activation for speech with bursts of activity associated with lip rounding gestures (Goffman & Smith 1994; Ruark & Moore, 1997; Wohlert & Goffman 1994). We also observed robust activity bilaterally in the LL EMGs of the preschool children.
One question we wanted to address was whether early stuttering is associated with a disturbance in bilateral coordination of oral muscles. An earlier study from our laboratory, Goffman & Smith (1994), employed spectral coherence analysis to determine if the muscle fibers belonging to a single motor unit were found in a single, ipsilateral quadrant of the LL, or if motorneuron axons cross midline to innervate contralateral perioral muscle fibers. Results from this study indicate that the muscle fibers belonging to single motor units are ipsilaterally distributed. Therefore our right and left EMG recordings from the children’s lower lips sampled the activity of distinct groups of motor units. We also noted in Goffman and Smith (1994) that cross-talk (evidenced by high levels of broad band coherence in the lower frequency range) from contralateral LL recordings only arose at very high amplitudes of lip rounding activity. Given that speech does not involve high levels of perioral muscle activity, cross-talk between right and left recordings should not have been a confounding factor in the present experiment.
Given these observations, we can conclude that for right and left LL muscles to produce bilaterally synchronous activity for speech, the motor commands driving the right and left perioral motorneuron pools must be coordinated to produce the required amplitude and timing of lip muscle activity. The zero-lag cross-correlations for left and right LL we observed for the children’s conversational speech and sentence productions typically ranged from 0.5 to 0.9. This range is similar to those reported in earlier studies of the activation of synergistic jaw muscles during speech in adults (Moore et al., 1988; Moore, 1993) and of right upper and LL muscles in two-year-olds during speech (Ruark & Moore, 1997). Thus our preschoolers show bilaterally coordinated activity. We did not see a difference in the bilateral synchrony of LL muscle activation between our subject groups. This finding provides evidence that the nervous system in both CWS and CWNS is delivering similar command signals to the right and left sets of motorneuron pools that innervate muscle fibers in the right and left LL quadrants.
Right/left asymmetries in EMG amplitude
Given the findings of Choo et al. (2010) concerning a reversal of presumably normal, right dominant LL EMG asymmetry during speech in a small group of AWS, we compared the amplitudes of right and left EMG recordings and computed a laterality index for each participant for both speech tasks. No significant differences were observed between groups on the laterality index; both groups of children, on average, had significantly higher left LL EMG amplitudes. It should be noted that the individual L/R laterality indices were variable, with some children showing higher amplitude of activation of right LL quadrant (see Figure 5). There was a modest trend for more of the older children in our sample to have the L>R amplitude asymmetry, so this may reflect a developmental trend.
We are frankly unsure how to interpret the finding of higher left LL activation on average, nor the trend for higher left EMG amplitude laterality indices with increasing age in both groups. Choo et al. (2010) interpreted their results to indicate AWS have less left cerebral dominance for speech, as they activated left LL more than right, while the normally fluent controls showed the reverse pattern R>L. We suggest that this interpretation is overly simplistic in light of recent evidence from transcranial magnetic stimulation studies. Stimulation of primary motor facial areas in each hemisphere typically produces contralateral and ipsilateral activation of LL muscles in human subjects (Fischer, Hess, Rössler, 2005; Meyer, Werhahn, Rothwell, Roericht, & Fauth, 1994; Triggs, Ghacibeh, Springer, & Bowers, 2005; Urban, Beer, & Hopf, 1997). Triggs et al. (2005) reported considerable variability among the large number of adults they tested, but there was a trend for left LL muscle activity to be evoked at lower thresholds from both right and left primary motor areas. Related to developmental changes, understanding the trend for emerging L>R LL muscle activation for speech in young children must await further study, as we know of no extant studies of this phenomenon. Based on our findings it seems reasonable to conclude that an atypical laterality index for LL muscle activation for speech is not a candidate for a marker of early stuttering.
