Abstract
We show here that a cDNA library of normal tissue, expressed from a highly immunogenic viral platform, cures established tumors of the same histological type from which the cDNA library was derived. With suboptimal vaccination, immune escape was possible, but only when tumor cells were forced to acquire a radically new phenotype, readily treated by second line therapy. This approach has several major advantages. Use of the cDNA library leads to presentation of a broad repertoire of (undefined) tumor associated antigens, which reduces emergence of treatment resistant variants and also permits implementation of rational, combined modality approaches in the clinic. Finally, the viral vectors can be delivered systemically, without the need for tumor targeting, and are amenable to clinical grade production. Therefore, virus-expressed cDNA libraries represent a novel paradigm for cancer treatment by addressing many of the key issues which have undermined the efficacy of immuno/virotherapy to date.
Keywords: Vesicular Stomatitis Virus, cancer vaccines, tumor antigens, oncolytic virus, immune escape
Several key issues have undermined effective cancer immuno/virotherapy, including lack of identification of tumor associated antigens (TAA), poor coverage of the antigenic repertoire expressed by tumors and difficulties of vector targeting to tumors in vivo1–6. To allow for direct in vivo immune selection of appropriate TAA, we showed that killing normal cells in situ, with the adjuvant hsp70, generated T cell responses to antigens which mediated rejection of tumors of the same histological type7–11. With this approach12, 13, a broad repertoire of individually weak T cell responses is raised against multiple TAA imposing a cumulatively strong selective pressure against immune escape and no tumors have to be accessed by targeted vector delivery. However, injection of cytotoxic vectors into the normal tissues often resulted in toxicity8, 9. To alleviate these complications, we reasoned that the broad antigenic repertoire for in vivo immune selection of relevant TAA could be provided by expressing a cDNA library of a normal tissue from a systemically delivered, immunogenic vector to activate autoimmune/anti tumor T cell responses.
In this respect, viruses such as Vesicular Stomatitis Virus (VSV) can act as potent adjuvants for expression of antigens14–17, including TAA18–20, provided the TAA-expressing virus can access the draining lymph nodes (DLN)20, 21–23, 24.
Combining these observations, we hypothesized that cDNA from a normal tissue could be expressed from the VSV platform14–20, 23 to vaccinate against a wide range of TAA expressed on tumors of the same histological type. We also exploited the observation that altered self epitopes of TAA can be more immunogenic than the corresponding self epitopes25–27. We show here that a Viral Expressed Epitope Library (VEEL) from normal human prostate can induce rejection of established murine prostate tumors, without detectable autoimmunity. If tumors escaped immune selection, they adopted a radically new phenotype, which could be treated with second line therapy. Therefore, virus-expressed cDNA libraries represent a novel paradigm by which the ability of highly mutable tumor cells to escape selective pressures in vivo can be exploited to therapeutic advantage.
RESULTS
Viral Expressed Altered Self Epitope Library (VEASEL)
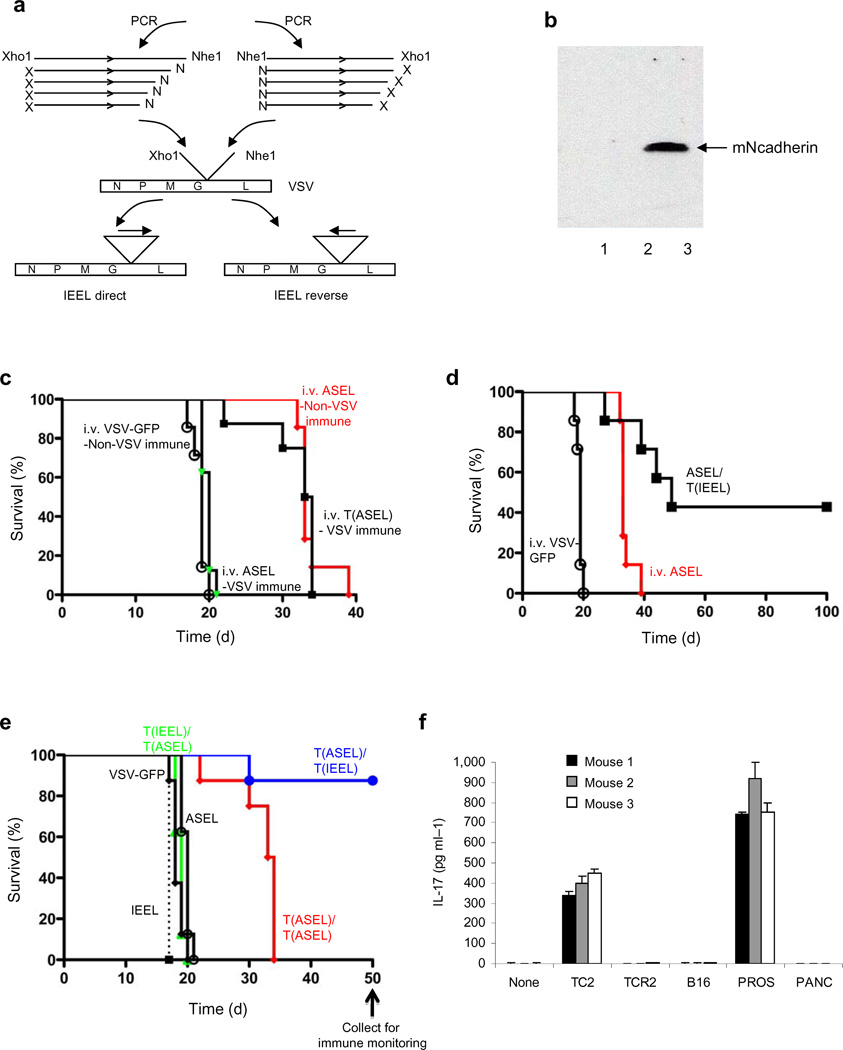
A cDNA library from normal human prostate, expressing Altered Self Antigens/Epitopes (in a murine context) from the Library (ASEL) was cloned into VSV (Fig.1a) in direct or reverse orientations. ASEL(Direct) and ASEL(Reverse) libraries had titers (1x107–6x107 pfu/ml) 1–2 log lower than VSV-GFP.
