Abstract
Purpose
To determine if young children who stutter have a basic motor timing and/or coordination deficit.
Method
Between-hand coordination and variability of rhythmic motor timing were assessed in 17 children who stutter (aged 4–6 years) and 13 age-matched controls. Children clapped in rhythm with a metronome with a 600 ms inter-beat interval and then attempted to continue to match this target rate for 32 unpaced claps.
Results
Children who stutter did not significantly differ from children who are typically developing on mean clapping rate or number of usable trials produced; however, they produced remarkably higher variability levels of inter-clap interval. Of particular interest was the bimodal distribution of the stuttering children on clapping variability. One subgroup of children who stutter clustered within the normal range, but 60% of the children who stutter exhibited timing variability that was greater than the poorest performing non-stuttering child. Children who stutter were not more variable in measures of coordination between the two hands (mean and median phase difference between hands).
Conclusion
We infer that there is a subgroup of young stuttering children who exhibit a non-speech motor timing deficit and discuss this result as it pertains to recovery or persistence of stuttering.
Introduction
Stuttering is a disorder involving breakdowns in the speech motor system. Most theories of the causes of stuttering postulate that many factors are involved in producing these motor breakdowns including genetic, linguistic, and psychosocial contributors (e.g., Conture, 1990; Smith, 1990, 1999; Starkweather, Gottwald & Halfond, 1993; Van Riper, 1982; Wall & Myers, 1995). Despite the complex interaction of underlying factors in accounts of the onset and development of stuttering, it is clear that abnormal speech motor output is an essential component of stuttering. During the disfluencies that characterize stuttering, the speech motor system fails to generate and/or send the motor commands to muscles that are necessary for fluent speech to continue. Thus disfluent intervals of speech in children and adults who stutter are clearly associated with breakdowns in the precise spatial and temporal control of movement necessary for fluent speech production. Also striking are findings that people who stutter often differ from controls in terms of the variability, speed, and relative timing of their articulatory movements when producing perceptually fluent speech (Kleinow & Smith, 2000; Max & Gracco, 2005; McClean & Runyan, 2000; McClean, Tasko & Runyan, 2004; Zimmermann, 1980). These studies provide evidence for persistent motor timing and coordination deficits that are present in the speech motor control systems of people who stutter, even when there are no perceptible stuttering behaviors in their speech.
Many investigators have hypothesized that people who stutter have a general motor deficit (Max, Caruso, & Gracco, 2003; Webster, 1985; Zelaznik, Smith, Franz, & Ho, 1997) or in some accounts, more specifically, a timing deficit (Boutsen, Brutten, & Watts, 2000; Kent, 1983) that contributes to the development and maintenance of the disorder. The underlying premise of this hypothesis is that motor control processes for speech, nonspeech oral, and limb movement share underlying neural substrates. Many neuroimaging studies have provided evidence for this overlap by identifying regions of the brain that are activated during speech and other motor behavior. Binkofski and Buccino (2004) observed activation in Broca’s area during speech production and complex hand movements. Similarly, increased blood flow to Broca’s area has been observed during performance of grasping gestures (Rizzolatti, Fadiga, Maelli, Bettinardi, & Paulesu, 1996). Regions near Broca’s area have also been identified as active during both rhythmic oral and limb movement (Bengtsson, Ehrsson, Forssberg, & Ullen, 2005).
Studies of speech and limb movements produced by normally fluent speakers have provided evidence of common characteristics and/or entrainment. For example, Smith, McFarland, and Weber (1986) found that when normally fluent participants spoke and tapped a finger at the same time, the pace and amplitude of the two activities spontaneously became coordinated. In addition, several studies of normally fluent participants have provided evidence that intra-subject timing, variability, and accuracy are similar for speech and non-speech rhythmic movements (Bengtsson et al., 2005; Franz, Zelaznik, & Smith, 1992; Tingley & Allen, 1975), suggesting that individuals have a single timing system that is utilized in performance of many different rhythmic motor tasks. Evidence of timing similarities across limb and speech tasks also has been observed in individuals who stutter. Cooper and Allen (1977) found that subjects who were good timers in comparison to other subjects for finger tapping were also good timers for sentence repetition and that this correlation was stronger in subjects who stuttered than in nonstuttering controls.