Children Who Stutter: Fluent vs. Disfluent Speech
From our study of 64 preschool CWS, we draw the conclusion that disfluencies are not the result of excessive perioral muscle activity. In fact, disfluent speech in the CWS was associated with reduced lip muscle activation compared to fluent speech. This finding seems counterintuitive given the often stated clinical impression that individuals who stutter manifest excessive orofacial tension especially during moments of disfluency. The perception of orofacial tension (or muscle tension in general) does not necessarily arise from excessive muscle activation. For example, no relationship between the perception of high levels of muscle tension and increased EMG activity in facial, neck, and shoulder muscles was found in people who suffer from headaches and myalgia (Bansevicius, Westgaard, & Jensen, 1997; Nilsen, Westgaard, Stovner, Helde, Rø, & Sand, 2006). Of course, we only recorded activity of LL muscles, and it could be the case that other muscle systems involved in speech, for example, respiratory and laryngeal, would show excessive activation, but there was no visible or audible evidence that this was the case. In addition, studies of AWS from our laboratory and others have not revealed excessive muscle activation in articulatory, laryngeal, or respiratory muscles during disfluencies (Denny & Smith, 1992; McClean et al., 1984; McLean & Cooper 1978; Smith et al., 1996). Given results of past studies and the present findings of fundamentally normal perioral muscle activation patterns in preschool CWS, we would conclude that disfluencies in young CWS and AWS arise as a result of an interruption of well-timed and coordinated command signals to the muscles involved in speech, rather than some properties inherent to the motorneurons (e.g., high gain motorneuron pools; Zimmermann 1980c; Smith & Luschei, 1983). Previous studies suggest that this involuntary interruption in command signals can be wide spread in adults who stutter, affecting articulatory, laryngeal, and respiratory systems (Denny & Smith, 1992; McClean et al., 1984; McLean & Cooper 1978; Smith et al., 1996; Zocchi, et al., 1990). Starkweather (1995) proposed “a simple theory of stuttering,” with the fundamental premise that “the proximal cause of stuttering is the elevated activity in the muscles involved in speech production” (p. 91). Based on the present results, we would argue the proximal cause of stuttering in preschoolers is not elevated muscle activation. Furthermore, there was no correlation observed between EMG amplitude during speech and severity of stuttering as measured by stuttering-like disfluencies per 100 syllables, also consistent with the idea that heightened muscle activation is not “the proximal cause of stuttering.”
What causes disruptions to command signals driving speech musculature that result in perceptible disfluencies? We have proposed that stuttering is dynamic and multifactorial in nature (Smith & Kelly, 1997). We hypothesize that these interruptions in motor commands are caused by the dynamic interplay of many neural networks involved in generating speech. Young children are developing the complex, interactive neural networks involved in language production—those involved in language processing, emotions, speech motor planning and production. CWNS have many normal disfluencies when these systems cannot operate quickly and accurately enough to meet the communication demands of the situation. The preschool child diagnosed as stuttering experiences qualitatively and quantitatively different breakdowns in fluency categorized as SLDs (Ambrose and Yairi, 1999). On the basis of the findings of the Illinois Stuttering Project, we expect that approximately half of the present sample of CWS will recover given that they were recruited at age four and five years (note that recovery rates are much higher, estimated at 65–80%, if children are ascertained closer to the onset of stuttering; Yairi & Seery, 2011). The present findings suggest that speech muscle systems of preschoolers who are stuttering are capable of age-appropriate, normal activation patterns in terms of amplitude and timing. The fact that approximately half of the present sample of 64 CWS will recover points to another significant issue. Our present analyses are based on means of the stuttering and non-stuttering groups, albeit the stuttering group actually comprises two groups: those children who will recover and those who will have a chronic stuttering problem. The question arises as to whether these two subgroups of CWS, persistent and recovered, would be distinguishable on some of the EMG measures we used. We cannot answer this question at this point in time, but at the end of our longitudinal investigation, we will be able to examine differences between recovered and persistent groups. Therefore it is important to note that although CWS/CWNS differences were not detected in the present cross-sectional analysis, the potential predictive value of EMG measures with regard to persistence or recovery has not yet been determined. For example, we can test the hypothesis that CWS with a higher left/right EMG laterality index are more likely to recover.
