Abstract
Chronic pain is influenced by biological, psychological, social, and cultural factors. The current study investigated potential roles for combinations of genetic and psychological factors in the development and/or maintenance of chronic musculoskeletal pain. An exercise-induced shoulder injury model was used and a priori selected genetic (ADRB2, COMT, OPRM1, AVPR1A, GCH1, and KCNS1) and psychological (anxiety, depressive symptoms, pain catastrophizing, fear of pain, and kinesiophobia) factors were included as predictors. Pain phenotypes were shoulder pain intensity (5-day average and peak reported on numerical rating scale), upper-extremity disability (5-day average and peak reported on the QuickDASH), and shoulder pain duration (in days). After controlling for age, sex, and race the genetic and psychological predictors were entered as main effects and interaction terms in separate regression models for the different pain phenotypes. Results from the recruited cohort (n = 190) indicated strong statistical evidence for interactions between the COMT diplotype and 1) pain catastrophizing for 5-day average upper-extremity disability and 2) depressive symptoms for pain duration. There was moderate statistical evidence for interactions for other shoulder pain phenotypes between additional genes (ADRB2, AVPR1A, and KCNS1) and depressive symptoms, pain catastrophizing, or kinesiophobia. These findings confirm the importance of the combined predictive ability of COMT with psychological distress, and reveal other novel combinations of genetic and psychological factors that may merit additional investigation in other pain cohorts.
Keywords: Chronic pain, muscle pain, pain candidate genes, psychological predictors, SNP, COMT
Introduction
Chronic pain is widely acknowledged to be a multi-factorial entity with input from various biological, psychological, social, and cultural factors.(26) This complexity makes it challenging to study the development of chronic pain conditions, but structure has been provided by recent theoretical models. In particular, Diatchenko et al(11) have proposed a model for idiopathic chronic pain conditions that takes into consideration many relevant factors involved in the transition from acute to chronic pain. In this model it was hypothesized that genetic factors provide a foundation for chronic pain development, while specific interactions between environmental factors, psychological distress, and pain amplification further increase the chances of developing a chronic pain condition.(11)
In our previous studies we have used this model as the framework for investigating variations in the catechol-O-methyltransferase (COMT) gene and psychological factors consistent with a Fear-Avoidance Model of Musculoskeletal Pain (FAM).(30) Results from exercise-induced injury and surgical shoulder pain cohorts indicated that an interaction between a COMT gene diplotype that confers high pain sensitivity due to low enzyme activity and elevated pain catastrophizing, resulted in higher shoulder pain intensity ratings.(16;17) Studies in patients with fibromyalgia have provided converging evidence to support an interaction between the COMT gene and pain associated psychological distress.(8;14) For example, Finan et al(14) reported that patients with a COMT single nucleotide polymorphism (SNP) associated with low enzyme activity had higher pain on days coinciding with elevated pain catastrophizing.
The interactions between the COMT gene and pain-associated psychological distress are compelling because it has been observed in different pain conditions, where replication is rare in such studies involving genetic predictors. Given the consistent nature of these findings, there is a need to further investigate the development and maintenance of chronic pain conditions by exploring interactions between other relevant genetic and pain-associated psychological factors.(35) COMT has been the most studied gene,(35;45) but there are other candidates to consider for interactions with pain-associated psychological factors. In 2004, Belfer et al(2) identified 19 other high priority candidate pain genes and since then many other promising genes have been identified in animal or human studies.(35;37) In addition, psychological factors other than pain catastrophizing merit further study in combination with candidate genes. For example, fear of pain, anxiety, and depressive symptoms have each been highlighted for their influence on the pain experience.(31)
The purpose of the current study was to investigate the influence of combinations of select genetic and psychological factors on different pain phenotypes. In addition to the COMT gene and pain catastrophizing, we studied other potential genetic and pain associated psychological predictors, to build on our previous studies.(16;17) An exercise-induced muscle injury model was used because it controls for mechanism of muscle injury and results in shoulder pain and disability that lasts for several days. We have successfully used this pain model in previous studies(3;4;15;16) and report on several pain phenotypes, including shoulder pain intensity, upper-extremity disability, and duration of shoulder pain. These pain phenotypes were selected a priori and each represents a different aspect of the pain experience.
Methods
Subjects
The University of Florida’s institutional review board for human subjects approved this study and all subjects provided informed consent prior to participating in this study. Subjects were otherwise healthy men and women of any racial/ethnic background recruited by fliers from undergraduate and graduate courses and from the surrounding community. Inclusion criteria included 1) being between 18–85 years old and 2) not currently performing strength training exercises for the upper extremity (operationally defined as no resistance exercise during the previous six weeks). Exclusion criteria included any one of the following: 1) currently experiencing neck or shoulder pain; 2) neurological impairment of the upper extremity (e.g. loss of sensation, muscle weakness, or reflex changes); 3) currently taking pain medication or 4) previous history of shoulder surgery. These eligibility criteria are the same as used in our previous exercise induced injury shoulder studies.(15;16) Subjects received up to $160.00 compensation (prorated for each completed session) for their research participation for the time it took to complete the exercise protocol and data collection sessions.
Procedures
All subjects underwent five testing sessions on consecutive days. During the first session subjects provided informed consent and completed a series of questionnaires assessing demographic and psychological data. Then subject DNA was collected via buccal swabs, and the concentric-eccentric isokinetic exercise-induced pain protocol was completed on their dominant shoulder. Subjects returned to the lab post-injury at 24-hour intervals for the next four days for collection of data related to their shoulder pain, including pain intensity and upper extremity disability. If shoulder pain continued after the fifth study day, subjects were sent an email prompting them to report pain intensity via a web based data collection tool. These procedures are explained in more detail in the subsequent sections.
Self Report Questionnaires
Demographic and psychological data were captured by self-report. The demographic data included sex, age, race, dominant hand, height, and weight. The psychological data included general negative mood constructs and constructs specific to the FAM. These questionnaires are described below and total scores were used for this study.
Negative Mood Measures
Depressive symptoms and anxiety were the negative mood constructs of interest for this study. The Patient Health Questionnaire (PHQ) is a 9-item measure that was used for assessment of depressive symptoms.(24;29) Anxiety was assessed with the State-Trait Anxiety Inventory (STAI) which is a 40-item measure for symptoms of anxiety.(43) Only the 20-item trait portion of the STAI was used in the data analysis to capture a dispositional construct.(38;47)
FAM Measures
Fear of pain, fear of re-injury/movement, and pain catastrophizing were the FAM-specific constructs of interest for this study. Fear of pain was assessed with the Fear of Pain Questionnaire (FPQ-III), a 30-item instrument that measures fear of specific situations that normally produce pain.(1;34;39) For sake of brevity we used a shortened 9-item version of the FPQ-III for ease of application and because we have found these 9 items to correlate highly with the original 30-item scale and have predictive validity in a previous study.(40) Fear of movement was assessed with the Tampa Scale of Kinesiophobia (TSK) and we used the previously validated 11-item version to quantify avoidance and re-injury beliefs.(18;48) Finally, pain catastrophizing was assessed with the Pain Catastrophizing Scale (PCS) which is a 13-item measure to quantify pain catastrophizing characterized by magnification and rumination of pain beliefs.(44;46)
Genetic Data Generation
Gene and SNP Selection
Genetic predictors for this paper were selected a priori based on allele frequencies, status as tagging SNPs, functional consequences relevant to processing of nociception, and promising findings in human association studies involving experimental or clinical pain phenotypes. All SNPs chosen were bi-allelic. In this paper we report findings for genes involved in peripheral or central pain modulation pathways, and in a separate report we will present findings for genes involved in the inflammatory process. The specific SNPs selected for each gene (Table 1) were in part based on our previous studies and had minor allele frequencies in Caucasian populations of European descent that ensured adequate power in statistical analyses. For two genes, established functional haplotypes/diplotypes were available for use in analysis, whereas the other genes had only individual SNPs.