Given these arguments, a generalized motor deficit in stuttering hypothetically would affect nonspeech oral movements as well as limb movements. However, studies comparing timing and coordination variability in oral motor behaviors (e.g., open/close oral movements, syllable repetition) and limb motor performance of individuals who stutter to that of individuals who do not stutter have produced equivocal results. In considering these mixed results it is notable that many different kinds of motor behaviors have been studied, from those involving processes postulated in the general motor control literature to recruit intrinsic (or emergent) timing processes to others, presumably involving extrinsic (or event) timing mechanisms (Spencer, Zelaznik, Diedrichsen, & Ivry, 2003). Some studies report no differences between individuals who stutter and controls in timing or coordination variability (e.g., Hulstijn, Summers, van Lieshout, & Peters, 1992; Max & Yudman, 2003; Zelaznik, Smith, & Franz, 1994). Others have reported that participants who stutter were more variable in timing and/or coordination measures (Boutsen, Brutten, & Watts, 2000; Cooper & Allen, 1977; Ward, 1997; Zelaznik et al., 1997), and one investigation of self-paced oral and finger movements demonstrated that adults who stutter were less variable timers than the control group (Brown, Zimmermann, Linville, & Hegmann, 1990). In discussions of these mixed results, the issue of task complexity is often raised (Max & Yudman, 2003; Zelaznik et al. 1997). It is possible that differences in nonspeech motor coordination and timing, as well as differences in speech movement variability (Kleinow & Smith, 2000) are more obvious in people who stutter when the task is more demanding. In fact, many studies of more complex tasks have revealed differences in the non-speech motor timing of adults who stutter. For example, when a finger sequencing task was used, people who stutter produced slower response initiations, made more errors (Webster, 1986), and were more variable than controls (Smits-Bandstra, De Nil, & Rochon, 2006).
Increasing task difficulty by requiring participants to produce bimanual, rather than unimanual movements also has resulted in observations of greater variability in performance in people who stutter. Differences in relative phase variability (a measure of inter-effector coordination that captures the relative timing of two effectors on repeated cycles of a rhythmic behavior) between adults who stutter and controls have been found with a bimanual finger tapping task (Zelaznik, Smith, Franz, & Ho, 1997) and a bimanual hand tapping task (Hulstijn, Summers, van Lieshout, & Peters, 1992). Hulstijn et al. (1992) also found greater variability in adults who stutter than in normally fluent controls when they performed a dual task of simultaneously synchronizing speech and hand movement to a metronome. These differences were not found when the same participants completed less complex timing tasks.
If a more generalized motor coordination and/or timing deficit contributes to the onset and development of stuttering, we should observe differences in performance of a nonspeech motor task in young children who stutter compared to their normally fluent peers. Therefore in the present study, we examine performance of 4 to 6-year-old children who stutter on a clapping task, which requires bimanual control. We chose this task because it has ecological validity, that is, young children clap their hands spontaneously and would be able to do the task. Furthermore, this task requires bimanual coordination, which based on earlier studies of adults, should increase the probability of observing differences between stuttering and nonstuttering groups. We also elected to use a classic paradigm from the motor behavior literature (Wing & Kristofferson, 1973), requiring the children to produce a series of paced claps in time to a metronome, followed by a continuation phase of unpaced claps.
We are aware of three earlier studies of the non-speech motor timing or coordination abilities of children who stutter. Westphal (1933) assessed 26 boys diagnosed as stuttering and 26 matched control participants (aged 8–18 years) on a battery of motor tests. She included grip strength, eye-hand coordination, hand writing while blindfolded, and hand steadiness while balancing a plate. She found no differences in the boys who stuttered compared to the nonstuttering boys on any of these measures. Riley and Riley (1980) administered tests of motor and psycholinguistic abilities and stuttering severity in 76 children who stutter aged 5–12 years. They completed a factor analysis of 19 variables that yielded four statistically useful factors implicating linguistic, oral motor, and auditory processing abilities as significant underlying components in stuttering. Finally, Howell, Au-Yeung, and Rustin (1997) reported a pilot investigation of children aged 9–10-years who stuttered and controls on a sinusoidal lip tracking task. For the children who could complete the task, they decomposed lip tracking variance into central clock and implementation variance according to the Wing and Kristoffersson (1973) model. They found that the children who stutter performed the task as well as the nonstuttering children on timing accuracy (equal clock variances), but that there was a trend for implementation variance to be higher in the stuttering group. Thus there is scant evidence concerning the issue of whether differences in nonspeech motor performance are a characteristic of children who stutter.
The existence of an impairment in a motor process shareable by speech and non-speech motor control systems in young children who stutter in comparison to children who do not stutter would be a critical piece of evidence needed to evaluate the hypothesis outlined above, that a generalized difference in motor capacities contributes to onset and development of stuttering in early childhood. Evidence of timing and or coordination deficits seen in the clapping movements of young children near the onset of stuttering would also further support the hypothesized common substrates for the neural processes controlling speech and non-speech motor behaviors.
Method
Participants
Participants were 17 children who stutter and 13 children who do not stutter. Consistent with methods used by Yairi and Ambrose (1999), a child was diagnosed as stuttering if he/she produced three or more stutter-like disfluencies (i.e. whole or part-word repetitions or disfluent phonations) per 100 syllables in two spontaneous language samples (one with a parent and one with an experimenter). Disfluencies were identified and coded using the methods described by Yairi and colleagues (Yairi & Ambrose, 1999). The group of stuttering children consisted of 13 males and 4 females between the ages of 4;0 and 6;10 (M = 5;0). The nonstuttering children were 9 males and 4 females between the ages of 4;0 and 6;2 (M = 4;9). The Handedness Inventory (subset of 5 tests adapted from Oldfield, 1971) identified 1 child in each group as left handed. Of the children who stutter, eight were currently undergoing speech therapy targeting fluency, while one was in language therapy.