From the therapeutic view point, the present results contain another encouraging finding. There was no evidence among our large sample of preschoolers who stutter that tremor-like oscillations of muscle activity were already present during disfluencies. As noted in the introduction, tremor is the only frankly aberrant sign of muscle activation observed during disfluencies in EMGs in older CWS and AWS (Denny & Smith, 1992; Fibiger, 1971; Kelly et al., 1995; McClean et al., 1984; McLean & Cooper 1978; Smith et al., 1996; Smith et al., 1993). Tremor should not be confused with secondary characteristics of stuttering (e.g., facial grimacing, nostril flaring, head jerks, eye blinks) that may be present in up to 75% of preschoolers who are stuttering (Yairi & Ambrose, 2005). Rather it is a neuromuscular oscillatory behavior that may or may not be visible in AWS. This synchronous, repetitive discharge of groups of motor units in the 5–15 Hz band is highly maladaptive. The forward flow of speech cannot be resumed while motor units are entrained in involuntary, repetitive, synchronous discharge. This repeated pattern of entrainment can also be present across muscle systems (e.g., laryngeal and orofacial, Smith et al., 1993) during disfluencies. The fact that these tremor-like oscillations of muscle activity have not yet emerged during stuttering, at least not in the perioral system in the children we assessed, is also a positive indication for early treatment.
As a final note, we do not want to encourage the conclusion that the speech motor systems of prechoolers who are stuttering are characterized by a completely typical developmental trajectory. The present findings regarding perioral muscle activation revealed no atypical patterns of activation, however other measures of speech motor processes in this age group suggest that many CWS are lagging their typically developing peers in interarticulator coordination consistency, especially when linguistic demands are higher (MacPherson & Smith, in press). This may indicate, as we and others have suggested earlier (Namasivayam & van Lieshout, 2011; Smits-Bandstra, DeNil, & Rochon, 2006; Zelaznik, Smith, Franz, & Ho, 1997), that differences between stuttering and nonstuttering individuals are more likely to be observed in measures that reflect multi-effector coordination, while measures of basic movement parameters, such as EMG or movement amplitudes are less likely to reflect the physiological bases of the disorder.
Acknowledgments
This research was supported by the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (R01 DC00559) and by a supplement to this grant awarded to the first author (. We are grateful to Janna Berlin, Barb Brown, and Tricia Zebrowski and her colleagues at the University of Iowa for their contributions to this project. We expressly wish to thank all of the children and their families whose participation in the study made this research possible.
Footnotes
We note that counting single-syllable whole word repetitions as SLDs is controversial. For example, Conture (1990b) maintains that the repetition of single syllable words is not stuttering; but indicates an expressive language delay. We elected to use the classification system established by Yairi, Ambrose, and colleagues due to the strong evidence that stuttering can be clearly differentiated from normal disfluencies in preschoolers using their weighted SLD method (Ambrose &Yairi, 1999; Yairi & Ambrose, 1999).
The absence of clear relations between target words and EMG patterns during an SLD is clearly illustrated in Figure 1 in which lip EMG amplitude during a non-labial part-word repetition (“ga”) is indistinguishable from EMG activity associated with a bilabial sound prolongation (“mmm”).
References
- Ambrose N, Yairi E. Normative data for early childhood stuttering. Journal of Speech, Language, & Hearing Research. 1999;42:895–909. doi: 10.1044/jslhr.4204.895. [DOI] [PubMed] [Google Scholar]
- Blair C. Interdigitating muscle fibers throughout orbicularis oris inferior. Journal of Speech and Hearing Research. 1986;29:266–269. doi: 10.1044/jshr.2902.266. [DOI] [PubMed] [Google Scholar]