Table 1.
Descrip tive statistic s for pain candid ate SNPs
| Gene | SNP | Genotype | Number, % | MAF Allele, Number, % |
|---|---|---|---|---|
| ADRB2# | rs1042713 | AA | 30, 16.0% | A, 152, 40.6% |
| AG | 92, 49.2% | |||
| GG | 65, 34.8% | |||
| rs1042714 | CC | 62, 34.1% | G, 127, 36.9% | |
| CG | 93, 51.1% | |||
| GG | 27, 14.8% | |||
| COMT# | rs4633 | CC | 59, 31.6% | T, 176, 47.0% |
| CT | 80, 42.8% | |||
| TT | 48, 25.7% | |||
| rs6269 | AA | 55, 30.2% | G, 157, 43.1% | |
| GA | 97. 53.3% | |||
| GG | 30, 16.5% | |||
| rs4818 | CC | 65, 36.3% | G, 142, 39.7% | |
| CG | 86, 48.0% | |||
| GG | 28, 15.6% | |||
| rs4680 | AA | 42, 23.2% | A, 170, 46.9% | |
| AG | 86, 47.5% | |||
| GG | 53, 29.3% | |||
| OPRM1* | rs1799971 | AA | 137, 72.5% | G, 62, 16.4% |
| AG | 42, 22.2% | |||
| GG | 10, 5.3% | |||
| AVPR1A* | rs1042615 | AA | 31, 16.7% | A, 146, 39.2% |
| AG | 84, 45.2% | |||
| GG | 71, 38.2% | |||
| rs10877969 | CC | 19, 10.2% | C, 104, 27.9% | |
| CT | 66, 35.5% | |||
| TT | 101, 54.3% | |||
| GCH1* | rs3783641 | AA | 6, 3.3% | A, 64, 17.7% |
| AT | 52. 28.7% | |||
| TT | 123, 68.0% | |||
| KCNS1* | rs734784 | CC | 26, 14.2% | C, 156, 42.6% |
| CT | 104, 56.8% | |||
| TT | 53, 29.0% |
- Included in data analysis as diplotype comprised of SNPs described in Table 1
- Included in data analysis as individual SNPs described in Table 1
MAF = Minor Allele Frequency
The COMT and μ-opioid receptor (OPRM1) genes were included based on their high priority status(2) through previous studies linking COMT and OPRM1 variants with experimental pain sensitivity and analgesic response.(10;13) (20;21) COMT was represented by the four established COMT SNPs (rs6269, rs4633, rs4818, and rs4680) that affect pain sensitivity(12;50), while OPRM1 was represented by a SNP (rs1799971, “A118G”) believed to be highly relevant for pain sensitivity,(32) including the first such report in humans from Drs. Fillingim and Wallace.(13) We also included the adrenergic receptor beta 2 (ADRB2) gene represented by 2 well-established SNPs (rs1042713 and rs1042714).(41) These two SNPs are in almost complete linkage disequilibrium, both encode amino acid substitutions, and are associated with catecholamine response(33) and risk of chronic pain.(9;23)
Three other genes were included because of their potential relevance for prediction in this pain model. Arginine vasopressin receptor 1A (AVPR1A) was represented by SNPs rs1042615 and rs10877969(36) and GTP cyclohydrase (GCH1) was represented by SNP rs2149482(5). These specific SNPs have been associated with enhanced experimental pain sensitivity in the previously referenced human studies, thus justifying their inclusion as potential predictors. Potassium voltage gated channel alpha subunit S1 (KCNS1) was represented by SNP rs734784. This variation, encoding an amino acid substitution, was reported to be a predictor of neuropathic pain states in a rat model and in human patients with lumbar disc herniation(7). This provided rationale for including this gene/SNP in our study.
Genotyping
Genotyping was performed through Dr. Wallace’s laboratory using techniques similar to those implemented in our previous studies.(13;16;17) Genomic DNA samples were extracted from buccal swabs using the PureGene system (Qiagen Inc., Valencia CA (USA)), and quantitated on a Nanodrop ND-2000 spectrophotometer (ThermoFisher Scientific Inc, Waltham, MA (USA)). High-throughput genotyping in a 96-well-plate format was accomplished using pre-designed TaqMan SNP assays (ABI/Invitrogen Inc, Carlsbad CA (USA)) genotyped on an ABI Prism 7900HT System in the UF Pharmacogenomics core. Quality control measures included analysis of duplicates, blanks, and spot-checked genotypes generated with DNA sequencing or restriction analysis (to also genotype any that failed the TaqMan assays). Hardy-Weinberg equilibrium was calculated and found acceptable for each SNP.
In our analysis, four genes (OPRM1, AVPR1A, GCH1, and KCNS1) were represented by their individual SNPs and two (COMT and ADRB2) by diplotypes. The descriptive data for each SNP is shown in Table 1 and data for haplotypes and diplotypes are presented in Table 2. COMT haplotypes were inferred as described by Diatchenko et al(12) using the four markers that are in very strong linkage disequilibrium and which denote the high pain sensitivity (HPS), average pain sensitivity (APS), and low sensitivity (LPS) established haplotypes. This 4-marker system readily identifies rare recombinant haplotypes for which relative pain sensitivity cannot be attributed. Furthermore, Dr. Wallace’s lab performed some phase analyses via PCR across multiple SNPs to verify haplotypes. Thus, rare-haplotype-bearing samples in our set (n= 22 out of 372 haplotypes) were not included in the COMT analysis. The 3 COMT haplotypes (HPS, APS, LPS) were again combined into high and low pain sensitivity diplotypes (Table 3) to represent subject genotypes per the Diatchenko et al(12) approach for risk of temporo-mandibular disorder (TMD) development and in our previous shoulder pain studies for increased pain intensity (16;17). Using the Diatchenko et al(9) functional study, ADRB2 diplotypes were constructed from the three common haplotypes associated with lower or higher ADRB2 expression and risk of TMD.(9) For our purposes the diplotype with either homozygotes for lower or higher ADRB2 expression and the one heterozygote for higher ADRB2 expression was labeled “A” (higher risk of TMD), while the diplotype with heterozygotes for low and high ADRB2 expression was labeled “B” (lower risk of TMD). The nomenclature we used differed from the Diatchenko et al paper(9), but refers to the same haplotypes and diplotypes (Table 2).
Table 2.
Descriptive statistics for pain candidate gene haplotypes and diplotypes
| Gene | Haplotype | Number | Frequency (%) |
|---|---|---|---|
| ADRB2 | 1 (GG) | 149 | 41.6% |
| 2 (AC) | 70 | 19.6% | |
| 3 (GC) | 139 | 38.8% | |
| COMT | LPS (GCGG) | 142 | 38.2% |
| APS (ATCA) | 164 | 44.1% | |
| HPS (ACCG) | 44 | 11.8% | |
| rare | 22 | 5.9% | |
| Gene | Diplotype | Number | Frequency (%) |
| ADRB2 | A* 11, 12 22, 33 |
90 | 50.3% |
| B* 13, 23 |
89 | 49.7% | |
| COMT | High APS/HPS HPS/APS HPS/HPS APS/APS |
72 | 38.7% |
| Low HPS/LPS APS/LPS LPS/LPS LPS/rare |
114 | 61.3% |
for our ADRB2 coding: 1 = H2 (high expression haplotype), 2 = H3 (high expression haplotype), and 3 = H1 (low expression haplotype) following the Diatchenko et al paper.
