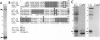Abstract
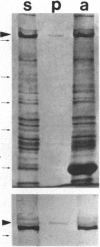
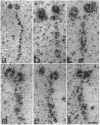
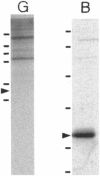
Organelles in the axoplasm from the squid giant axon move along exogenous actin filaments toward their barbed ends. An approximately 235-kDa protein, the only band recognized by a pan-myosin antibody in Western blots of isolated axoplasmic organelles, has been previously proposed to be a motor for these movements. Here, we purify this approximately 235-kDa protein (p235) from axoplasm and demonstrate that it is a myosin, because it is recognized by a pan-myosin antibody and has an actin-activated Mg-ATPase activity per mg of protein 40-fold higher than that of axoplasm. By low-angle rotary shadowing, p235 differs from myosin II and it does not form bipolar filaments in low salt. The amino acid sequence of a 17-kDa protein that copurifies with p235 shows that it is a squid optic lobe calcium-binding protein, which is more similar by amino acid sequence to calmodulin (69% identity) than to the light chains of myosin II (33% identity). A polyclonal antibody to this light chain was raised by using a synthetic peptide representing the calcium binding domain least similar to calmodulin. We then cloned this light chain by reverse transcriptase-PCR and showed that this antibody recognizes the bacterially expressed protein but not brain calmodulin. In Western blots of sucrose gradient fractions, the 17-kDa protein is found in the organelle fraction, suggesting that it is a light chain of the p235 myosin that is also associated with organelles.
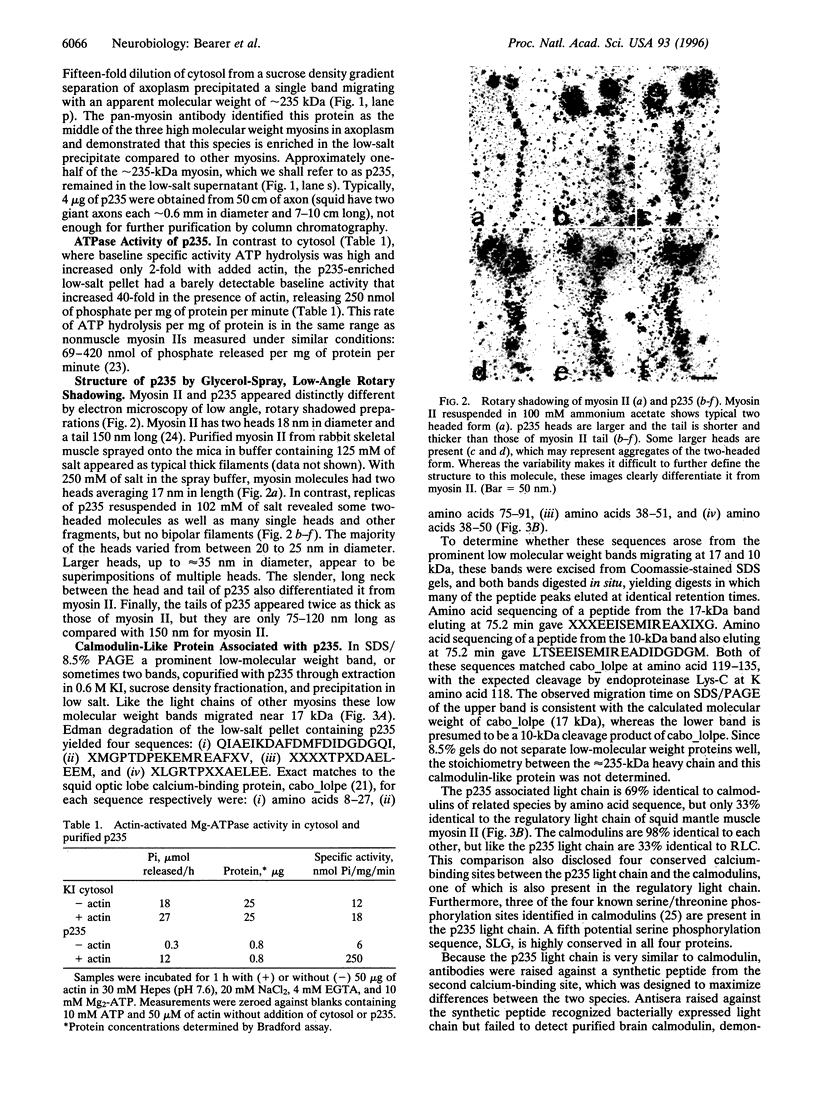
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearer E. L., DeGiorgis J. A., Bodner R. A., Kao A. W., Reese T. S. Evidence for myosin motors on organelles in squid axoplasm. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11252–11256. doi: 10.1073/pnas.90.23.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer E. L., DeGiorgis J. A., Medeiros N. A., Reese T. S. Actin-based motility of isolated axoplasmic organelles. Cell Motil Cytoskeleton. 1996;33(2):106–114. doi: 10.1002/cm.970330202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer E. L. Direct observation of actin filament severing by gelsolin and binding by gCap39 and CapZ. J Cell Biol. 1991 Dec;115(6):1629–1638. doi: 10.1083/jcb.115.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement W. M., Hasson T., Wirth J. A., Cheney R. E., Mooseker M. S. Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell types. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6549–6553. doi: 10.1073/pnas.91.14.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beushausen S., Kladakis A., Jaffe H. Kinesin light chains: identification and characterization of a family of proteins from the optic lobe of the squid Loligo pealii. DNA Cell Biol. 1993 Dec;12(10):901–909. doi: 10.1089/dna.1993.12.901. [DOI] [PubMed] [Google Scholar]