- Blair C, Smith A. EMG recording in human lip muscles: Can single muscles be isolated? Journal of Speech and Hearing Research. 1986;29:256–266. doi: 10.1044/jshr.2902.256. [DOI] [PubMed] [Google Scholar]
- Bansevicius D, Westgaard RH, Jensen C. Mental stress of long duration: EMG activity, perceived tension, fatigue, and pain development in pain-free subjects. Headache. 1997;37:499–510. doi: 10.1046/j.1526-4610.1997.3708499.x. [DOI] [PubMed] [Google Scholar]
- Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, Ludlow CM. Altered patterns of cerebral activity during speech and language production during developmental stuttering: An H215O positron emission tomography study. Brain. 1997;120:761–784. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- Burgemeister BB, Blum LH, Lorge I. Columbia Mental Maturity Scale. 3. New York, NY: Harcourt Brace Jovanovich; 1972. [Google Scholar]
- Choo A, Robb MP, Dalrymple-Alford JC, Huckabee ML, O’Beirne GA. Different lip asymmetry in adults who stutter: Electromyographic evidence during speech and non-speech. Folia Phoniatrica et Logopaedica. 2010;62:143–147. doi: 10.1159/000287213. [DOI] [PubMed] [Google Scholar]
- Conture EG. Stuttering. 2. Englewood Cliffs, NJ: Prentice-Hall; 1990b. [Google Scholar]
- de Felício CM, Freitas RL, Vitti M, Regalo SC. Comparison of upper and lower lip muscle activity between stutterers and fluent speakers. International Journal of Pediatric Otorhinolaryngology. 2007;71:1187–1192. doi: 10.1016/j.ijporl.2007.04.008. [DOI] [PubMed] [Google Scholar]
- De Nil LF, Kroll RM, Kapur S, Houle S. A positron emission tomography study of silent and oral single word reading in stuttering and nonstuttering adults. Journal of Speech, Language, and Hearing Research. 2000;43:1038–1053. doi: 10.1044/jslhr.4304.1038. [DOI] [PubMed] [Google Scholar]
- Denny M, Smith A. Gradations in a pattern of neuromuscular activity associated with stuttering. Journal of Speech and Hearing Research. 1992;35:1216–1229. doi: 10.1044/jshr.3506.1216. [DOI] [PubMed] [Google Scholar]
- Fibiger S. Stuttering explained as a physiological tremor. Speech Transmission Laboratory Quarterly Progress and Status Report. 1971;2–3:1–24. [Google Scholar]
- Fischer R, Hess CW, Rössler KM. Uncrossed cortico-muscular projections in humans are abundant to facial muscles of the upper and lower face, but may differ between sexes. Journal of Neurology. 2005;252:21–26. doi: 10.1007/s00415-005-0592-7. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C, Lancaster JL. A PET study of the neural systems of stuttering. Nature. 1996;382:158–161. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- Freeman FJ, Ushijima T. Laryngeal muscle during stuttering. investigations. Journal of Speech and Hearing Research. 1978;21:538–562. doi: 10.1044/jshr.2103.538. [DOI] [PubMed] [Google Scholar]
- Goffman L, Smith A. Motor unit territories in the human perioral musculature. Journal of Speech and Hearing Research. 1994;37:975–984. doi: 10.1044/jshr.3705.975. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. A Four-factor index of social status. Department of Sociology, Yale University; New Haven, CT: 1975. Unpublished manuscript. [Google Scholar]
- Kelly EM, Smith A, Goffman L. Orofacial muscle activity of children who stutter: A preliminary study. Journal of Speech and Hearing Research. 1995;38:1025–1036. doi: 10.1044/jshr.3805.1025. [DOI] [PubMed] [Google Scholar]
- Kleinow J, Smith A. Influences of length and syntactic complexity on the speech motor stability of the fluent speech of adults who stutter. Journal of Speech, Language, and Hearing Research. 2000;43:548–559. doi: 10.1044/jslhr.4302.548. [DOI] [PubMed] [Google Scholar]
- Lapatki BG, Oostenveld R, Van Dijk JP, Jonas IE, Zwarts MJ, Stegeman DF. Optimal placement of bipolar surface EMG electrodes in the face based on single motor unit analysis. Psychophysiology. 2010;47:299–314. doi: 10.1111/j.1469-8986.2009.00935.x. [DOI] [PubMed] [Google Scholar]
- MacPherson MK, Smith A. Influences of sentence length and syntactic complexity on the speech motor control of children who stutter. Journal of Speech, Language, and Hearing Research. doi: 10.1044/1092-4388(2012/11-0184). in press. [DOI] [PMC free article] [PubMed]