Table 3.
Descriptive statistics for baseline demographic and psychological factors
| Variable | Mean±SD or Frequency | Median [Min, Max] or Percent | |
|---|---|---|---|
| Age | 23.0±6.0 | 21 [18, 58] | |
| Gender | Female | 116 | 61% |
| Male | 74 | 39% | |
| Race | White | 153 | 81% |
| Black or African American | 12 | 6% | |
| Other | 24 | 13% | |
| Dominant Hand | Right | 171 | 90% |
| Left | 19 | 10% | |
| FPQ_total | 23.4±5.8 | 24 [9, 38] | |
| PCS_total | 9.9±7.7 | 10 [0, 38] | |
| PHQ_total | 2.7±3.2 | 2 [0, 22] | |
| STAI_total | 45.6±3.1 | 46 [37, 53] | |
| TSK_total | 18.0±4.2 | 17 [11, 34] | |
Exercise Induced Muscle Injury Protocol
Exercise induced muscle injury was induced using a Kin-Com isokinetic dynamometer (Chattanooga Group., Chattanooga, TN). A brief description of the specific protocol is provided with this paper with more detailed methods available from our previous studies.(15;16;40) During the first session maximum voluntary isometric contraction (MVIC) was determined by having the subjects perform three repetitions of maximal isometric shoulder external rotation. The highest torque value was then recorded as the MVIC and used as a baseline reference. During the muscle injury protocol subjects were verbally encouraged to complete maximal isokinetic concentric/eccentric external rotation repetitions for their dominant shoulder. The exercise speed was set at 60°/s for 3 sets of 10 repetitions and subjects were given 30 seconds rest between sets. Following the first set of 3 isokinetic repetitions, MVIC was measured and if subjects could still generate greater than 50% of their initial MVIC, they performed additional sets of 10 repetitions at 60°/s (range = 1 to 8 additional sets). Subjects were re-assessed after each additional set until their peak torque was lower than 50% of the initial MVIC. This criterion was selected because previous research has indicated that the inability to achieve 50% of baseline peak MVIC is an indicator of muscle micro-trauma resulting in characteristic inflammatory and proprioceptive responses.(6)
Shoulder Pain Outcomes
Shoulder Pain Intensity
The Brief Pain Inventory (BPI) was used to measure pain intensity as it has been found to have good test-rest reliability over short intervals.(28) The BPI consists of rating pain on an 11-point numerical rating scale ranging from 0 (no pain) to 10 (worst pain imaginable). The BPI asks subjects to rate current pain and pain at its worst, best and average over the past 24 hours. For our data analysis the current, worst, and best BPI ratings were averaged for each day, consistent with our previous study(17). Then these averaged daily ratings were averaged over the 5 days to represent the 5-day shoulder pain intensity phenotype. The highest worst pain intensity recorded during the 5 day period was used as a separate phenotype for the peak shoulder pain intensity variable.
Upper Extremity Disability
The Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) was used to assess upper-extremity disability.(25) In this study we used a validated abridged version of the DASH (the QuickDASH) which consists of 11 functional items, with total scores ranging from 0 (not disability) to 100 (complete disability).(19) We used the QuickDASH because shoulder pain can also affect distal function of the arm and hand, and we wanted to obtain an assessment of global upper extremity disability. Similar to the 5-day shoulder pain intensity ratings, QuickDASH scores were averaged over the 5 days to represent the 5-day upper extremity disability phenotype and the highest average disability score recorded during the 5 day period was used as a separate phenotype for the peak upper extremity disability intensity variable.
Duration of Shoulder Pain
Not all subjects were pain free at the assessment on the 5th day. These subjects were sent an email each subsequent day prompting them to enter the BPI through a secure, web-based data collection. Subjects continued to receive an email each day until they rated their current pain at 0/10 and their worst pain was rated less than 2/10. The number of days it took to reach this criterion was recorded as the phenotype for duration of shoulder pain.
Data Analysis
All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, North Carolina). Summary statistics were calculated for all demographic, psychological, genetic, and shoulder pain outcome measures. For every pain candidate gene, a general linear model was fitted to assess its main effect (genotype level) and a series of expanded models were fitted to study its interaction with five psychological factors for each shoulder pain outcome. The pain candidates genes were included in the regression models as previously established diplotypes (ADRB2 and COMT) or individual SNPs (OPRM1, AVPR1A, GCH1, and KCNS1). Psychological factors were kept in the original continuous metric in the regression models and also included as main effects in the regression models. Therefore, each model had the same structure with 4 increments including 1) demographic data (age, gender, and race), 2) genotype, 3) psychological factor, and 4) the gene by psychological factor interaction. In this approach the gene by psychological interaction effect was determined individually after accounting for the other predictor variables, to identify its unique prediction of variability in the respective shoulder pain phenotype.
In our linear modeling, we conducted a total of 40 independent tests to determine if interactions (8 genetic factors by 5 psychological factors) improved prediction for each shoulder pain outcome. Bonferroni correction would yield a threshold alpha level of 0.0013 for each outcome. While this might be a conservative correction for genetic studies (42), the value of 0.0013 provides a convenient benchmark in assessing the outcome of the analyses reported below. In what follows then, interaction terms with p values <0.0013 were considered “strong” statistical evidence for predicting the pain phenotype of interest, while those with p values ≥0.0013 but <0.05 as showing “moderate” statistical evidence for predicting the pain phenotype of interest. Interaction terms with p values ≥0.05 were not further considered.
Results
One hundred ninety subjects completed the study and descriptive statistics for demographic and psychological in Table 3. Exercise-induced muscle pain resulted in average shoulder pain intensity scores (mean±sd) of 0.4±0.8 (day of injury protocol), 2.0±1.4 (24 hours after injury protocol), 2.4±1.7 (48 hours after injury protocol), 2.1±1.7 (72 hours after injury protocol) and 1.2±1.2 (96 hours after injury protocol) respectively. The average peak pain intensity rating (mean±sd) was 5.0±2.4. The corresponding upper extremity disability reported (mean±sd) was 2.7±4.6 (day of injury protocol), 11.6±11.6 (24 hours after injury protocol), 16.5±13.8 (48 hours after injury protocol), 15.5±13.8 (72 hours after injury protocol) and 10.8±10.8 (96 hours after injury protocol). The average peak upper extremity disability was 19.6±15.0. The average duration of shoulder pain in days (mean±sd) was 6.1±1.8, with all subjects providing complete data on shoulder pain duration.
Correlations between the variables used as shoulder pain outcomes in this study ranged from r = 0.25 to r = 0.95, with 8/10 of the correlations being below r = 0.60. The outcome measures that correlated below 0.60 were retained as different phenotypes since they shared less than 36% of variance. As expected, the highest correlations were between 5-day shoulder pain intensity and peak shoulder pain intensity (r = 0.86) and 5-day upper extremity disability and peak upper extremity disability (r = 0.95). Since we had planned a priori to consider these as separate measures on conceptual grounds, these phenotypes were also analyzed separately.
Regression models included age, sex, and race to control for these effects on the shoulder pain phenotypes, as well as the individual main effects for the genotype and psychological factor of interest. Models meeting our criterion for strong or moderate statistical evidence are summarized in Table 4 and highlighted below in more detail for each shoulder pain phenotype.
Table 4.