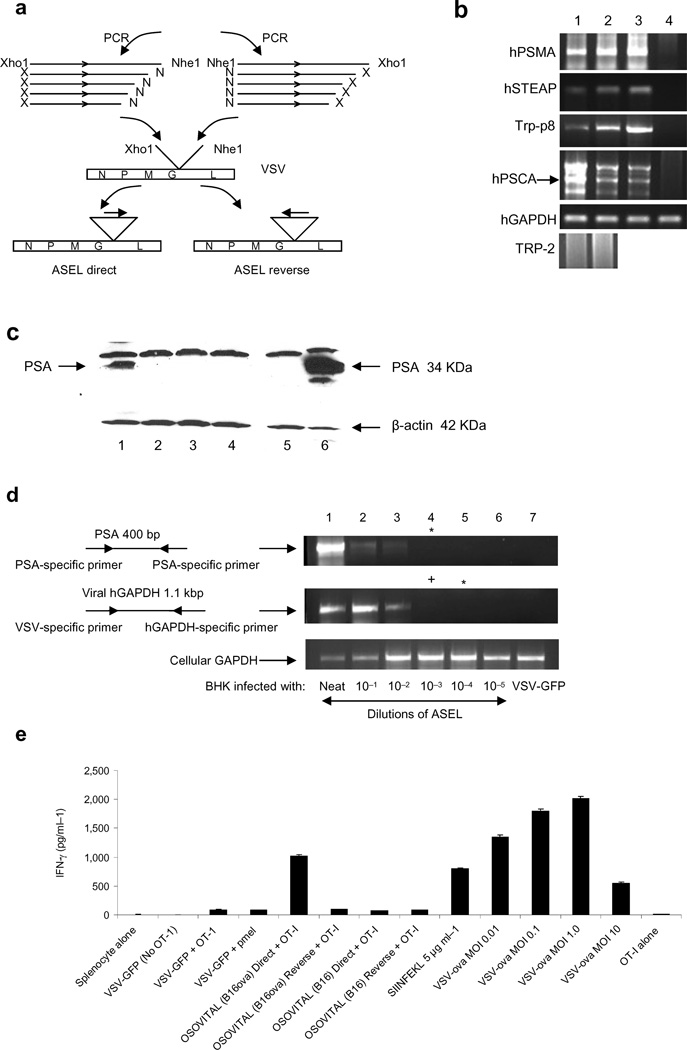
Figure 1. VSV-expressed cDNA libraries.
a. The ASEL VSV-expressed cDNA library contains cDNA from normal human prostate cloned into VSV in direct, or reverse, orientation. b. Human prostate specific genes37–40, but not the melanocyte-specific TRP-211, detected by PCR in the original human prostate plasmid library (lane 1) and in the VSV-cDNA plasmid library (2). rtPCR from cDNA of HT1080 cells infected with ASEL (MOI 0.1) (3) compared to uninfected cells (4) (predicted size for hPSCA shown with an arrow). c. BHK cells infected with ASEL Direct (1) or Reverse (2), or with control viruses (lanes 3&4) (MOI ~10) assayed for human PSA by Western Blot. Lane 5, uninfected BHK cells; Lane 6, 104 human prostate LnCap cells. d. BHK cells infected with 10 fold dilutions of ASEL virus (1–6) assayed by rtPCR for PSA or human GAPDH. No PSA-specific signal was detected at dilutions lower than 1:100 of the original virus stock. (Expression of GFP from 100 pfu of VSV-GFP could be detected by this assay). +Positive/*negative for PCR upon nested PCR. e. Splenocytes infected with VSV-GFP or VSV-cDNA libraries from cells which did, or did not, express OVA (MOI 0.1), co-cultured with naive OT-I T cells and assayed for IFN-γ28. Lane 1, splenocytes alone; (2–4), splenocytes infected with VSV-GFP, without OT-I (2), with OT-I (3) or with irrelevant T cells41; (5&6), splenocytes infected with VSV-cDNA library from B16ova cells in Direct (5) or Reverse (6) orientation with OT-I; (7&8), splenocytes infected with VSV-cDNA library from B16 cells (no OVA) in Direct (7) or Reverse (8) orientations with OT-I. (9), OT-I activated by SIINFEKL peptide. (10–13), splenocytes infected with VSV-ova at MOI 0.01 (10), 0.1 (11), 1.0 (12) and 10 (13). (14), OT-I (no splenocytes, no VSV).
Sequences of human prostate antigens, but not the melanocyte/melanoma associated TRP-2 (Fig.1b), gp100 or tyrosinase antigens (not shown), were present in the ASEL libraries (Fig.1b, 1&2). Expression of prostate-specific sequences (Fig.1b, 3&4), as well as, in some cases, full-length protein (PSA), was transferred by ASEL virus (Fig.1c). Using rtPCR (Fig.1d), we estimated that the positive PSA signal in 105 infected BHK cells (Fig.1c) derived from infection by about 200 VSV-PSA particles, and yielded PSA at 10 fold lower levels than in 104 LN-Cap human prostate cells (Fig.1c). When VSV-expressed cDNA libraries from murine cells which either did, or did not, express chicken ovalbumin (OVA), were used to infect H2-Kb splenocytes, naive OT-I T cells were activated to secrete IFN-γ, indicating transfer of expression of the SIINFEKL epitope28 at levels similar to infection with VSV-ova at an MOI of 0.01 (Fig.1e). However, the PCR assay of Fig.1d estimated that the VSV-cDNA(ova) virus was present in the splenocyte infections of Fig.1e at a MOI of about one log lower.
Intravenous injection of ASEL does not induce autoimmunity
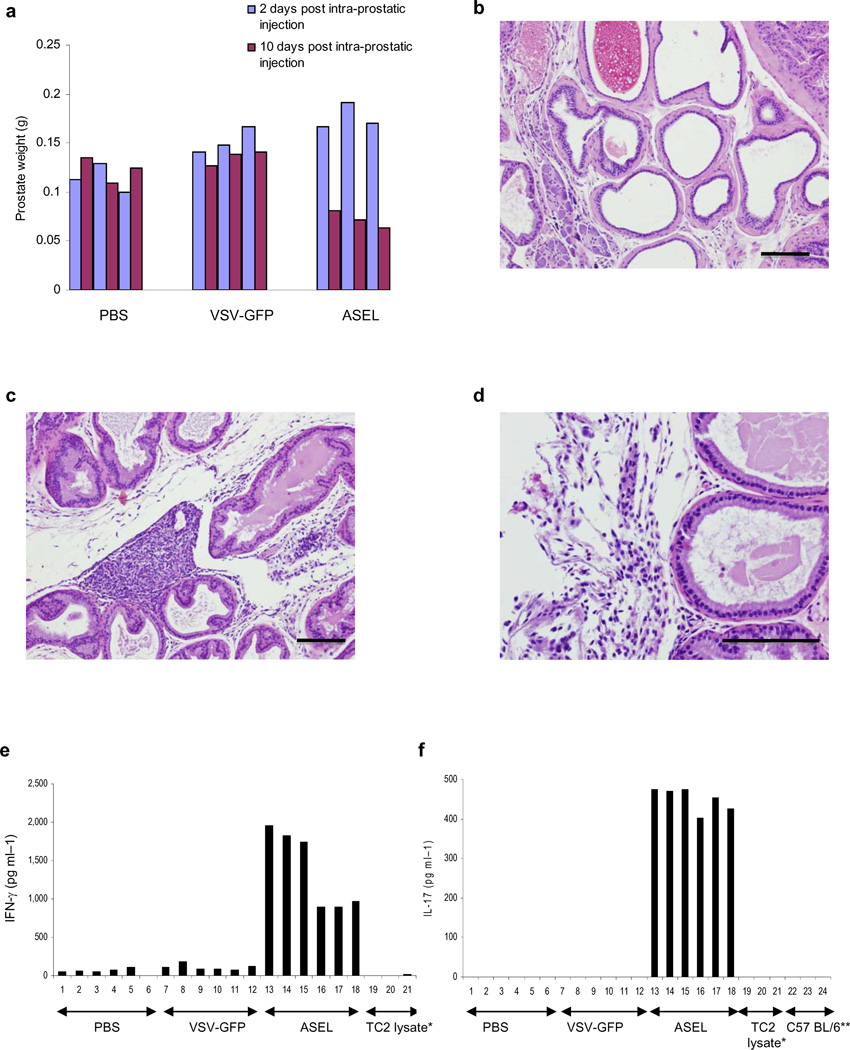
Within two days, prostates injected with VSV-GFP or ASEL were enlarged compared to PBS-injected controls (p=0.04 or 0.01) (Fig.2a). Within 10 days, prostate weights were significantly lower in mice injected with ASEL compared to controls (p<0.001) (Fig.2a), associated with loss of tissue architecture and immune infiltration (Figs.2b-d). Splenocytes from mice injected intra-prostatically with ASEL secreted both IFN-γ (Fig.2e) and IL-17 (Fig.2f) in response to normal prostate (not shown) or TC2 murine prostate tumor cells (Fig.2e,f), but not to murine B16 melanoma cells (not shown). In contrast, after 60 days, prostates from mice injected i.v. with ASEL were not significantly different from controls in either weight (Fig.3a) or histology (not shown).