- Max L, Caruso AJ, Gracco VL. Kinematic analyses of speech, orofacial nonspeech, and finger movements in stuttering and nonstuttering adults. Journal of Speech, Language, and Hearing Research. 2003;46:215, 232. doi: 10.1044/1092-4388(2003/017). [DOI] [PubMed] [Google Scholar]
- McClean M, Goldsmith H, Cerf A. Lower-lip EMG and displacement during bilabial disfluencies in adult stutterers. Journal of Speech and Hearing Research. 1984;27:342–349. doi: 10.1044/jshr.2703.342. [DOI] [PubMed] [Google Scholar]
- McClean MD, Runyan CR. Variations in the relative speeds of orofacial structures with stuttering severity. Journal of Speech, Language, and Hearing Research. 2000;43:1524–1531. doi: 10.1044/jslhr.4306.1524. [DOI] [PubMed] [Google Scholar]
- McClean MD, Tasko SM, Runyan CR. Orofacial movements associated with fluent speech in persons who stutter. Journal of Speech, Language, and Hearing Research. 2004;47:294–303. doi: 10.1044/1092-4388(2004/024). [DOI] [PubMed] [Google Scholar]
- McLean AE, Cooper EB. Electromyographic indications of laryngeal-area activity during stuttering expectancy. Journal of Fluency Disorders. 1978;3:205–219. [Google Scholar]
- Meyer BU, Werhahn K, Rothwell JC, Roericht S, Fauth C. Functional organisation of corticonuclear pathways to motoneurones of lower facial muscles in man. Experimental Brain Research. 1994;101:465–472. doi: 10.1007/BF00227339. [DOI] [PubMed] [Google Scholar]
- Moore CA. Symmetry of mandibular muscle activity as an index of coordinative strategy. Journal of Speech and Hearing Research. 1993;36:1145–1157. doi: 10.1044/jshr.3606.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Ruark JL. Does speech emerge from earlier appearing oral motor behaviors? Journal of Speech and Hearing Research. 1996;39:1034–1047. doi: 10.1044/jshr.3905.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Smith A, Ringel RL. Task-specific organization of human jaw muscles. Journal of Speech and Hearing Research. 1988;31:670–680. doi: 10.1044/jshr.3104.670. [DOI] [PubMed] [Google Scholar]
- Namasivayam AK, van Lieshout P. Speech motor skill and stuttering. Journal of Motor Behavior. 2011;43:477–489. doi: 10.1080/00222895.2011.628347. [DOI] [PubMed] [Google Scholar]
- Nilsen KB, Westgaard RH, Stovner LJ, Helde G, Rø M, Sand TH. Pain induced by low-grade stress in patients with fibromyalgia and chronic shoulder/neck pain, relation to surface electromyography. European Journal of Pain. 2006;10:615–627. doi: 10.1016/j.ejpain.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Raab P, Neumann K, Euler HA, von Gudenberg AW, Gall V, Zanella F. Event –related fMRI for the suppression of speech-associated artifacts in stuttering. NeuroImage. 2003b;19:1076–1084. doi: 10.1016/s1053-8119(03)00157-5. [DOI] [PubMed] [Google Scholar]
- Ruark JL, Moore CA. Coordination of lip muscle activity by 2-year-old children during speech and nonspeech tasks. Journal of Speech, Language, and Hearing Research. 1997;40:1373–1385. doi: 10.1044/jslhr.4006.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. Childhood Autism Rating Scale. Los Angeles, CA: Western Psychological Services; 1988. [Google Scholar]
- Shapiro AI. An electromyographic analysis of the fluent and disfluent utterances of several types of stutterers. Journal of Fluency Disorders. 1980;5:203–231. [Google Scholar]
- Smith A. Neural drive to muscles in stuttering. Journal of Speech and Hearing Research. 1989;32:252–264. doi: 10.1044/jshr.3202.252. [DOI] [PubMed] [Google Scholar]
- Smith A, Denny M, Shaffer LA, Kelly EM, Hirano M. Activity of intrinsic laryngeal muscles in fluent and disfluent speech. Journal of Speech and Hearing Research. 1996;39:329–348. doi: 10.1044/jshr.3902.329. [DOI] [PubMed] [Google Scholar]
- Smith A, Kelly E. Stuttering: A dynamic, multifactorial model. In: Curlee R, Siegel G, editors. Nature and Treatment of Stuttering: New Directions. Boston: Allyn & Bacon; 1997. pp. 204–217. [Google Scholar]
- Smith A, Luschei E. Assessment of oral-motor reflexes in stutterers and normal speakers: Preliminary observations. Journal of Speech and Hearing Research. 1983;26:322–328. doi: 10.1044/jshr.2603.322. [DOI] [PubMed] [Google Scholar]