Regression analyses for prediction of shoulder pain phenotypes by genetic and psychological factors
| Model Description | R-Square Increment | R-Square Full Model | p-value Full Model | ||||
|---|---|---|---|---|---|---|---|
| Age, Sex, and Race | Add Genotype | Add Psychological Factor | Add Interaction Term | P-value Interaction Term | |||
| Prediction of 5-Day Average Pain Intensity | |||||||
| Age, sex, and race + COMT + PHQ + COMT*PHQ | 0.034 | 0.047^ | 0.043 | 0.084 | 0.0105 | 0.208 | 0.003 |
| Prediction of Peak Pain Intensity | |||||||
| Age, sex, and race + ADRB2 + TSK + ADRB2*TSK | 0.054^ | 0.007 | 0.054 | 0.037 | 0.0182 | 0.152 | 0.022 |
| Prediction of 5-Day Average Upper Extremity Disability | |||||||
| Age, sex, and race + COMT+ PCS + COMT*PCS | 0.078^ | 0.038^ | 0.140^ | 0.091 | 0.0011 | 0.347 | <0.0005 |
| Age, sex, and race + COMT+ PHQ + COMT*PHQ | 0.115^ | 0.039^ | 0.140 | 0.0299 | 0.295 | <0.0005 | |
| Age, sex, and race + KCNS1 (rs734784) + PCS + KCNS1 (rs734784)*PCS | 0.079 | 0.062^ | 0.123 | 0.0108 | 0.342 | <0.0005 | |
| Prediction of Peak Upper Extremity Disability | |||||||
| Age, sex, and race + COMT+ PCS + COMT*PCS | 0.063^ | 0.025^ | 0.098^ | 0.070 | 0.0043 | 0.256 | <0.0005 |
| Age, sex, and race + KCNS1 (rs734784) + PCS + KCNS1 (rs734784) *PCS | 0.066 | 0.038^ | 0.104 | 0.0259 | 0.208 | 0.002 | |
| Prediction of Shoulder Pain Duration | |||||||
| Age, sex, and race + COMT+ PHQ + COMT* PHQ | 0.033 | 0.000 | 0.052 | 0.068 | 0.0010 | 0.153 | 0.008 |
| Age, sex, and race + COMT+ TSK + COMT*TSK | 0.000 | 0.001 | 0.077 | 0.0094 | 0.111 | 0.099 | |
| Age, sex, and race + ADRB2 + PHQ + ADRB2 *PHQ | 0.060^ | 0.015 | 0.097 | 0.0153 | 0.205 | 0.014 | |
| Age, sex, and race + AVPR1 (rs1042615) + PHQ + AVPR1 (rs1042615) *PHQ | 0.045 | 0.020 | 0.108 | 0.0065 | 0.206 | 0.016 | |
| Age, sex, and race + AVPR1 (rs10877969) + PHQ + AVPR1 (rs10877969)*PHQ | 0.043 | 0.017 | 0.106 | 0.0069 | 0.199 | 0.021 | |
| Age, sex, and race + AVPR1 (rs10877969) + PCS + AVPR1 (rs10877969)*PCS | 0.043 | 0.004 | 0.088 | 0.0314 | 0.168 | 0.113 | |
Models in bold font meet criterion for strong statistical evidence of interaction term;
indicates p-valued for R-Square main effect is < 0.05;
Gene without rs number is the associated diplotype; PHQ - Patient Health Questionnaire (depressive symptoms); PCS – Pain Catastrophizing Scale, TSK – Tampa Scale of Kinesiophobia;
5-Day Shoulder Pain Intensity
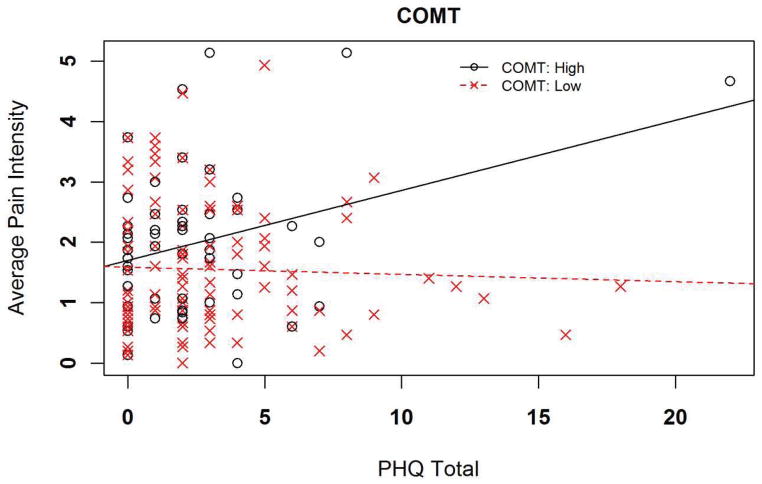
Two different genetic and psychological interactions had moderate statistical evidence for explaining additional variance in 5-day shoulder pain intensity ratings. In the full regression model for the COMT diplotype and the PHQ, total variance explained was an estimated 20.8%, with the interaction term independently accounting for 8.4% of the overall variance (p = 0.011 for the interaction term). Increasing PHQ scores were more strongly associated with pain intensity for the high pain sensitivity COMT diplotype in comparison to the low pain sensitivity diplotype (Figure 1). The interaction resulted in an increased pain intensity rating of 0.45 for every 1 standard deviation increase in PHQ score among individuals with the high pain sensitivity COMT diplotype.
Figure 1.
Interaction for prediction of 5-day shoulder pain intensity
Key: PHQ = Patient Health Questionnaire for assessment of depressive symptoms (scale truncated for display purposes)
Peak Shoulder Pain Intensity
One genetic and psychological interaction had moderate statistical evidence for involvement in the peak shoulder pain intensity phenotype. The full model including the ADRB2 diplotype and TSK interaction explained an estimated 15.2% overall variance in this pain phenotype, with the interaction term independently accounting for 3.7% of the overall variance (p = 0.018). TSK scores were associated with peak pain intensity for the B ADRB2 diplotype. The interaction increased peak pain intensity rating of 0.59 for each 1 standard deviation increase in the TSK.
5-Day Upper Extremity Disability
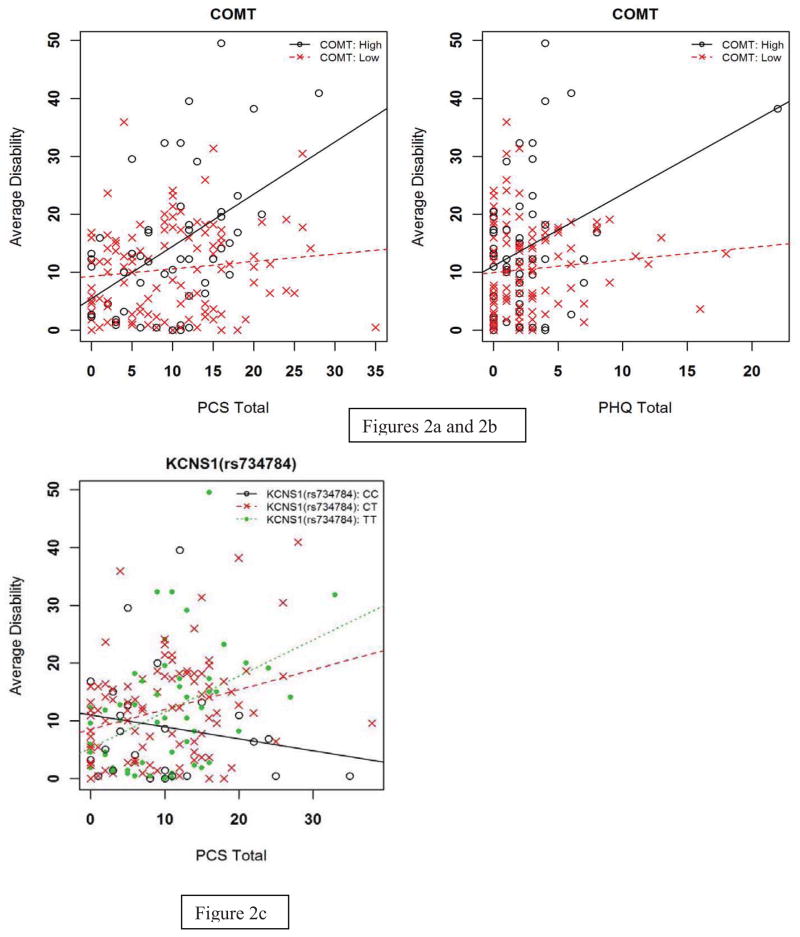
One genetic-psychological interaction had strong statistical evidence for the upper-extremity disability phenotype. The full model including the COMT gene diplotype and PCS explained an estimated 34.7% of the overall variance in the pain phenotype, with the interaction term independently accounting for 9.1% of the overall variance (p <0.001). Increasing PCS scores were associated with disability in individuals with the COMT high pain sensitivity diplotype in comparison to the low pain sensitivity diplotype (Figure 2a). The high pain sensitivity diplotype was predictive of increased disability (5.47) for each 1 standard deviation increase in the PCS.