- Burridge K., Bray D. Purification and structural analysis of myosins from brain and other non-muscle tissues. J Mol Biol. 1975 Nov 25;99(1):1–14. doi: 10.1016/s0022-2836(75)80154-9. [DOI] [PubMed] [Google Scholar]
- Cheney R. E., O'Shea M. K., Heuser J. E., Coelho M. V., Wolenski J. S., Espreafico E. M., Forscher P., Larson R. E., Mooseker M. S. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993 Oct 8;75(1):13–23. doi: 10.1016/S0092-8674(05)80080-7. [DOI] [PubMed] [Google Scholar]
- Cheney R. E., Riley M. A., Mooseker M. S. Phylogenetic analysis of the myosin superfamily. Cell Motil Cytoskeleton. 1993;24(4):215–223. doi: 10.1002/cm.970240402. [DOI] [PubMed] [Google Scholar]
- Collins K., Sellers J. R., Matsudaira P. Calmodulin dissociation regulates brush border myosin I (110-kD-calmodulin) mechanochemical activity in vitro. J Cell Biol. 1990 Apr;110(4):1137–1147. doi: 10.1083/jcb.110.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A., Offer G., Burridge K. Electron microscopy of myosin molecules from muscle and non-muscle sources. Proc R Soc Lond B Biol Sci. 1976 Mar 30;193(1110):45–53. doi: 10.1098/rspb.1976.0030. [DOI] [PubMed] [Google Scholar]
- Fernandez J., DeMott M., Atherton D., Mische S. M. Internal protein sequence analysis: enzymatic digestion for less than 10 micrograms of protein bound to polyvinylidene difluoride or nitrocellulose membranes. Anal Biochem. 1992 Mar;201(2):255–264. doi: 10.1016/0003-2697(92)90336-6. [DOI] [PubMed] [Google Scholar]
- Goodson H. V., Spudich J. A. Molecular evolution of the myosin family: relationships derived from comparisons of amino acid sequences. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):659–663. doi: 10.1073/pnas.90.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J. F. Amino acid sequence of a low molecular weight, high affinity calcium-binding protein from the optic lobe of the squid Loligo pealei. J Biol Chem. 1989 May 5;264(13):7202–7209. [PubMed] [Google Scholar]
- Kawasaki H., Emori Y., Suzuki K. Production and separation of peptides from proteins stained with Coomassie brilliant blue R-250 after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1990 Dec;191(2):332–336. doi: 10.1016/0003-2697(90)90227-z. [DOI] [PubMed] [Google Scholar]
- Kelley C. A., Takahashi M., Yu J. H., Adelstein R. S. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem. 1993 Jun 15;268(17):12848–12854. [PubMed] [Google Scholar]
- Kuznetsov S. A., Langford G. M., Weiss D. G. Actin-dependent organelle movement in squid axoplasm. Nature. 1992 Apr 23;356(6371):722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Langford G. M., Kuznetsov S. A., Johnson D., Cohen D. L., Weiss D. G. Movement of axoplasmic organelles on actin filaments assembled on acrosomal processes: evidence for a barbed-end-directed organelle motor. J Cell Sci. 1994 Aug;107(Pt 8):2291–2298. doi: 10.1242/jcs.107.8.2291. [DOI] [PubMed] [Google Scholar]