Screening/Testing Procedures
The children spoke standard American English as their first language and passed a pure tone hearing screening (20 dB HL at 500, 100020 dB HL at 500, 2000 and 4000 Hz). The parents of the children reported negative histories for motor delays, neurological problems, and serious illnesses. As an index of socio-economic status, the mother’s highest year of education was recorded (4-high school, 5-partial college, 6-college graduate, 7-post graduate work; Hollingshead, 1975). The median educational score for each group was 6.
All participants underwent speech (Bankson-Bernthal Test of Phonology), language (Test for Auditory Comprehension of Language-3 and Structured Photographic Expressive Language Test-3), oral-motor (Robbins & Klee, 1987), and cognitive (Columbia Mental Maturity Scale and Test of Auditory-Perceptual Skills) testing. All nonstuttering children scored no lower than 1 standard deviation below the mean for same-aged peers on all of the tests. Of the 17 children who stutter, 3 scored lower than 1 sd below the mean for same age peers on speech testing, 3 scored < 1sd on language testing, and 1 scored < 1sd on both. These participants were included in the current study, because the population of stuttering children exhibits high rates of co-occurrence of other speech and language disorders (Arndt & Healey, 2001). Characteristics of the participants, including age of onset and severity of stuttering for the children who stutter, are summarized in Table 1. The estimates of stuttering severity were based on a combination of the results of the parents’ severity rating on a 0–7 scale, the project clinician’s severity rating on the same scale, and the average number of disfluencies per 100 syllables in the child’s speech.
Table 1.
Description of Participants
| Subject | Sex | Age at Testing | Hand | Age of Onset | Severity | Pass Speech? | Pass Language? | In Therapy? |
|---|---|---|---|---|---|---|---|---|
| S1 | M | 5;6 | R | 30 | Mild | Yes | Yes | Yes |
| S2 | M | 6;3 | R | 48 | Mod | Yes | Yes | Yes |
| S3 | M | 5;7 | R | 30 | Mod/Severe | No | Yes | Yes |
| S4 | F | 4;1 | L | 24 | Mod | Yes | No | Yes |
| S5 | M | 4;1 | R | 36 | Mild | No | Yes | No |
| S6 | M | 4;0 | R | 36 | Mild | No | No | Yes |
| S7 | M | 4;2 | R | 36 | Mild | Yes | Yes | No |
| S8 | M | 5;7 | R | 48 | Mod | Yes | No | Yes |
| S9 | M | 4;7 | R | 42 | Mild | Yes | Yes | No |
| S10 | F | 6;11 | R | 36 | Mild | Yes | Yes | Yes |
| S11 | M | 4;0 | R | 24 | Mod | Yes | Yes | No |
| S12 | M | 5;1 | R | 48 | Mod/Severe | Yes | Yes | No |
| S13 | M | 4;9 | R | 46 | Mod | No | Yes | Yes |
| S14 | M | 4;11 | R | 30 | Mod/Severe | Yes | No | Yes |
| S15 | M | 6;7 | R | 52 | Severe | Yes | Yes | Yes |
| S16 | F | 4;10 | R | 46 | Mild | Yes | Yes | No |
| S17 | F | 4;10 | R | 48 | Mild | Yes | Yes | No |
| C1 | M | 5;1 | R | |||||
| C2 | M | 5;0 | R | |||||
| C3 | M | 5;5 | R | |||||
| C4 | F | 5;5 | R | |||||
| C5 | F | 4;6 | R | |||||
| C6 | F | 4;8 | R | |||||
| C7 | M | 4;0 | R | |||||
| C8 | M | 4;5 | R | |||||
| C9 | F | 4;9 | R | |||||
| C10 | M | 4;9 | R | |||||
| C11 | M | 5;4 | R | |||||
| C12 | M | 4;8 | L | |||||
| C13 | M | 6;2 | R |
Testing Locations
Two testing sites were employed, Purdue University and the University of Iowa. Identical testing procedures were used at both sites. Subjects also performed a number of other tasks as part of a larger study, including electroencephalographic and electromyographic recording sessions as well as sentence and word repetition tasks. These results will not be reported in this paper.
Apparatus
A Northern Digital Optotrak 3020 system was used to record hand movements during the clapping task. In the Optotrak system the motion of infrared light emitting diodes (IREDs) is tracked by a set of three fixed cameras. Children sat in view of the cameras with IREDs attached to both middle fingers. IREDs were connected to a small splint that was taped to the distal end of each middle finger so that the diode could remain in view of the camera for the entire clapping motion. Wires extending from the IREDs were secured to the hands with tape to prevent them from interfering with clapping. Movements were analyzed in the medial-lateral dimension. The position of each IRED was sampled at 250 samples per second.