Figure 2.
Figure 2a-c. Prediction of 5-day upper extremity disability: interactions between genetic and psychological factors
Key: PHQ = Patient Health Questionnaire for assessment of depressive symptoms; PCS = Pain Catastrophizing Scale (all scales truncated for display purposes)
In addition, there was moderate statistical evidence for two other genetic and psychological interactions for this disability pain phenotype. First, the COMT gene diplotype also interacted with the PHQ, with the full model accounting for an estimated 29.5% of the overall variance and the interaction term independently contributed 14.0% (p = 0.030) variance. Increasing PHQ scores were associated with disability for the COMT high pain sensitivity diplotype in comparison to the low pain sensitivity diplotype (Figure 2b). The high pain sensitivity diplotype was predictive of increased disability (3.20) for each 1 standard deviation increase in the PHQ. Second, there was a moderate interaction between the KCNS1 gene and the PCS with the full model explaining an estimated 34.2% variance and the interaction term accounting for 12.3% variance (p = 0.011). The PCS score had a stronger association with upper extremity disability when the KCNS1 gene had a TT genotype compared to a CC genotype (Figure 2c). This interaction resulted in an increase of upper extremity disability of 6.08 units for each standard deviation increase in the PCS.
Peak Upper Extremity Disability
Two genetic and psychological interactions had moderate statistical evidence to supporting the prediction of peak upper extremity disability. In the full model for the PCS with the COMT diplotype the total variance explained was an estimated 25.6%, with the interaction term independently accounting for 7.0% (p = 0.004). PCS scores had an increasing association with peak disability for those with the COMT high pain sensitivity diplotype in comparison to those with the COMT low pain sensitivity diplotype. The interaction resulted in an increase peak disability of 8.47 for each 1 standard deviation increase in the PCS. Second, the full model with the interaction term for PCS and KCNS1 (rs734784) explained an estimated total of 20.8% variance, with the interaction term accounting for 10.2% of the variance independently (p = 0.026). PCS scores had an increasing association with peak disability for those with the TT genotype compared to a CC genotype. This interaction resulted in an increase of peak disability of 9.09 units for each standard deviation increase in the PCS.
Duration of Shoulder Pain
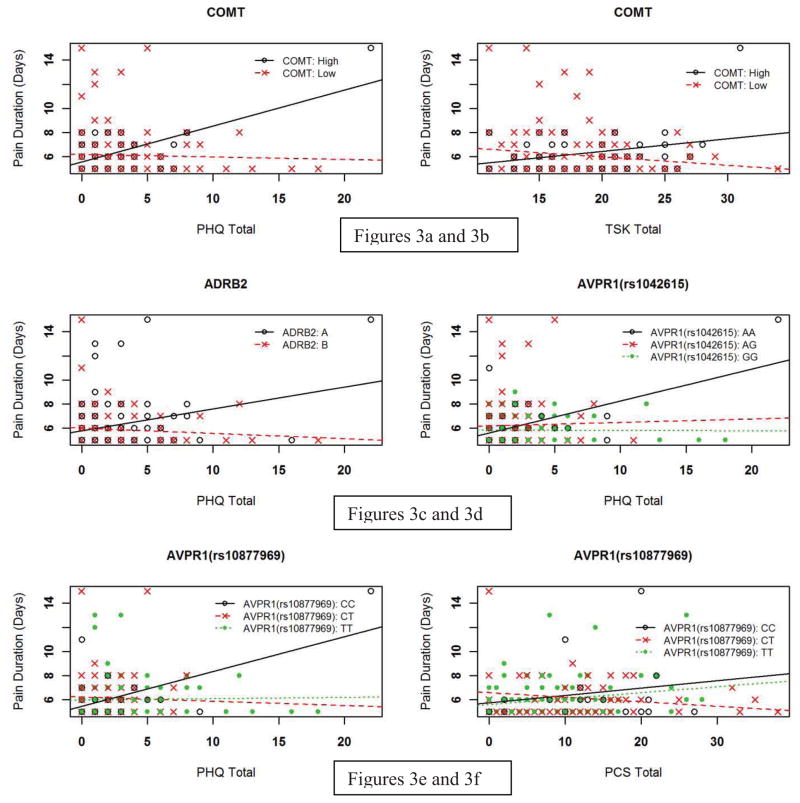
There was one genetic and psychological interaction that met our criterion for strong statistical evidence for the duration pain phenotype. The full regression model including the COMT diplotype and the PHQ accounted for an estimated 15.3% of the total variance, with the interaction term independently contributing 6.8% (p < 0.001). PHQ scores were more strongly associated with shoulder pain duration for the high pain sensitivity COMT diplotype in comparison to the low pain sensitivity diplotype (Figure 3a). This interaction increased shoulder pain duration by 0.99 days for each 1 standard deviation increase in the PHQ.
Figure 3.
Prediction of shoulder pain duration: interactions between genetic and psychological factors
Key: PHQ = Patient Health Questionnaire for assessment of depressive symptoms; PCS = Pain Catastrophizing Scale; TSK = Tampa Scale of Kinesiophobia (all scales truncated for display purposes)
In addition, there were 5 other genetic and psychological interactions that met our criterion for moderate statistical evidence for the pain duration phenotype. The full model with the COMT diplotype and TSK scores accounted for an estimated 11.1% variance, with the interaction term contributing 7.7% independently (p = 0.009). TSK scores were more strongly associated with shoulder pain duration for the high pain sensitivity diplotype in comparison to the low pain sensitivity diplotype (Figure 3b), increasing 0.17 days for each 1 unit increase in the TSK. The full model containing the interaction of the ADRB2 diplotype and PHQ scores explained an estimated 20.5% of overall variance in shoulder pain duration, with the interaction term accounting for 9.7% variance (p = 0.015). PHQ scores were more strongly associated with shoulder pain duration for the A ADRB2 diplotype (Figure 3c). The interaction increased shoulder pain duration by 0.67 days for each 1 standard deviation increase in the PHQ (p = 0.012).