Figure 2. Intra-prostatic injection of ASEL induces autoimmunity.
a. Prostate weights of mice injected intra-prostatically with PBS, VSV-GFP or ASEL 2 or 10 days after injection (n=3). b-d. Histology of prostates 10 days after intra-prostatic injection of PBS (b) or ASEL (c,d). Scale bars, 100µm. e,f. 10 days following intra-prostatic injection of PBS, or 107pfu VSV-GFP or ASEL (6 mice per group) splenocytes from each mouse co-cultured with lysates of TC2 cells were assayed for IFN-γ (e) or IL-17 (f). Mean values of cytokine from two ELISA wells per sample are shown for each mouse.
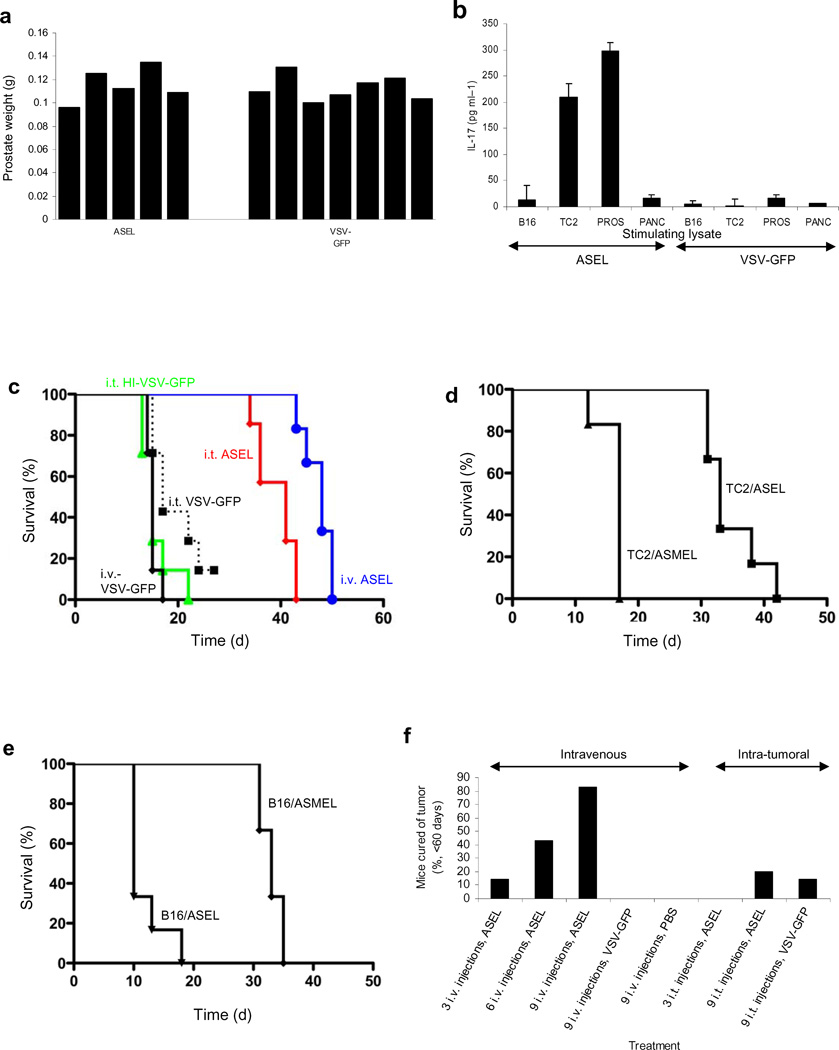
Figure 3. Intravenous injection of ASEL has anti-tumor efficacy.
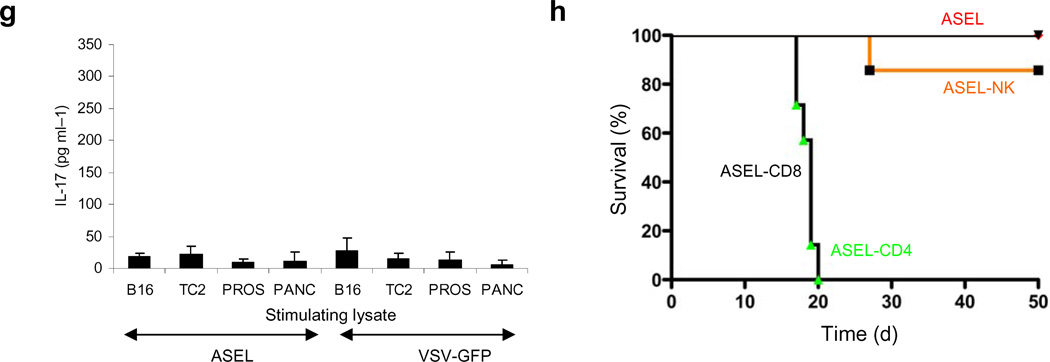
a. Prostate weights 60d following 107 pfu i.v. of ASEL (n=5) or VSV-GFP (n=7). b. Mean levels of IL-17 secreted by splenocytes from these mice co-cultured with lysates of B16 melanoma, TC2 prostate, normal mouse prostate or pancreas. c. Survival of mice bearing 7d TC2 tumors (n=7–8) injected intra-tumorally or intravenously with 107pfu of VSV-GFP, ASEL, or heat inactivated VSV-GFP (days 7,9,11). d,e. Survival of mice bearing 7d TC2 (d) or B16 (e) tumors injected intravenously with ASEL, or with a VSV-cDNA library from human melanoma cells (Altered Self Melanoma Epitope Library, ASMEL) (days 7,9,11). f. Cumulative percentages of mice cured of 7d TC2 tumors when administered 3, 6 or 9 injections of ASEL or VSV-GFP i.t. or i.v. every other day. g. IL-17 secreted by splenocytes from the three mice cured of TC2 tumors by 9 intra-tumoral injections of ASEL in f., as well as from 3 mice treated similarly with VSV-GFP, co-cultured with lysates of B16, TC2, normal mouse prostate or pancreas. h. Survival of mice bearing 7d TC2 tumors (n=7/8), mock depleted, or depleted of CD4+, CD8+ or NK cells, injected intravenously with ASEL on days 7,9,11,14,16,18,21,23,25.