Procedures
The experimental protocol began after the IREDs were attached. A metronome (computer generated piano tone) with an inter-beat interval of 600 ms was played, and children were instructed to complete a clap coincident with the metronome beat. The children were instructed that when the metronome stopped (after 12 beats), they should keep clapping as though the metronome were still on (this phase is called continuation). Child friendly language was used (e.g., “clap in time with the piano beat, when the beat goes off, keep clapping and try to keep the beat.”) The continuation phase lasted long enough for 32 claps, after which the children were instructed to stop clapping. Up to three practice trials were given to ensure participants understood and could complete the task correctly. Children clapped along with the experimenter on the first practice trial and then were required to complete the task themselves before data collection began. Participants completed the task independently during the data collection trials, but, if necessary, were prompted to continue clapping when the beat stopped. To keep the IREDS in view, children were instructed to point their fingers toward “Ernie,” a doll seated on top of the cameras. If they clapped so that the markers went out of view of the cameras, they were told to “Make sure that they aimed at Ernie.” In some cases children produced such large claps that the IREDS went outside the camera view, and they were asked “not to clap so hard” or “to make softer claps”. All subjects were encouraged to complete at least six trials.
Data Analysis
All signal conditioning and data analysis were completed in the Matlab signal processing program. Displacement records were low pass filtered in both backward and forward directions with a cut off of 8 Hz using a fifth order Butterworth filter. The velocity of each clapping movement was calculated using a three point difference technique. Motions of each hand were measured separately. The starting point for each clap was defined as the point at which the velocity of the hand slowed to 3% of the peak velocity while moving towards the midline. The 3% velocity toward the midline value corresponds almost exactly to the point in time when the hands first make contact. The starting point of each clapping cycle also served as the ending point of the previous clapping cycle. A Matlab algorithm automatically computed the starting point for each clap, for each hand, based on this 3% velocity criterion. The displacement of both hands and the automatically defined claps were graphically displayed for each trial (see Figure 1). If the Matlab algorithm obviously picked an erroneous starting point, it was manually moved to the correct location using a mouse-driven cursor. Likewise, if a clap was missed by the algorithm, it was added in the correct location. Similar techniques have been shown to be accurate and reliable in previous studies in which rhythmic movements were measured (Robertson et al., 1999; Zelaznik et al., 1997). Trials were excluded if the child stopped clapping during the trial for 2 seconds or more. Trials were also analyzed to ensure that the same number of claps was recorded for each hand, because a clap by definition requires that both hands reach midline. Of 38 children tested on this protocol, 8 participants (5 children who stutter and 3 normally fluent) were excluded from the present study, because they did not produce at least two useable clapping trials. Thus the task was clearly challenging for our young participants.
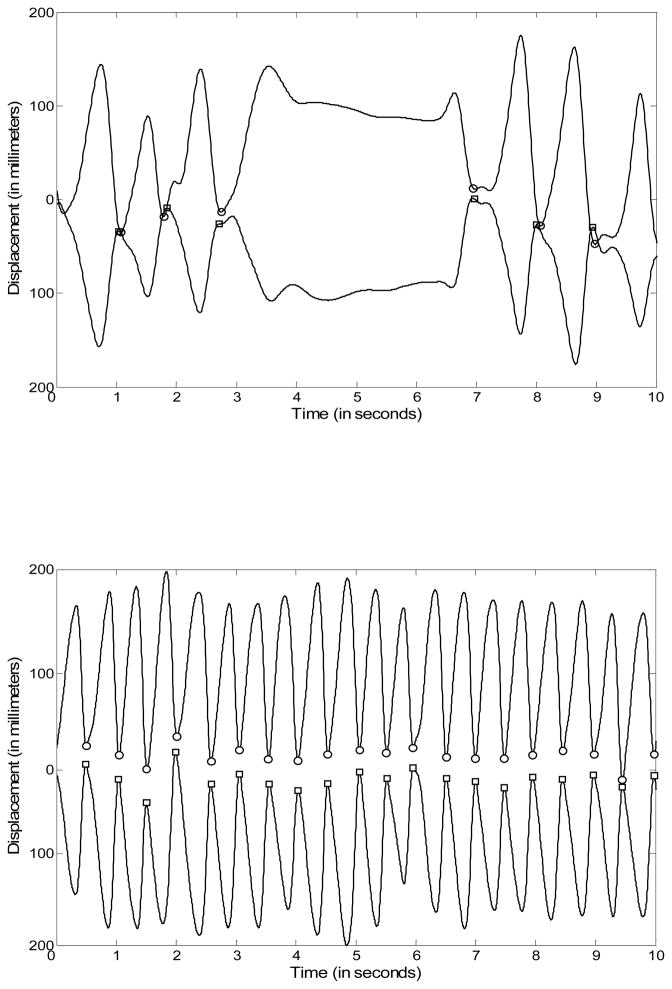
Figure 1.
Displacements of each hand from the mid-line during the clapping task. The top line represents the left hand and the bottom line represents the right hand. The circles (for the left hand) and the squares (for the right hand) represent the starting/ending points of each clapping cycle. The top record was unusable, because the child stopped for over 2 seconds. The bottom record shows a trial that was usable.