The two AVPR1A SNPs (rs1042615 and rs10877969, not in linkage disequilibrium) separately interacted with PHQ scores in full regression models, with estimated variance explained being 20.6% and 19.9% respectively. In these models the interaction terms independently accounted for 10.8% and 10.6% of the variance (p = 0.007 for both interaction terms). PHQ scores were more strongly associated with shoulder pain duration for those with the AA genotype (GG as comparison, rs1042615) and CC genotype (with TT as comparison, rs10877969) (Figures 3d and 3e). The effects of these interactions were very similar – an increase of 0.90 and 0.86 days for each 1 standard deviation increase in the PHQ respectively. The rs10877969 SNP of AVPR1A also interacted with PCS scores with estimated overall variance explained of 16.8%, with the interaction term accounting for 8.8% of the overall variance independently (p = 0.031). PCS scores were more strongly associated with shoulder pain duration for the TT genotype in comparison to the CT genotype (Figure 3f). This interaction resulted in an increase of 0.77 days for each 1 standard deviation increase in the PCS.
Influence of Extreme PHQ Data Point
Visual inspection of the data provided an indication that there was one extreme PHQ score. This point was confirmed to be more than 3 standard deviations beyond the mean PHQ scores for this sample. There was consideration in dropping this point from the analyses, but we had made no a priori decision rules for eliminating extreme points. Furthermore, although this point was “extreme” for this sample it did not represent the end range of the PHQ scale (observed value = 20, maximum value = 27) and is potentially more representative of PHQ scores from clinical pain samples. Therefore we decided to keep the data point in the primary analyses and report separately on the influence of this point. When this point was removed, only the interaction with the COMT diplotype (p = 0.049) remained for the 5-day upper extremity disability phenotype, with the KCNS1 interaction losing significance. No other statistical support for interactions with the PHQ was evident.
Discussion
The purpose of this study was to investigate how certain pain candidate genes interact with key psychological factors to predict different pain phenotypes from an exercise-induced muscle injury protocol. This particular pain model was selected because it allows for control of mechanism of injury and results in pain that lasts for several days, unlike more discrete experimental methods (e.g. thermal or pressure stimuli). There is also evidence that exercise induced muscle injury produces clinically-relevant responses as we have found biopsychosocial influences on pain intensity and disability reports from induced muscle injury comparable to those in clinical cohorts.(4;15;16;40) We investigated the effects of established diplotypes for COMT and ADRB2, as well as exploring the effects of SNPs for other previously identified pain candidate genes. Results from the current study advance previous work by confirming the importance of the COMT high pain sensitivity diplotype in combination with pain catastrophizing or depressive symptoms. We also identified other genes (ADRB2, AVPR1A, and KCNS1) that provided moderate statistical evidence of interaction with psychological factors and may merit future study when investigating the development of chronic musculoskeletal pain.
The COMT high pain sensitivity diplotype (12) was a consistent genetic marker when strong statistical evidence of an interaction was reported. The combined effect of the COMT diplotype with pain catastrophizing predicted the average upper extremity disability phenotype, and the combined effect with depressive symptoms predicted the shoulder pain duration phenotype. In addition, there was also moderate statistical evidence for an interaction between the COMT diplotype and depressive symptoms for the phenotypes for average pain intensity average upper-extremity disability, as well as pain catastrophizing for peak upper extremity disability. These results are a partial replication of our previous studies reporting a COMT diplotype by pain catastrophizing interaction for increased pain intensity in both exercised-induced and clinical shoulder surgical pain.(16;17) In the current study we report more interactions with depressive symptoms, which may not be surprising given the high correlation between depressive symptoms and catastrophizing(22) and the inclusion of different shoulder pain phenotypes. Collectively our findings converge with other reports in the literature that used different pain models to demonstrate that COMT diplotypes appear to be a robust genetic predictor of the pain experience, especially when considered in combination with depressive symptoms or pain catastrophizing.(8;14)
Only two genetic and psychological combinations met our criterion for strong statistical evidence of an interaction, but other models containing the ADRB2, AVPR1A and KCNS1 genes met the criterion for moderate statistical evidence. The ADRB2 gene has been relatively understudied in pain research,(35) but its variations have been linked to common chronic pain conditions like TMD,(9) widespread body pain,(23) and disabling neck and back pain.(41) Previous studies have not explicitly considered interactions of the ADRB2 diplotype with pain relevant psychological factors for influence on exercise induced muscle injury pain phenotypes. Diatchenko et al(9) reported an association between the same ADRB2 haplotypes as in this study and psychological profile, as well as risk of developing TMD in female subjects; however, combined influences of genetic and psychological predictors were not considered in predicting TMD onset. Interestingly the diplotype for ADRB2 had differing associations with pain phenotypes based on the psychological factor it interacted with. The B diplotype (haplotypes for both high and low ADRB2 expression) interacted with kinesiophobia for increased peak pain reports, while the A diplotype (haplotypes for either high or low ADRB2 expression) interacted with depressive symptoms for increased duration of shoulder pain. This finding should be viewed with some caution, however, as the interaction with depressive symptoms was affected by the extreme PHQ value from this sample. Future studies are necessary to determine whether ADRB2 actually has differing influence on pain phenotypes based on psychological factors, or whether this was a spurious finding based on the distribution of our sample. Notably, we did not detect a gender effect in the ADRB2 outcome in our study and it may be necessary in future analyses to separate the high and low ADRB2 expression homozygotes when investigating interactions with psychological factors. Further, OPRM1 was not significantly involved in any interactions, which is perhaps not surprising given its primary involvement with analgesic response, a variable we did not directly measure in this study.
SNPs from the AVPR1A gene were identified as predictors for the duration of shoulder pain phenotype, and their effects emerged when considered in combination with depressive symptoms and pain catastrophizing. Previous translational work involving capsaicin induced pain in humans identified that endogenous analgesia activated by vasopressin can be diminished in male subjects that report stress during pain testing.(36) Our results corroborate and advanced these previous findings by indicating that the same SNP of the AVPR1A gene (rs10877969) and another (rs1042615) interact with psychological factors to prolong shoulder pain duration in a clinically relevant pain model. Also, a SNP from KCNS1 was predictive of two shoulder pain phenotypes in combination with pain catastrophizing (5 day upper extremity disability and peak upper extremity disability). The translational precedent for KCNS1 was from peripheral neuropathic pain models, as variations in this gene affect potassium channel activity and subsequent neuronal excitability such that increased low back pain was associated with the risk allele (C), which encodes the amino acid substitution of a valine in place of the more common isoleucine).(7) Our results indicated that the KCNS1 influence on muscle pain phenotypes was related to not only the SNP, but also the concurrent presence of pain catastrophizing.
The primary purpose of this study was to identify genetic and psychological interactions predictive of exercise-induced shoulder pain phenotypes; however there are neurobiological observations that can be made from these data. First, the interaction between COMT and pain catastrophizing or depressive symptoms suggests that normal levels of COMT enzyme activity supporting healthy catecholaminergic neurotransmission may buffer the adverse effects of these psychological processes on pain and disability. Similarly, the interaction between ADRB2 and depressive symptoms or kinesiophobia extends the established link between beta adrenergic receptor function and negative mood symptoms(27;49) and suggests that beta adrenergic function moderates psychological influences on the pain experience following exercise-induced muscle injury. The current study represents the first we are aware of to investigate AVPR1A and KCNS1 in a musculoskeletal pain model. The neurobiological implications of these findings are that following muscle injury, both vasopressin-mediated endogenous analgesia and neuronal excitability can influence the pain-related impact of emotional or cognitive states, thereby supporting future investigation to determine the clinical relevance of these interactions for predicting treatment outcomes. It should be noted that these mechanistic observations are speculative because we did not include direct measures of physiological processes. However, these pre-clinical findings could provide future direction for those interested in studying how neurobiological mechanisms are influenced by psychological state.