Intravenous injection of ASEL cures established tumors
Intravenous ASEL generated a prostate-specific Th17, but not an IFN-γ, response (Fig.3b). Moreover, 3 i.v. injections of ASEL gave the best survival compared to either i.t. ASEL (p=0.01) or i.t. VSV-GFP (p<0.0001) (Fig.3c). Neither i.v. VSV-GFP (Fig.3c), nor i.v. ASEL(Reverse) (not shown), generated therapy. In contrast, the ASEL had no effects against B16 melanomas (Fig.3d,e). Similarly, a VSV-cDNA library from human melanoma cells significantly slowed murine B16 melanomas, but had no effect against TC2 tumors (Fig.3d,e).
With more injections of ASEL, tumors were cured more effectively with i.v., compared to i.t., treatment (Fig.3f). Thus, 9 i.v. injections of ASEL cured over 80% of mice compared to 0% with VSV-GFP (p<0.0001) or ASEL(Reverse) (not shown) (Fig.3f) - with no detectable autoimmune prostatitis. Unlike i.v. (Fig.3b), no mice treated i.t. with ASEL, including three cures, developed Th17 (Fig.3g) or IFN–γ (not shown) responses to prostate. Consistent with an immune, as opposed to oncolytic, mechanism for i.v. ASEL, therapy was dependent upon CD4+ T cells but not CD8+ T cells or NK cells (Fig.3f).
In vivo selection of immune escape variants
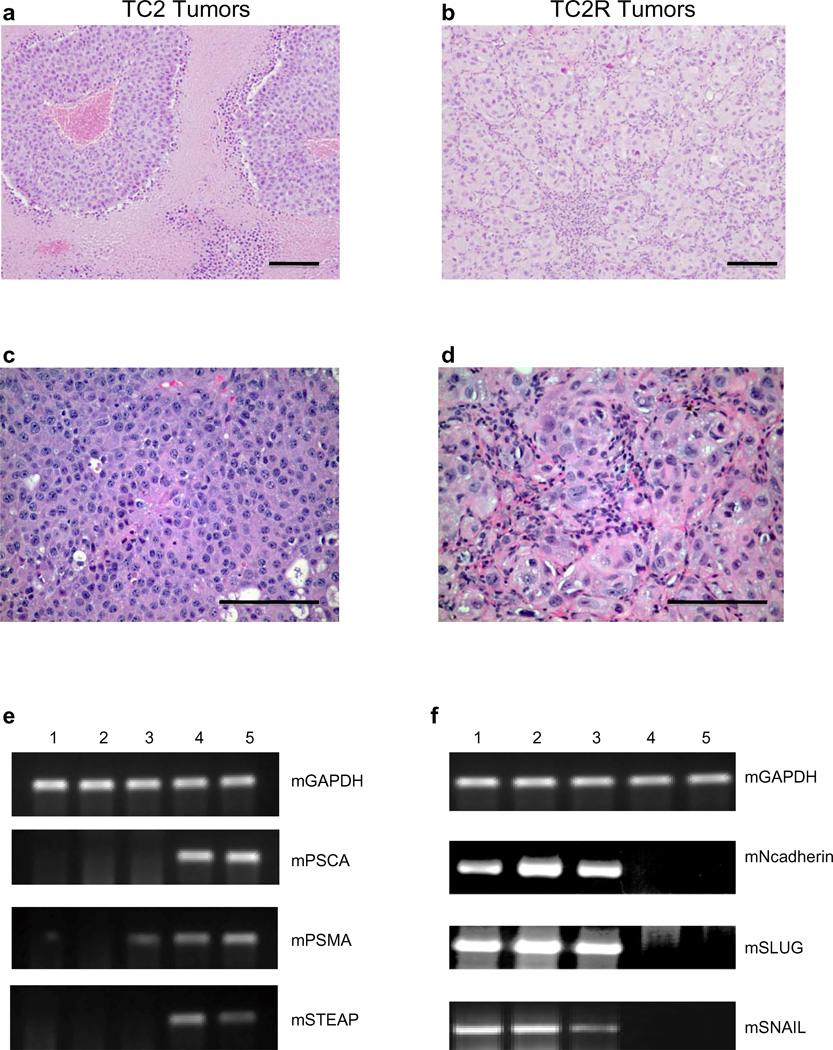
3 i.v. injections of ASEL (Figs.3c-f) typically induced initial regression with subsequent aggressive recurrence. Recurrent TC2R tumors were significantly different from parental TC2 tumors histologically with extensive interstitial lymphocyte infiltrates (Figs.4a-d). Similarly, TC2R tumors had lost, or reduced, expression of murine homologues of the human prostate-specific antigens in the ASEL (Fig.4e), as well as increased expression of N-Cadherin, SNAIL and SLUG, associated with an epithelial-mesenchymal-like transition (Fig.4f)29–32.
Figure 4. Sub optimal vaccination induces immune escape variants.
a-d. H&E staining of tumors (1.0cm diameter) from mice bearing 7d TC2 tumors treated i.v. with PBS (a,c) or ASEL (b,d) (days 7,9,11) (typically d20 for PBS (TC2) or d50 for ASEL (TC2R)). Scale bars, 100µm. e,f. Three TC2R tumors from mice treated with ASEL (1–3), one tumor from a mouse treated with PBS (4), as in a-d. above, and in vitro cultured TC2 cells (5) analyzed by rtPCR for murine prostate specific genes PSCA, PSMA and STEAP42 (e) or for N-Cadherin, SLUG or SNAIL29, 31, 32, 30 (f). *Cultured TC2 cells positive for N-Cadherin by nested PCR and weakly positive by Western Blot at lower levels than detected in TCR2 tumors.
Sequential vaccination cures recurrences
Viral Expressed Immune Escape Epitope Libraries (IEEL), constructed from TC2R tumors from mice treated with ASEL (Fig.5a), contained sequences of TC2R-characteristic genes (SNAIL and SLUG) (not shown) and transferred expression of TC2R-expressed N-Cadherin (Figs.4f,5b).