Only claps produced during the continuation phase of 32 claps (without metronome) were analyzed to determine timing variability. Although analysis of the paced claps would be of interest to compare the abilities of the two groups to synchronize a motor activity to an external beat, there were not enough pacing beats (only 12 per trial) to conduct a meaningful analysis. The first two claps and the final clap of the continuation phase were not included in the analysis. Each trial ideally included 28 claps. The average cycle duration, its variance, and the coefficient of variation of the inter-clap interval were computed and averaged across trials for each participant’s left and right hand. These variables served as measures of timing variability for each hand. Detrended variance was calculated to remove the influence of any drift in average clapping rate on variability, and the square root of the detrended variance was used to calculate the coefficient of variation [CV%= (Detrended SD/Mean Interclap Interval)*100]. Phase difference was calculated by finding the difference between the right and left hand in the onset of each clap and dividing that difference by the duration of the following inter-clap interval of the right hand (Right hand onset - Left hand onset)/ following inter-clap interval for the right hand) (Zelaznik et al., 1997). A positive value indicated that the right hand reached midline before the left hand, while a negative value indicated that the left hand reached midline before the right hand. Mean and median phase difference, which served as measures of the coordination of timing between the hands were determined and converted into degrees.
It was our plan to report clock and implementation variance for the unpaced clapping sequences following the methods of Wing and Kristofferson (1973), but the children’s inter-clap-interval data series violated the model’s basic assumption of negative lag 1 covariance, and the model could not be applied. It is notable that in a study of inter-response-interval data series of adults who stutter performing similar rhythmic tasks, the data met the negative lag 1 covariance assumption, and the model was applied (Max & Yudman, 2003).
Results
Results are reported for 17 children who stutter and 13 normally fluent children who produced at least 2 useable clapping trials. The range of useable trials/per child was 2–5, with a median of 4 useable trials/child for both groups. To address the statistical concern that clapping variability would be greater in the children for whom fewer usable trials were completed, we computed the correlation between the number of usable trials and the coefficient of variability of the inter-clap interval. This correlation (number of trials vs. CV right hand) was zero (−.075), thus alleviating this concern. Table 2 contains means and standard deviations for the variables measured in this study for the two groups of participants.
Table 2.
Dependent variable means and SD for children who stutter and normally fluent controls.
| Dependent Variable | Mean (SD)
|
|
|---|---|---|
| CWS | CNORM | |
| Duration of Inter-Clap Interval (ms) | 427(80) | 464(56) |
| Detrended Variance of Inter-Clap Interval | 4137(4135) | 1421(673) |
| R. Hand Coefficient of Variation (%) of Inter-Clap Interval | 13.4 (6.3) | 7.6 (2.4) |
| Mean Phase Difference (degrees) Between Hands | −0.49 (9.7) | 0.89 (10.9) |
| Median Phase Difference (degrees) Between Hands | −2.90 (8.0) | 0.72 (9.9) |
| SD of Phase | 22.9 (10.8) | 15.7 (8.0) |
Inter-Clap Interval
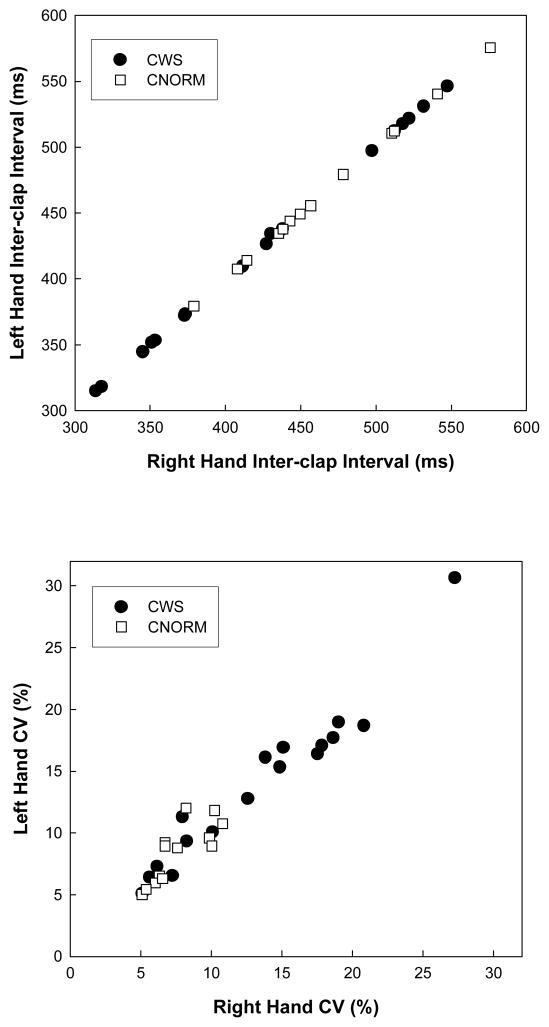
Figure 2 contains scatter plots of inter-clap intervals (top plot) and the variability of the inter-clap intervals for all 30 children for whom clapping data were analyzable. From the inter-clap interval plot it is apparent that the distribution of the children’s mean clapping intervals is overlapping for the two groups of participants. A repeated measures ANOVA revealed no significant difference between average inter-clap intervals of stuttering and nonstuttering children [F (1,28) = 2.05, p = .16] and no significant main effect of hand [F < 1] or interaction between hand and group [F < 1]. These results indicate that the stuttering and nonstuttering children were clapping at the same rate, and both groups apparently clapped at a rate faster than the target 600ms inter-beat interval.