- Smith A, Luschei E, Denny M, Wood JL, Hirano M, Badylak S. Spectral analyses of activity in laryngeal and orofacial muscles in stutterers. Journal of Neurology, Neurosurgery, and Psychiatry. 1993;56:1303–1311. doi: 10.1136/jnnp.56.12.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, McFarland DA, Weber CM, Moore CA. Spatial organization of human perioral reflexes. Experimental Neurology. 1987;98:233–248. doi: 10.1016/0014-4886(87)90239-1. [DOI] [PubMed] [Google Scholar]
- Smith A, Sadagopan N, Walsh B, Weber-Fox C. Increasing phonological complexity reveals heightened instability in interarticulatory coordination adults who stutter. Journal of Fluency Disorders. 2010;35:1–18. doi: 10.1016/j.jfludis.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Zelaznik HN. Development of functional synergies for speech motor coordination in childhood and adolescence. Developmental Psychobiology. 2004;45:22–33. doi: 10.1002/dev.20009. [DOI] [PubMed] [Google Scholar]
- Smits-Bandstra S, DeNil L, Rochon E. The transition to increased automaticity during finger sequence learning in adult males who stutter. Journal of Fluency Disorders. 2006;31:22–42. doi: 10.1016/j.jfludis.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Starkweather CW. A simple theory of stuttering. Journal of Fluency Disorders. 1995;20:91–116. doi: 10.1016/s0094-730x(02)00144-4. [DOI] [PubMed] [Google Scholar]
- Stepp C. Surface electromyography for speech and swallowing systems: Measurement, analysis, and interpretation. Journal of Speech, Language, and Hearing Research. 2012;55:1232–1246. doi: 10.1044/1092-4388(2011/11-0214). [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Ghacibeh G, Springer U, Bowers D. Lateralized asymmetry of facial motor evoked potentials. Neurology. 2005;65:541–544. doi: 10.1212/01.wnl.0000172916.91302.e7. [DOI] [PubMed] [Google Scholar]
- Urban PP, Beer S, Hopf HC. Cortico-bulbar fibers to orofacial muscles: Recordings with enoral surface electrodes. Electroencephalography and Clinical Neurophysiology. 1997;105:8–14. doi: 10.1016/s0924-980x(96)96584-4. [DOI] [PubMed] [Google Scholar]
- van Lieshout PHHM, Peters HFM, Starkweather CW, Hulstijn W. Physiological differences between stutterers and nonstutterers in perceptually fluent speech: EMG amplitude and duration. Journal of Speech and Hearing Research. 1993;36:55–63. doi: 10.1044/jshr.3601.55. [DOI] [PubMed] [Google Scholar]
- Walsh B, Smith A. Articulatory movements in adolescents: Evidence for protracted development of speech motor control processes. Journal of Speech, Language, and Hearing Research. 2002;45:1119–1133. doi: 10.1044/1092-4388(2002/090). [DOI] [PubMed] [Google Scholar]
- Weber C, Smith A. Reflex responses in human jaw, lip, and tongue muscles elicited by mechanical stimulation. Journal of Speech and Hearing Research. 1987;30:70–79. doi: 10.1044/jshr.3001.70. [DOI] [PubMed] [Google Scholar]
- Wohlert AB, Goffman L. Human perioral muscle activation patterns. Journal of Speech and Hearing Research. 1994;37:1032–1040. doi: 10.1044/jshr.3705.1032. [DOI] [PubMed] [Google Scholar]
- Yairi E, Ambrose N. Early childhood stuttering I: Persistency and recovery rates. Journal of Speech, Language, & Hearing Research. 1999;42:1097–1112. doi: 10.1044/jslhr.4205.1097. [DOI] [PubMed] [Google Scholar]
- Yairi E, Ambrose N. Early Childhood Stuttering. Austin, TX: Pro-Ed; 2005. [Google Scholar]
- Yairi E, Seery CH. Stuttering foundations and clinical applications. Boston, MA: Pearson; 2011. [Google Scholar]
- Zelaznik HN, Smith A, Franz EA, Ho M. Differences in bimanual coordination associated with stuttering. Acta Psychologica. 1997;96:229–243. doi: 10.1016/s0001-6918(97)00014-0. [DOI] [PubMed] [Google Scholar]
- Zemlin WR. Speech and Hearing Science: Anatomy and Physiology. 4. Boston, MA: Allyn & Bacon; 1998. [Google Scholar]
- Zimmermann G. Stuttering: A disorder of movement. Journal of Speech and Hearing Research. 1980c;23:122–136. [PubMed] [Google Scholar]
- Zocchi L, Estenne M, Johnston S, Del Ferro L, Ward ME, Macklem PT. Respiratory muscle incoordination in stuttering speech. American Review of Respiratory Disease. 1990;141:1510–1515. doi: 10.1164/ajrccm/141.6.1510. [DOI] [PubMed] [Google Scholar]