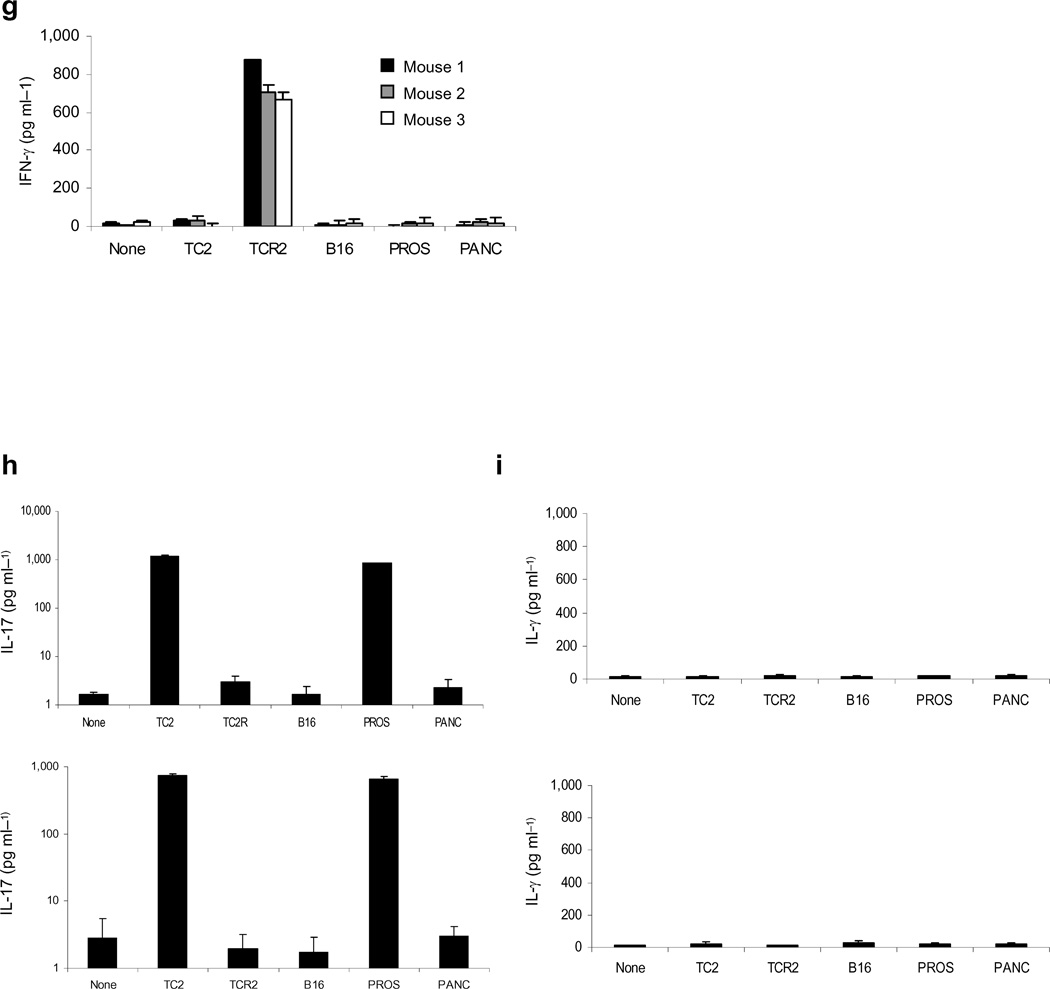
Figure 5. TC2R tumors can be treated with a second vaccination.
a. The IEEL contained cDNA from three TCR2 tumors cloned into VSV. b. Western blot for murine N-Cadherin from BHK cells infected with VSV (1), IEEL Reverse (2) or Direct (3) (MOI ~10). Equal loading confirmed by ß-actin probing. c. Survival of mock, or VSV-GFP,-vaccinated mice bearing 7d TC2 tumors treated with three i.v. injections of VSV-GFP or ASEL, either as viral supernatant (107pfu) or as virus pre-loaded onto CD8+ T cells [T(ASEL)]. d. Mice bearing 7d TC2 tumors were treated i.v. with VSV-GFP or ASEL (days 7,9,11). On days 25,27,29 mice initially treated with ASEL received i.v. IEEL virus pre-loaded onto CD8+ T cells [T(IEEL)]. e. VSV-vaccinated mice bearing 7d TC2 tumors were injected intravenously with VSV-GFP, ASEL or IEEL (107pfu) or with virus pre-loaded onto CD8+ T cells [T(ASEL)/T(IEEL)] (d 7,9,11). On days 20,22,24, surviving mice were treated i.v. with T cells loaded with IEEL [T(ASEL)/T(IEEL)] or ASEL [T(ASEL)/T(ASEL)]. f-i. Splenocytes from mice which either did (f,g) or did not (h,i) reject TC2/TC2R tumors following i.v. ASEL + T(IEEL) (d) or T(ASEL) + T(IEEL) (e) were co-cultured with lysates of TC2, TC2R, B16, normal mouse prostate or pancreas and assayed for (f,g) IL-17 or (h,i) IFN-γ. Results are from three survivor mice (f,g) or two mice which succumbed to TC2R tumors (h,i).
TC2 tumor-bearing mice treated with ASEL (days 7,9,11) followed by IEEL (days 25, 27 & 29) had no survival advantage over ASEL alone, probably due to neutralization of IEEL virus by NAb against VSV raised by injections of ASEL23 (Fig.5c). Consistent with reports that VSV is protected from NAb by loading onto PBL at low MOI 21–23, treatment of VSV-immune, TC2-bearing mice with PBL pre-loaded with ASEL restored therapy (Fig.5c). Therefore, we repeated sequential ASEL/IEEL treatment with IEEL virus pre-loaded onto PBL [T(IEEL)]. In Figs.5c,d, 4/7 mice treated with ASEL/T(IEEL) developed recurrences, but slower than with ASEL alone (Fig.5d). The 3 remaining mice never developed recurrences were tumor-free for over 100 days (Fig.5d) (p=0.001 compared to ASEL).
For prolongation of survival in the presence of NAb from the start of therapy, delivery of ASEL required pre-loading onto PBL but still induced recurrence (Fig.5e). Sequential T(ASEL)/T(IEEL) generated long-term cures with, in Fig.5e, only a single recurrence. Starting IEEL at day 20 (Fig.5e) consistently led to more long term cures compared to starting at day 27 (Fig.5d). The sequence of vaccination was also critical as neither T(IEEL) nor IEEL alone delayed growth of TC2 tumors (Fig.5e).
Sequential vaccination induces IFN-γ responses to TC2R
Long-term survivors of ASEL/IEEL (Figs.5d,e) developed a prostate-specific, Th17 response against TC2 and normal prostate (Fig.5f, 2&5) but not against TC2R cells (Fig.5f, 3). Conversely, splenocytes from survivors did not secrete IFN-γ in response to TC2 or normal prostates (Fig.5g, 2&5), but consistently produced IFN-γ in response to TC2R tumors (Fig.5g, 3). In contrast, non-survivors of ASEL/IEEL did not secrete IFN-γ in response to TC2R tumors (Figs.5g,i). Over two separate experiments, 7/14 (50%) of mice treated with ASEL (days 7,9,11), followed by IEEL (days 25,27,29), as in Fig.5d, were cured long term, compared to no cures (0%) with ASEL alone. In contrast, 0/14 mice (0%) treated with ASEL followed by IEEL, but with CD8+ T cell depletion (days 34,35), were cured.