Figure 2.
Graphs of mean inter-clap interval for each child’s right and left hand (top plot) and the coefficient of variation of the inter-clap interval computed for each child’s right and left hand (lower plot).
Variability of Inter-Clap Intervals
In contrast to the plot of inter-clap intervals in the top panel of Figure 2, the plot of the coefficient of variation of the inter-clap intervals for the right and left hands (lower plot) for each participant reveals that the distributions of scores are not overlapping. A subgroup of children who stutter performed outside the range of variability observed in the normally fluent group. Specifically, 10 of the 17 stuttering children show clapping variability higher than that observed in the most variable nonstuttering child (a CV of about 12%). Given that the distribution of the CVs for the stuttering children does not approximate the normal distribution and that the variance of the stuttering group is clearly much larger than that of the normally fluent children, an ANOVA could not be used to test for differences between groups. A nonparametric test for differences between two independent samples was calculated for the CV of the inter-clap interval for the right and left hands. Children who stutter had higher CV for the inter-clap intervals for right (Mann-Whitney U = 50, p = .01) and left hands (Mann-Whitney U = 46, p < .01).
Coordination Between Hands
Children who stutter did not significantly differ from the nonstuttering children in terms of mean [t (28) = 0.37, p = .72] or median [t (28) = 1.1, p = .28] phase difference between the hands, indicating that children who stutter, on average, did not show higher levels of dyssynchrony in coordinating the movements of the two hands toward midline. The standard deviation of the phase difference was significantly different between the two groups [t (28)=2.02, p = .05].
Potential Effects of Testing Site
As indicated by t-tests computed separately for each group, there was no effect of testing site (Purdue vs. Iowa) on any of the dependent variables. While differences in performance among the stuttering children related to speech/language status, therapy history, and severity would be of potential interest, any subgrouping of the 17 stuttering subjects would result in very small subject numbers; therefore we did not pursue these issues with any statistical analyses.
Discussion
Rhythmic Timing Variability in Children Who Stutter
The present study is the first to investigate non-speech motor timing and coordination in young children who stutter. Examining potential timing and coordination differences in children who stutter, near the age of stuttering onset, is a critical step towards providing evidence of an underlying neural overlap between speech and non-speech motor control processes and understanding if the motor differences often observed in adults who stutter are adaptive or causal in nature. The most striking finding of the present study is that timing variability during a clapping task significantly differed between children who stutter and age-matched typically developing children. The variance and coefficient of variation of the inter-clap interval were much higher in the children who stutter, despite the fact that both groups responded to the task similarly with no significant differences in average clapping rates or number of usable trials produced. This suggests that both groups of children matched the metronome rate with the same level of accuracy and completed the task with similar levels of success. However, the children who stutter clearly did not maintain a consistent rate of clapping as well as normally fluent children, even when the data were detrended in order to remove the influence of any steady rate change on variability levels. Furthermore, 10 of the 17 children who stutter performed outside the normal range in clapping variability. These results provide clear evidence that in its early years, developmental stuttering is associated with a fundamental deficit in the child’s ability to generate consistent, rhythmic motor behaviors. This suggests that children who stutter have atypical development of neural networks in the brain involved in the control of speech and other complex movements.
The results of the current study support the theory put forth by Max et al., (2003) and Zelaznik et al. (1997) that differences in the timing, coordination and synchronization of the articulators of adults who stutter are influenced by an underlying motor timing deficit. The fact that timing differences were found during performance of a clapping task suggests that they are not specific to the speech motor system and that motor timing differences for speech and non-speech movements may share a common neural substrate (Binkofski & Buccino, 2004; Bengtsson et al., 2005). Further support for this argument is provided by a recent neuroimaging study by Chang, Kenny, Loucks, and Ludlow (2009) who reported that the same atypical neural activations observed in adults who stutter during speech tasks were also present when they performed nonspeech oral motor tasks. The presence of significantly increased timing variability in 4, 5 and 6-year-old children who stutter, in comparison to age-matched nonstuttering children also provides evidence that these timing differences are present close to the onset of stuttering rather than developed, over time as an adaptation to chronic stuttering, as hypothesized by McClean et al. (2004). Within the scope of a multi-factorial model of stuttering (Smith, 1999), the present results lend strong support to the hypothesis that a general motor timing deficit is one of the factors contributing to the abnormal speech and non-speech motor output observed in a significant portion of individuals who stutter.