A strength of this study included its use of a clinically relevant pain model and the monitoring of several different resultant pain phenotypes. Another strength of the current study was that we were able to monitor subjects as they progressed from a pain free to a painful state and back. Direct observation of this transition is rare in the pain literature and our study provides a unique perspective on how combined genetic and psychological effects may contribute to early pain responses following muscle injury. These data may eventually inform early identification of those likely to have an elevated response to muscle injury, which in turn could be a risk factor for the development of a chronic musculoskeletal pain condition.
The major limitation of this study is that there was an extreme value for the PHQ, and this value had a strong influence on the results. This is not an ideal situation but we decided to report the data including this observation as we did not have a priori decision guidelines to remove data, and although this value is extreme for our sample, it does not represent the end scale value for the PHQ. Readers that do not agree with this decision may have a different interpretation of the study, based on our description of the outcomes with that point removed. In sum, 13 genetic by psychological interactions were identified in these analyses, with two providing strong statistical evidence and 11 providing moderate statistical evidence. Six of these interactions involved the PHQ, five involved the PCS, and two involved the TSK. If the extreme PHQ point is removed, only one PHQ interaction remains as meeting our criterion for moderate statistical evidence (the interaction with COMT for average disability). A more conservative overall interpretation of these analyses is that nine interaction terms were identified, with only one (COMT with pain catastrophizing) providing strong statistical evidence.
Another limitation of this study is that by using only 1–4 SNPs per gene, we cannot make inferences for contributions to these pain phenotypes from gene variations not in linkage disequilibrium with the selected SNPs. Also, since the majority of our participants were white (non-Hispanic), we may have missed subtle influences of race on the pain phenotypes. Other limitations of this study include low overall levels of fear and catastrophizing on the psychological measures. While these levels are typical in healthy subjects, they may not reflect the influence of the higher pain associated psychological distress observed in clinical populations. Another limitation is the self-resolving nature of exercise induced muscle injury which is different from clinical musculoskeletal pain conditions that have more varied outcomes. Also, the number of genes studied here was only a subset of all possible pain candidate genes. And the lack of “in vivo” physiological measures for the functional effects of the genetic markers is another limitation to consider. Even though we purposefully used previously-established SNPs with known physiological effects, our study offers no direct proof of the physiological mechanisms involved in pain processing in this model for any of the identified genetic predictors.
Collectively these findings of genetic and psychological interactions lend additional support for a theoretical model of chronic pain development(11) and indicate that future research into these, and other, pain candidate genes should simultaneously consider psychological status when predicting pain phenotypes of interest. Overall interpretation of these results largely depends on what the reader perceives as the most representative phenotype for a chronic musculoskeletal pain condition. In our opinion, duration of shoulder pain potentially represents the most clinically relevant phenotype for the exercise-induced injury model, as it reflects continued symptoms beyond expected time of resolution. Typically, exercise-induced muscle injury resolves within 96 hours, but we observed variation in the duration of pain reported after exercise lasting up to 15 days. The duration of shoulder pain phenotype had the most complex pattern of predictors, including 3 genes (COMT, ADRB2, and AVPR1A) and 3 psychological factors (depressive symptoms, kinesiophobia, and pain catastrophizing). If this is the level of complexity that emerges from prediction of responses to a controlled muscle injury, the complexity of predictive factors and their patterns of interaction must be much greater for clinical conditions that can include involvement of skin, muscle, nerve, connective tissue, and/or bone and have much more variation in the pain phenotypes generated.
This study informs the growing literature on genetic contributions to pain, because most previous studies investigated main effects of pain candidate genes.(35) Our findings indicate that a focus on main effects may miss important information for better prediction of the pain experience. The consideration of psychological predictors may indirectly explain why some pain candidate gene studies have been reported with mixed results. If a particular gene’s “true” effect is via interaction with pain associated psychological processes, then unmeasured variation in psychological characteristics across cohorts may help explain non-replication of genetic associations. Future studies will investigate these same genetic and psychological predictors in a surgical cohort to determine the ecological validity of these predictors for clinical pain phenotypes and to eventually allow for development of tailored pain management strategies based on a combined genetic and psychological profile.
Perspective.
Interactions between genetic and psychological factors were investigated as predictors of different exercise-induced shoulder pain phenotypes. The strongest statistical evidence was for interactions between the COMTdiplotype and pain catastrophizing (for upper extremity disability) or depressive symptoms (for pain duration). Other novel genetic and psychological combinations were identified that may merit further investigation.
Acknowledgments
Alberto Bursian, Brianna Castillo, Lauren Hardin, Andy Hogan, Kelly Larkin Kaiser, Natalie Martinez, Pamela McCurdy, Rachel Montgomery, Hannah Spilker, and Nhi Thieu assisted with exercise-induced injury protocol and data collection
We thank Will Eaton and Michelle Burch for assisting with genetic analyses.
This study was completed with funding from the National Institutes of Health - NIAMS (AR055899) and NINDS (NS045551).
Footnotes
Disclosures
The authors have no financial or other conflict of interest to report for the data reported in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Albaret MC, Munoz Sastre MT, Cottencin A, Mullet E. The Fear of Pain questionnaire: factor structure in samples of young, middle-aged and elderly European people. Eur J Pain. 2004;8:273–281. doi: 10.1016/j.ejpain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Belfer I, Wu T, Kingman A, Krishnaraju RK, Goldman D, Max MB. Candidate gene studies of human pain mechanisms: methods for optimizing choice of polymorphisms and sample size. Anesthesiology. 2004;100:1562–1572. doi: 10.1097/00000542-200406000-00032. [DOI] [PubMed] [Google Scholar]
- 3.Bishop MD, Horn ME, George SZ. Exercise-induced Pain Intensity Predicted by Pre-exercise Fear of Pain and Pain Sensitivity. Clin J Pain. 2011;27:398–404. doi: 10.1097/AJP.0b013e31820d9bbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop MD, Horn ME, George SZ, Robinson ME. Self-reported pain and disability outcomes from an endogenous model of muscular back pain. BMC Musculoskelet Disord. 2011;12:35. doi: 10.1186/1471-2474-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell CM, Edwards RR, Carmona C, Uhart M, Wand G, Carteret A, Kim YK, Frost J, Campbell JN. Polymorphisms in the GTP cyclohydrolase gene (GCH1) are associated with ratings of capsaicin pain. Pain. 2009;141:114–118. doi: 10.1016/j.pain.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 7.Costigan M, Belfer I, Griffin RS, Dai F, Barrett LB, Coppola G, Wu T, Kiselycznyk C, Poddar M, Lu Y, Diatchenko L, Smith S, Cobos EJ, Zaykin D, Allchorne A, Gershon E, Livneh J, Shen PH, Nikolajsen L, Karppinen J, Männikkö M, Kelempisioti A, Goldman D, Maixner W, Geschwind DH, Max MB, Seltzer Z, Woolf CJ. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519–2527. doi: 10.1093/brain/awq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desmeules J, Piguet V, Besson M, Chabert J, Rapiti E, Rebsamen M, Rossier MF, Curtin F, Dayer P, Cedraschi C. Psychological distress in fibromyalgia patients: a role for catechol-O-methyl-transferase Val158met polymorphism. Health Psychol. 2012;31:242–29. doi: 10.1037/a0025223. [DOI] [PubMed] [Google Scholar]