Altered Self Libraries Afford Better Protection than Self Libraries
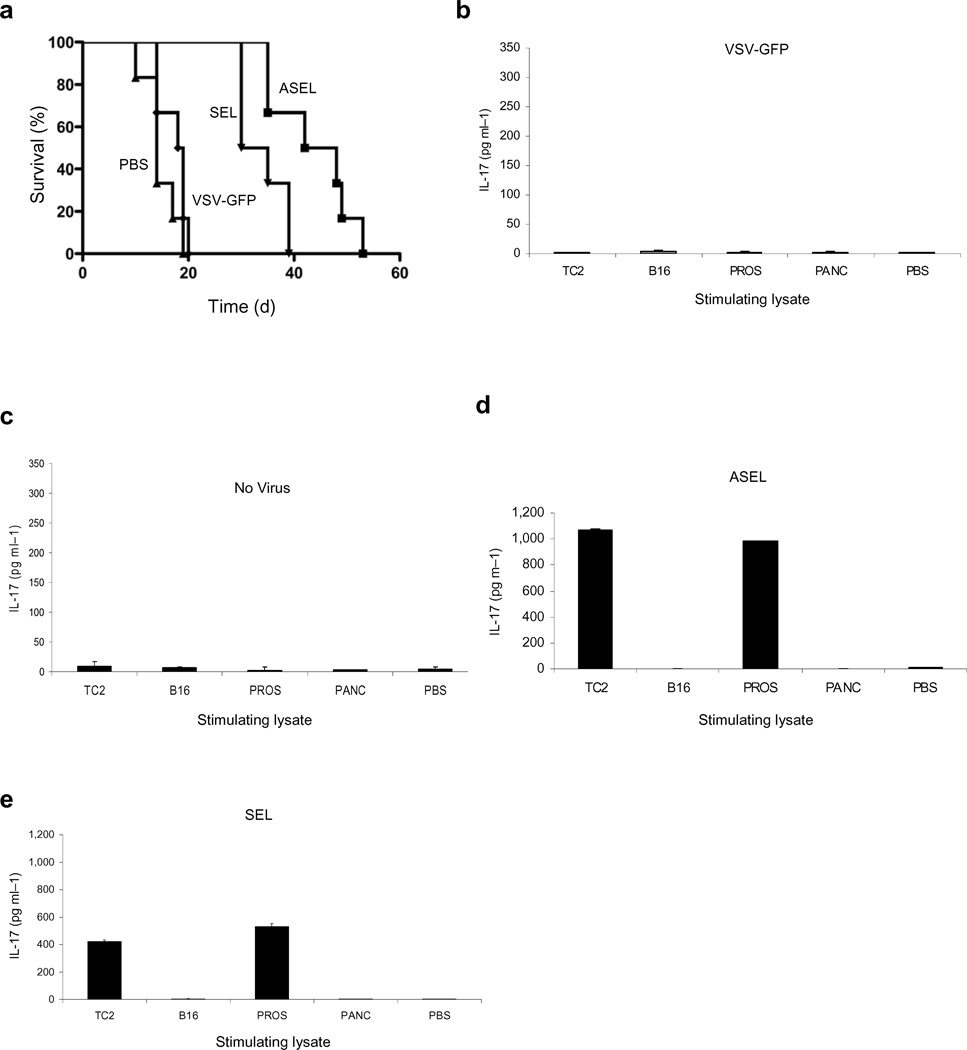
Finally, to investigate the contribution of responses against xenogeneic proteins, we constructed the Self Epitope expressed Library, SEL, from normal mouse prostate. The ASEL conferred significantly better protection against TC2 than the SEL (p=0.04), although both ASEL and SEL were better than PBS (p=0.0064, SEL vs PBS) or VSV-GFP (p=0.0029 SEL vs VSV-GFP) (Fig.6a). Similarly, in vitro stimulation/infection of splenocytes by the SEL induced lower levels of Th17 against prostate targets compared to the ASEL (Figs.6b-e).
Figure 6. Immunogenicity of Altered Self/Self Libraries.
a. Survival of mice bearing 7d TC2 tumors (n=7–8) injected intravenously with 107 pfu VSV-GFP, ASEL, or SEL (days 7,9,11). b-e. IL-17 secreted from lymph node cells/splenocytes infected with (b) VSV-GFP, (c) no virus, (d) ASEL or (e) SEL (MOI 1) for 2 weeks and co-cultured with lysates of TC2, B16, normal mouse prostate, pancreas or PBS.
DISCUSSION
We show here that cDNA libraries transferred into VSV express tissue-specific sequences and proteins (Figs.1b-d) and are immunologically functional (Fig.1e). Using human prostate cDNA to express altered self epitopes25, 26, 33, we generated VEASEL (Viral Expressed Altered Self Antigen/Epitope Library) virus. Intra-prostatic ASEL induced Th17 and IFN-γ responses against prostate antigens (Figs.2e,f) which were probably responsible for the associated prostatitis (Figs.2a-d)9, 34. In contrast, i.v. ASEL induced only Th17, and not IFN-γ, responses to prostate antigens (Fig.3b) and no overt signs of prostatitis9, 34 (Fig.3a), probably because of a lack of inflammatory signals within the prostate induced by intraprostatic injection.
We observed both a Th17 response against prostate antigens (Fig.3b) and cures of established TC2 tumors (Fig.3f) mediated by CD4+ T cells (Fig.3h), upon repeated i.v. injections of ASEL. It is tempting to speculate that a CD4+/Th17 response was responsible for these cures, although further depletion studies are required to confirm this. We believe that the direct oncolytic activity of VSV may have contributed to, but was not responsible for, these cures, because i.v. VSV-GFP had no similar efficacy (Fig.3f) and intra-tumoral ASEL was less effective against TC2 tumors than intravenous injection (Fig.3c). This is probably because intravenous injection of virus allows for significantly better access to the lymph nodes for cross priming of APC.
Emergence of treatment-resistant tumor variants is of great clinical significance1–3, 6. When vaccination was insufficient to clear tumors (Fig.3c), rapid re-growth of aggressive recurrent TC2R tumors was observed, which had induced new differentiation programs similar to an epithelial to mesenchymal type transition29–32 (Figs.4a-d,f). As a result, ASEL-treated mice could be vaccinated early during the immune-driven evolution of TC2 to TC2R tumors against emergence of aggressive, TC2R tumors by Virus Expressed IEEL, which transferred expression of TC2R, as opposed to TC2, characteristic genes (Figs.4f, 5a,b,e), using PBL loading to protect IEEL viruses from NAb21–23, 35, 36 (Figs.5c,d). Efficacy depended upon the sequence in which the libraries were given (Fig.5E), early treatment with IEEL (Figs.5d,e), and upon two distinct, but synergistic, immune re-activities differing in antigen specificity and effector phenotypes (Fig.5f-i). The first ASEL-primed response (Th17-associated, CD4-dependent, TC2/prostate specific, TC2R ‘blind’) forced TC2 tumors to evolve into a phenotype against which the second, IEEL-primed response (IFN-γ-associated, CD8-dependent, prostate ‘blind’, TC2R specific), could clear recurrent tumors as they emerged.