Influence of Task
We used clapping to produce a target rhythm to assess timing differences in young children who stutter, because rhythmic clapping is a difficult task for young children. It is, however, a task that can be understood and performed with limited amounts of practice. Furthermore, clapping is complex enough to reveal subtle differences in the motor timing abilities between groups. Normally developing 4-year-old children are able to perform rhythmic clapping but are more variable in timing than adults (Fitzpatrick, Schmidt, & Carello, 1996; Getchell, 2006) and older children (Getchell & Whithall, 2003). Children as old as 10 years have also been reported to be less accurate in rhythmic motor timing than adults (McAuley, Jones, Holub, Johnston, & Miller, 2006). Rhythmic clapping may be challenging because it is bimanual (Serrien, 2008) and requires the spatial control necessary to make the hands come together on each cycle. In previous studies examining articulatory movements in adults who stutter, variability differences were observed more often when speech tasks were linguistically more complex (Kleinow & Smith, 2000). Hence, the complexity of the clapping task used in this study was critical in preventing a ceiling effect in subject performance. The actual clapping rates produced by the children in this study, which missed the target rate typically by 15–50%, clearly indicate the high difficulty level of the task. There were no differences between groups on mean clapping rates, and only 9 of the 30 children clapped within 100ms of the target rate produced by the metronome. Many of the tasks used in previous studies (Max & Yudman, 2003; Webster, 1985) with adults have likely not been challenging enough to elicit timing differences between adults who stutter and controls.
With regard to relative phase, both groups of children produce the same mean and median phase differences between the two hands in the clapping task. We interpret this finding to suggest that the groups were equal in terms of the relative time at which the two hands arrived at midline. Between hands coordination differences may not have been found in this study because clapping requires the hands to come together on each timing cycle, making it less likely for the hands to be out of phase. The children who stutter, however, were significantly more variable in maintaining a consistent phase relationship between the hands. This result is consistent with those of previous studies in which adults who stutter performed rhythmic tapping tasks with higher relative phase variability, a measure of consistency of between fingers coordination, than controls (Hulstijn et al. 1992; Zelaznik et al., 1997).
Differences in Inter-Clap Interval Variability Levels Among Children Who Stutter
An important finding is that 41% of the children who stutter exhibited inter-clap interval variability levels within the range produced by the group of normally fluent children, while 59% were so variable that they were outside the normal range. It is possible that only a proportion of children who stutter have a motor timing deficit in comparison to same-aged peers. These two subgroups may still be present in adults who have persisted in stuttering. If this is the case, an additional explanation may be provided for the lack of differences in non-speech motor timing found between adults who stutter and controls in some previous studies (e.g. Max & Yudman, 2003; Webster, 1985). These mixed results may simply reflect sampling differences in these studies with small sample sizes. It is also possible that some children who spontaneously recover from developmental stuttering continue to show subtle differences in motor timing. In fact, Chang et al. (2008) reported that children between the ages of 9 and 12 years who had recovered from developmental stuttering continued to show less gray matter volume in Broca’s area and in the supplementary motor area when compared to children who had never stuttered. This may explain why the current study revealed significant differences in variability between children who stutter and controls, despite the fact that approximately half of the subjects included will not persist in stuttering.
An intriguing speculation arising from the present work is that the presence of a general motor timing deficit in children who stutter may be an early indicator of persistent stuttering. In other words, children who stutter who do not show evidence of a general timing deficit might spontaneously recover, while those who do show abnormally high motor timing variability will persist. Currently there is no way to determine if a child who stutters will continue to do so into adulthood. Findings of the Illinois Stuttering project (Ambrose & Yairi, 1999; Paden, Yairi, & Ambrose, 1999; Watkins, Yairi, & Ambrose, 1999; Yairi & Ambrose, 1999), a four year longitudinal study of children who stutter revealed a 75% spontaneous recovery rate for 3-year-olds who stutter. Because the children in the current study were 4, 5 and 6-year-olds, and as a result had already been stuttering for 1 to 3 years, recovery rates for this group should be approximately 50%. The distribution of clapping variability levels produced by children who stutter in the present study (41% of the children who stutter falling within the range produced by CNS and 59% producing higher variability levels than children who stutter roughly matches expected recovery rates.
Although it is beyond the scope of the present study to strongly suggest clapping variability is an early marker of stuttering persistence versus recovery, the bimodal-looking distribution in the coefficient of variation in the children who stutter provides a promising possibility for the early identification of persistent stuttering and will continue to be investigated in future work in our laboratory. A procedure for the early detection of persistent stuttering, would clearly be beneficial for speech language pathologists and families of children who stutter when deciding whether a young child who is stuttering should enter therapy.
Conclusion
The results of the present study reveal that a subgroup of children who stutter are more variable timers than typically developing children during a bimanual rhythmic timing task. This provides evidence that speech and non-speech motor timing may share a common neural substrate and that abnormalities in this shared brain region may lead to a generalized motor control deficit that is one of the factors contributing to the abnormal speech motor output observed individuals who stutter. The fact that only a portion of children who stutter performed with higher inter-clap interval variability levels than controls also provides a possible early indicator of persistent stuttering that warrants further investigation.