- 9.Diatchenko L, Anderson AD, Slade GD, Fillingim RB, Shabalina SA, Higgins TJ, Sama S, Belfer I, Goldman D, Max MB, Weir BS, Maixner W. Three major haplotypes of the beta2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:449–462. doi: 10.1002/ajmg.b.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, Goldman D, Maixner W. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125:216–224. doi: 10.1016/j.pain.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123:226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 13.Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, Wallace MR. The a118g single nucleotide polymorphism of the ì-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia. Pain. 2011;152:300–307. doi: 10.1016/j.pain.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George SZ, Dover GC, Fillingim RB. Fear of Pain Influences Outcomes After Exercise-induced Delayed Onset Muscle Soreness at the Shoulder. Clin J Pain. 2007;23:76–84. doi: 10.1097/01.ajp.0000210949.19429.34. [DOI] [PubMed] [Google Scholar]
- 16.George SZ, Dover GC, Wallace MR, Sack BK, Herbstman DM, Aydog E, Fillingim RB. Biopsychosocial influence on exercise-induced delayed onset muscle soreness at the shoulder: pain catastrophizing and catechol-o-methyltransferase (COMT) diplotype predict pain ratings. Clin J Pain. 2008;24:793–801. doi: 10.1097/AJP.0b013e31817bcb65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George SZ, Wallace MR, Wright TW, Moser MW, Greenfield WH, III, Sack BK, Herbstman DM, Fillingim RB. Evidence for a biopsychosocial influence on shoulder pain: Pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 2008;136:53–61. doi: 10.1016/j.pain.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goubert L, Crombez G, Van Damme S, Vlaeyen JW, Bijttebier P, Roelofs J. Confirmatory factor analysis of the Tampa Scale for Kinesiophobia: invariant two-factor model across low back pain patients and fibromyalgia patients. Clin J Pain. 2004;20:103–110. doi: 10.1097/00002508-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:44. doi: 10.1186/1471-2474-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastie BA, Riley JL, III, Kaplan L, Herrera DG, Campbell CM, Virtusio K, Mogil JS, Wallace MR, Fillingim RB. Ethnicity interacts with the OPRM1 gene in experimental pain sensitivity. Pain. 2012;153:1610–1619. doi: 10.1016/j.pain.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henker RA, Lewis A, Dai F, Lariviere WR, Meng L, Gruen GS, Sereika SM, Pape H, Tarkin IS, Gowda I, Conley YP. The Associations Between OPRM1 and COMT Genotypes and Postoperative Pain, Opioid Use, and Opioid-Induced Sedation. Biol Res Nurs. 2012;15:309–317. doi: 10.1177/1099800411436171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsh AT, George SZ, Riley JL, III, Robinson ME. An evaluation of the measurement of pain catastrophizing by the coping strategies questionnaire. Eur J Pain. 2007;11:75–81. doi: 10.1016/j.ejpain.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Hocking LJ, Smith BH, Jones GT, Reid DM, Strachan DP, Macfarlane GJ. Genetic variation in the beta2-adrenergic receptor but not catecholamine-O-methyltransferase predisposes to chronic pain: results from the 1958 British Birth Cohort Study. Pain. 2010;149:143–151. doi: 10.1016/j.pain.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21:547–552. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.IOM (Institute of Medicine) Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 27.Kang EH, Yu BH. Anxiety and beta-adrenergic receptor function in a normal population. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:733–737. doi: 10.1016/j.pnpbp.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Annals. 2002;32:509–521. [Google Scholar]
- 30.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 31.Linton SJ, Shaw WS. Impact of Psychological Factors in the Experience of Pain. Phys Ther. 2011;91:700–711. doi: 10.2522/ptj.20100330. [DOI] [PubMed] [Google Scholar]
- 32.Lotsch J, Geisslinger G. Relevance of frequent mu-opioid receptor polymorphisms for opioid activity in healthy volunteers. Pharmacogenomics J. 2006;6:200–210. doi: 10.1038/sj.tpj.6500362. [DOI] [PubMed] [Google Scholar]
- 33.McLean RC, Baird SW, Becker LC, Townsend SN, Gerstenblith G, Kass DA, Tomaselli GF, Schulman SP. Response to catecholamine stimulation of polymorphisms of the beta-1 and beta-2 adrenergic receptors. Am J Cardiol. 2012;110:1001–1007. doi: 10.1016/j.amjcard.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 34.McNeil DW, Rainwater AJ. Development of the Fear of Pain Questionnaire--III. J Behav Med. 1998;21:389–410. doi: 10.1023/a:1018782831217. [DOI] [PubMed] [Google Scholar]
- 35.Mogil JS. Pain genetics: past, present and future. Trends Genet. 2012;28:258–266. doi: 10.1016/j.tig.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Mogil JS, Sorge RE, LaCroix-Fralish ML, Smith SB, Fortin A, Sotocinal SG, Ritchie J, Austin JS, Schorscher-Petcu A, Melmed K, Czerminski J, Bittong RA, Mokris JB, Neubert JK, Campbell CM, Edwards RR, Campbell JN, Crawley JN, Lariviere WR, Wallace MR, Sternberg WF, Balaban CD, Belfer I, Fillingim RB. Pain sensitivity and vasopressin analgesia are mediated by a gene-sex-environment interaction. Nat Neurosci. 2011;14:1569–1573. doi: 10.1038/nn.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moller AT, Jensen TS. Pain and genes: Genetic contribution to pain variability, chronic pain and analgesic responses. European Journal of Pain Supplements. 2010;4:197–201. [Google Scholar]
- 38.Munafo MR, Stevenson J. Anxiety and surgical recovery. Reinterpreting the literature. J Psychosom Res. 2001;51:589–596. doi: 10.1016/s0022-3999(01)00258-6. [DOI] [PubMed] [Google Scholar]
- 39.Osman A, Breitenstein JL, Barrios FX, Gutierrez PM, Kopper BA. The Fear of Pain Questionnaire-III: further reliability and validity with nonclinical samples. J Behav Med. 2002;25:155–173. doi: 10.1023/a:1014884704974. [DOI] [PubMed] [Google Scholar]
- 40.Parr JJ, Borsa PA, Fillingim RB, Tillman MD, Manini TM, Gregory CM, George SZ. Pain-related fear and catastrophizing predict pain intensity and disability independently using an induced muscle injury model. J Pain. 2012;13:370–378. doi: 10.1016/j.jpain.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skouen JS, Smith AJ, Warrington NM, O’Sullivan PB, McKenzie L, Pennell CE, Straker LM. Genetic variation in the beta-2 adrenergic receptor is associated with chronic musculoskeletal complaints in adolescents. Eur J Pain. 2012;16:1232–1242. doi: 10.1002/j.1532-2149.2012.00131.x. [DOI] [PubMed] [Google Scholar]
- 42.Smith SB, Maixner DW, Greenspan JD, Dubner R, Fillingim RB, Ohrbach R, Knott C, Slade GD, Bair E, Gibson DG, Zaykin DV, Weir BS, Maixner W, Diatchenko L. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J Pain. 2011;12:T92–101. doi: 10.1016/j.jpain.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for the state and trait anxiety inventory (form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 44.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 45.Tammimaki A, Mannisto PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenet Genomics. 2012;22:673–691. doi: 10.1097/FPC.0b013e3283560c46. [DOI] [PubMed] [Google Scholar]
- 46.Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96:319–324. doi: 10.1016/S0304-3959(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 47.Vaughn F, Wichowski H, Bosworth G. Does preoperative anxiety level predict postoperative pain? AORN J. 2007;85:589–604. doi: 10.1016/S0001-2092(07)60130-6. [DOI] [PubMed] [Google Scholar]
- 48.Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117:137–144. doi: 10.1016/j.pain.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 49.Yu BH, Dimsdale JE, Mills PJ. Psychological states and lymphocyte beta-adrenergic receptor responsiveness. Neuropsychopharmacology. 1999;21:147–152. doi: 10.1016/S0893-133X(98)00133-X. [DOI] [PubMed] [Google Scholar]
- 50.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;21;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]