We observed poorer protection with the SEL (self antigens) compared to the ASEL (xenogeneic, altered self antigens) (Fig.6a) and weaker stimulation against prostate targets by the SEL in vitro (Fig.6b-e). Significantly, the ASEL protected mice against TC2 tumors, but not B16 melanomas, and failed to stimulate splenocyte reactivity against non-prostate targets (Fig.5). This suggests that immune reactivity generated by the ASEL is directed not simply against xenogeneic household proteins (on B16 cells and normal pancreas) but against lineage-specific antigens - possibly reflecting a difference in the levels of T cell precursors to these two classes of antigens which survive both thymic and peripheral selection. Conversely, since xenogeneic antigens associated with rejection of allogeneic transplants include minor histocompatibility antigens (not lineage specific), the antigenic targets of rejection of TC2 tumors may turn out to be more widely expressed than simply in the prostate.
In summary, we show here that it is possible to vaccinate mice against established tumors using a wide repertoire of self7, 13, or near self25, 26, 33, epitopes encoded by a cDNA library expressed from the platform of a highly immunogenic virus. This allows for in vivo immune selection of TAA relevant for tumor rejection, but does not require their identification. In addition, systemic delivery of VSV-based libraries is possible, prevents damage inherent in delivering vectors directly to normal tissues8, 9 and did not induce autoimmunity.
METHODS
Cells and viruses
TRAMP-C2 (TC2) cells are derived from a prostate tumor that arose in a TRAMP mouse, grow in an androgen independent manner and are routinely grown as tumors in C57BL/6 male mice9. Murine B16ova melanoma cells (H2-Kb) were derived from B16 cells by transduction with a cDNA encoding the chicken ovalbumin gene 43.
cDNA from normal human prostate (Biochain, Hayward, CA) was amplified from the BioExpress shuttle vector by PCR and cloned into the VSV genomic plasmid pVSV-XN2 44 between the G and L genes. VSV-cDNA libraries were generated from the human prostate cDNA by size fractionating PCR cDNA molecules to below 4kbp, since lower sized cDNA inserts were associated with both higher viral titers and lower proportions of Defective Interfering particles. The complexity of the ASEL cDNA library cloned into pVSV-XN2 between the Xho1-Nhe1- sites was 4.75x106 colony forming units (at dilutions of 10−6 and 10−5 there were 5 and 45 colonies respectively). Of 20 colonies picked at random, 3 had no insert, 5 had an insert of less than 0.5kbp and 12 had inserts between 0.5kbp and 4kbp. Virus was generated from BHK cells by co-transfection of pVSV-XN2-cDNA library DNA along with plasmids encoding viral genes as described in44. Virus was expanded by a single round of infection of BHK cells and purified by sucrose gradient centrifugation.
Additional cDNA libraries from tumor cells (TC2R, B16ova, B16) were cloned into the pCMV.SPORT6 vector (Invitrogen, CA), amplified by PCR, and cloned into pVSV-XN2 before being packaged and amplified.
VSV-GFP was generated by cloning the cDNA for GFP into pVSV-XN2, as described44. Monoclonal VSV-GFP was plaque purified on BHK-21 cells and concentrated by sucrose gradient centrifugation. Titers were measured by plaque assays on BHK-21 cells44.
Histopathology
Prostates or tumors were fixed in 10% Formalin in PBS, paraffin embedded and sectioned. H&E-stained sections were prepared for analysis of tissue destruction and gross infiltrate.
Preparation of lymphocytes
The OT-I mouse strain, on a C57BL/6 background (H-2Kb), expresses a transgenic T-cell receptor Vα2 specific for the SIINFEKL peptide of ovalbumin in the context of MHC class I, H-2Kb 28. Spleen and lymph nodes from OT-I or C57BL/6 mice were crushed through a 100µm filter to prepare single-cell suspensions. RBC were removed by a 2-min incubation in ACK buffer. CD8+ T cells were isolated using the MACS CD8α (Ly-2) Microbead magnetic cell sorting system (Miltenyi Biotec). FACS analysis demonstrated cultures typically of >98% CD8+ T cells, <2% CD4+ T cells, <0.1% NK1.1+ve cells. Viable cells were purified using Lympholyte-M (Cedarlane Laboratories).
Loading of lymphocytes with VSV
CD8+ lymphocytes were pelleted and incubated with VSV in 100µl for 4h at 4°C as described in23. Cells were washed x3 in ice cold PBS and used directly for in vivo adoptive transfer23.
In vivo studies
All procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee. C57BL/6 mice were purchased from Jackson Laboratories at 6–8 weeks of age. To establish subcutaneous tumors, 2×106 TrampC2 cells in 100µl PBS were injected into the flank. Intra-tumoral injections were performed in a total of 50µl. Intravenous injections of virus were administered in 100µl volumes. For adoptive transfer experiments, mice were i.v. administered normal lymphocytes, or lymphocytes pre-loaded with VSV, typically at 2x106 cells in 100µl. For survival studies, tumor diameter in two dimensions, was measured three times weekly using calipers, and mice were killed when tumor size was approximately 1.0x1.0 cm in two perpendicular directions.
Immune cell depletions were performed by i.p. injections (0.1mg per mouse) of anti-CD8 (Lyt 2.43), anti-CD4 (GK1.5), (Monoclonal Antibody Core Facility, Mayo Clinic); anti-NK (anti-asialo-GM-1, Cedarlane) and IgG control (ChromPure Rat IgG, Jackson ImmunoResearch). FACS analysis of spleens and lymph nodes confirmed subset specific depletions.
Reverse transcriptase PCR
RNA was prepared from cells with the QIAGEN RNA extraction kit. 1µg total cellular RNA was reverse transcribed in a 20µl volume using oligo-(dT) as a primer. A cDNA equivalent of 1ng RNA was amplified by PCR with gene specific primers (details of primers on request).
Protein expression
Expression of human PSA was detected with the Prostate Specific Antigen antibody ab53774 (abcam) (rabbit polyclonal) and murine N-Cadherin with the 32/N-Cadherin mouse anti-N-Cadherin antibody (BD Biosciences) using standard Western Blot procedures.
ELISA analysis
Typically, 106 splenocytes were incubated at 37°C with freeze thaw lysates from tumor cells, or normal tissues, in triplicate, every 24 hours for 3 days. 48hrs later, cell-free supernatants were tested by specific ELISA for IL-17 (R&D Systems) or IFN-γ (BD OptEIA IFN-γ; BD Biosciences).
Statistics
Survival data was analyzed using the log rank test, and the two-sample unequal variance student’s t test analysis was applied for in vitro assays. Statistical significance was determined at the level of p < 0.05.
ACKNOWLEDGEMENTS
We thank Toni Higgins for expert secretarial assistance. This work was supported by The Richard M. Schulze Family Foundation, the Mayo Foundation, Cancer Research UK, the National Institutes of Health grants R01 CA107082, R01 CA130878, and R01 CA132734, and a grant from Terry and Judith Paul.