- McCollum D., Balasubramanian M. K., Pelcher L. E., Hemmingsen S. M., Gould K. L. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J Cell Biol. 1995 Aug;130(3):651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metuzals J., Tasaki I. Subaxolemmal filamentous network in the giant nerve fiber of the squid (Loligo pealei L.) and its possible role in excitability. J Cell Biol. 1978 Aug;78(2):597–621. doi: 10.1083/jcb.78.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. R., Lasek R. J. Stable polymers of the axonal cytoskeleton: the axoplasmic ghost. J Cell Biol. 1982 Jan;92(1):192–198. doi: 10.1083/jcb.92.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. Purification of nonmuscle myosins. Methods Enzymol. 1982;85(Pt B):331–356. doi: 10.1016/0076-6879(82)85033-7. [DOI] [PubMed] [Google Scholar]
- Quadroni M., James P., Carafoli E. Isolation of phosphorylated calmodulin from rat liver and identification of the in vivo phosphorylation sites. J Biol Chem. 1994 Jun 10;269(23):16116–16122. [PubMed] [Google Scholar]
- Reinhard J., Scheel A. A., Diekmann D., Hall A., Ruppert C., Bähler M. A novel type of myosin implicated in signalling by rho family GTPases. EMBO J. 1995 Feb 15;14(4):697–704. doi: 10.1002/j.1460-2075.1995.tb07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S., Bechtold R. Kinesin is bound with high affinity to squid axon organelles that move to the plus-end of microtubules. J Cell Biol. 1992 Oct;119(2):389–399. doi: 10.1083/jcb.119.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A., Schnapp B. J., Reese T. S., Sheetz M. P. The role of kinesin and other soluble factors in organelle movement along microtubules. J Cell Biol. 1988 Nov;107(5):1785–1792. doi: 10.1083/jcb.107.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See Y. P., Metuzals J. Purification and characterization of squid brain myosin. J Biol Chem. 1976 Dec 10;251(23):7682–7689. [PubMed] [Google Scholar]
- Sheldon A., Head J. F. Calcium-binding properties of two high affinity calcium-binding proteins from squid optic lobe. J Biol Chem. 1988 Oct 5;263(28):14384–14389. [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Takahashi M., Kawamoto S., Adelstein R. S. Evidence for inserted sequences in the head region of nonmuscle myosin specific to the nervous system. Cloning of the cDNA encoding the myosin heavy chain-B isoform of vertebrate nonmuscle myosin. J Biol Chem. 1992 Sep 5;267(25):17864–17871. [PubMed] [Google Scholar]
- Tan J. L., Ravid S., Spudich J. A. Control of nonmuscle myosins by phosphorylation. Annu Rev Biochem. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980 May;71(2):95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Reese T. S., Sheetz M. P. Movement of organelles along filaments dissociated from the axoplasm of the squid giant axon. Cell. 1985 Feb;40(2):449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Szent-Györgyi A. G. An immunological approach to myosin light-chain function in thick filament linked regulation. 1. Characterization, specificity, and cross-reactivity of anti-scallop myosin heavy- and light-chain antibodies by competitive, solid-phase radioimmunoassay. Biochemistry. 1981 Mar 3;20(5):1176–1187. doi: 10.1021/bi00508a020. [DOI] [PubMed] [Google Scholar]