REFERENCES
- 1.Drake CG, Jaffee EM, Pardoll DM. Mechanisms of immune evasion by tumors. Adv. Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 2.Koos D, et al. Tumor vaccines in 2010: need for integration. Cell. Immunol. 2010;263:138–147. doi: 10.1016/j.cellimm.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Le DT, Pardoll DM, Jaffee EM. Cellular vaccine approaches. Cancer J. 2010;16:304–310. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parato KA, Lichty BD, Bell JC. Diplomatic immunity: turning a foe into an ally. Curr. Opin. Mol. Ther. 2009;11:13–21. [PubMed] [Google Scholar]
- 5.Prestwich RJ, et al. Oncolytic viruses: a novel form of immunotherapy. Expert Rev. Anticancer Ther. 2008;8:1581–1588. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 7.Daniels G, et al. A simple method to cure established tumors by inflammatory killing of normal cells. Nat. Biotechnol. 2004;22:1125–1132. doi: 10.1038/nbt1007. [DOI] [PubMed] [Google Scholar]
- 8.Kottke T, et al. Antitumor immunity can be uncoupled from autoimmunity following heat shock protein 70-mediated inflammatory killing of normal pancreas. Cancer Res. 2009;69:7767–7774. doi: 10.1158/0008-5472.CAN-09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kottke T, et al. Induction of hsp70-mediated, Th17 autoimmunity can be exploited as immunotherapy for metastatic prostate cancer. Cancer Res. 2007;67:11970–11979. doi: 10.1158/0008-5472.CAN-07-2259. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Perez L, et al. Killing of normal melanocytes, combined with hsp70 and CD40L expression, cures large established melanomas. J. Immunol. 2006;177:4168–4177. doi: 10.4049/jimmunol.177.6.4168. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Perez L, et al. Potent selection of antigen loss variants of B16 melanoma following inflammatory killing of melanocytes in vivo. Cancer Res. 2005;65:2009–2017. doi: 10.1158/0008-5472.CAN-04-3216. [DOI] [PubMed] [Google Scholar]
- 12.Ferrone S. Immunotherapy dispenses with tumor antigens. Nat. Biotechnol. 2004;22:1096–1098. doi: 10.1038/nbt0904-1096. [DOI] [PubMed] [Google Scholar]
- 13.Turk MJ, Wolchok JD, Guevara-Patino JA, Goldberg SM, Houghton AN. Multiple pathways to tumor immunity and concomitant autoimmunity. Immunol. Rev. 2002;188:122–135. doi: 10.1034/j.1600-065x.2002.18811.x. [DOI] [PubMed] [Google Scholar]
- 14.Braxton CL, Puckett SH, Mizel SB, Lyles DS. Protection against lethal vaccinia virus challenge by using an attenuated matrix protein mutant vesicular stomatitis virus vaccine vector expressing poxvirus antigens. J. Virol. 2010;84:3552–3561. doi: 10.1128/JVI.01572-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobleigh MA, Bouonocore L, Uprichard SL, Rose JK, Robek MD. A vesicular stomatitis virus-based hepatitis B virus vaccine vector provides protection against challenge in a single dose. J. Virol. 2010;84:7513–7522. doi: 10.1128/JVI.00200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geisbert TW, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J. Virol. 2009;83:7296–7304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz JA, et al. Potent vesicular stomatitis virus-based avian influenza vaccines provide long-term sterilizing immunity against heterologous challenge. J. Virol. 2010;84:4611–4618. doi: 10.1128/JVI.02637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridle BW, et al. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol. Ther. 2009;17:1814–1821. doi: 10.1038/mt.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridle BW, et al. Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther. 2010;18:1430–1439. doi: 10.1038/mt.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz RM, et al. Oncolytic immunovirotherapy for melanoma using Vesicular Stomatitis Virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 21.Ilett EJ, et al. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 2009;16:689–699. doi: 10.1038/gt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prestwich RJ, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin. Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao J, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat. Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- 24.Flatz L, et al. Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8+ T cell immunity. Nat. Med. 2010;16:339–345. doi: 10.1038/nm.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guevara-Patino JA, et al. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J. Clin. Invest. 2006;116:1382–1390. doi: 10.1172/JCI25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guevara-Patino JA, Turk MJ, Wolchok JD, Houghton AN. Immunity to cancer through immune recognition of altered self: studies with melanoma. Adv. Cancer Res. 2003;90:157–177. doi: 10.1016/s0065-230x(03)90005-4. [DOI] [PubMed] [Google Scholar]
- 27.Overwijk W, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogquist KA, et al. T cell receptor antagonistic peptides induce positive selection. Cell. 1994;76:17. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 29.Berx G, van Roy R. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb. Perspect. Biol. 2009;1:a003129. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moody SE, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Santisteban M, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–2895. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motrich RD, Maccioni M, Riera CM, Rivero VE. Autoimmune prostatitis: State of the art. Scand. J. Immunol. 2007;66:217–227. doi: 10.1111/j.1365-3083.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- 35.Thorne SH, Contag CH. Integrating the biological characteristics of oncolytic viruses and immune cells can optimize therapeutic benefits of cell-based delivery. Gene Ther. 2008;15:753–758. doi: 10.1038/gt.2008.42. [DOI] [PubMed] [Google Scholar]
- 36.Willmon C, et al. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol. Ther. 2009;17:1667–1676. doi: 10.1038/mt.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumas F, et al. Molecular expression of PSMA mRNA and protein in primary renal tumors. Int. J. Cancer. 1999;80:799–803. doi: 10.1002/(sici)1097-0215(19990315)80:6<799::aid-ijc1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 38.Kiessling A, et al. Advances in specific immunotherapy for prostate cancer. Eur. Urol. 2008;53:694–708. doi: 10.1016/j.eururo.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 39.Reiter RE, et al. Prostate stem cell antigen: A cell surface marker overexpressed in prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61:3760–3769. [PubMed] [Google Scholar]
- 41.Tanaka S, et al. Target killing of carcinoembryonic antigen (CEA) - producing cholangiocarcinoma cells by polyamidoamine dendrimer-mediated transfer of an Epstein-Barr virus (EBV)-based plasmid vector carrying the CEA promoter. Cancer Gene Ther. 2000;7:1421–1249. doi: 10.1038/sj.cgt.7700219. [DOI] [PubMed] [Google Scholar]
- 42.Yang D, Holt GE, Velders MP, Kwon ED, Kast WM. Murine six-transmembrane epithelial antigen of the prostate, prostate stem cell antigen, and prostate-specific membrane antigen: Prostate-specific cell-surface antigens highly expressed in prostate cancer of transgenic adenocarcinoma mouse prostate mice. Cancer Res. 2001;61:5857–5860. [PubMed] [Google Scholar]
- 43.Sanchez-Perez L, et al. Synergy of adoptive T-cell therapy with intratumoral suicide gene therapy is mediated by host NK cells. Gene Ther. 2007;14:998–1009. doi: 10.1038/sj.gt.3302935. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: Application for treatment of malignant disease. J. Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]