Acknowledgments
This work was supported by grant DC00559 from the NIH’s National Institute on Deafness and Other Communication Disorders. Our thanks to Barbara Brown and Janna Berlin for their help in subject recruitment and testing.
Contributor Information
Lindsey Olander, Department of Speech, Language, and Hearing Sciences, Purdue University, West Lafayette, IN.
Anne Smith, Department of Speech, Language, and Hearing Sciences, Purdue University, West Lafayette, IN.
Howard Zelaznik, Department of Health and Kinesiology, Purdue University, West Lafayette, IN.
References
- Ambrose N, Yairi E. Normative data for early childhood stuttering. Journal of Speech, Language, & Hearing Research. 1999;42:895–909. doi: 10.1044/jslhr.4204.895. [DOI] [PubMed] [Google Scholar]
- Arndt J, Healey CE. Concomitant disorders in school-age children who stutter. Language, Speech, and Hearing Services in Schools. 2001;32:68–78. doi: 10.1044/0161-1461(2001/006). [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Ehrsson HH, Forssberg H, Ullen F. Effector-independent voluntary timing: Behavioral and neuroimaging evidence. European Journal of Neuroscience. 2005;22:3255–3265. doi: 10.1111/j.1460-9568.2005.04517.x. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G. Motor functions of the Broca’s region. Brain and Language. 2004;89:362–369. doi: 10.1016/S0093-934X(03)00358-4. [DOI] [PubMed] [Google Scholar]
- Boutsen FR, Brutten GJ, Watts CR. Timing and intensity variability in the metronomic speech of stuttering and nonstuttering speakers. Journal of Speech, Language, and Hearing Disorders. 2000;43:513–520. doi: 10.1044/jslhr.4302.513. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Zimmerman JP, Hegmann JP, Linville RN. Variations in self-paced behaviors in stutterers and nonstutterers. Journal of Speech and Hearing Research. 1990;33:307–316. doi: 10.1044/jshr.3302.317. [DOI] [PubMed] [Google Scholar]
- Chang S, Erickson K, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. NeuroImage. 2008;39:1333–1344. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Kenney MK, Loucks T, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. NeuroImage. 2009;46:201–212. doi: 10.1016/j.neuroimage.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MH, Allen GD. Timing control accuracy in stutterers and nonstutterers. Journal of Speech and Hearing Research. 1977;20:55–71. doi: 10.1044/jshr.2001.55. [DOI] [PubMed] [Google Scholar]
- Conture EG. Stuttering. 2. Englewood Cliffs, NJ: Prentice Hall; 1990. [Google Scholar]
- De Nil LF, Kroll RM, Houle S. Functional neuroimaging of cerebellar activation during single word reading and verb generation in stuttering and nonstuttering adults. Neuroscience Letters. 2001;392:77–80. doi: 10.1016/s0304-3940(01)01671-8. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick P, Schmidt RC, Carello C. Dynamical patterns in clapping behavior. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:707–724. [Google Scholar]
- Franz EA, Zelaznik HN, Smith A. Evidence of common timing processes in the control of manual, orofacial, and speech movements. Journal of Motor Behavior. 1992;24:281–187. doi: 10.1080/00222895.1992.9941623. [DOI] [PubMed] [Google Scholar]
- Getchell N. Age and task-related differences in timing stability, consistency, and natural frequency of children’s rhythmic motor coordination. Developmental Psychobiology. 2006;48:675–685. doi: 10.1002/dev.20186. [DOI] [PubMed] [Google Scholar]
- Getchell N, Whitall J. How do children coordinate simultaneous upper and lower extremity tasks? The development of dual motor task coordination. Journal of Experimental Child Psychology. 2003;85:120–140. doi: 10.1016/s0022-0965(03)00059-6. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Unpublished manuscript. Department of Sociology, Yale University; New Haven, Connecticut: 1975. Four factor index of social status. [Google Scholar]
- Howell P, Au-Yeung J, Rustin L. Clock and motor variances in lip-tracking: A comparison between children who stutter and those who do not. In: Peters HFM, van Lieshout PH, editors. Speech production: Motor control, brain research and fluency disorders. Amsterdam, the Netherlands: Elsevier; 1997. pp. 573–578. [Google Scholar]
- Hulstijn W, Summers JJ, van Lieshout PHM, Peters HFM. Timing in finger tapping and speech: A comparison between stutterers and fluent speakers. Human Movement Science. 1992;11:113–124. [Google Scholar]
- Kent RD. The segmental organization of speech. In: MacNeilage PF, editor. The production of speech. New York: Springer-Verlag; 1983. pp. 57–89. [Google Scholar]
- Kleinow J, Smith A. Influences of length and syntactic complexity on the speech motor stability of the fluent speech of adults who stutter. Journal of Speech, Language, and Hearing Research. 2000;43:548–559. doi: 10.1044/jslhr.4302.548. [DOI] [PubMed] [Google Scholar]
- Max L, Caruso A, Gracco V. Kinematic analyses of speech, orofacial nonspeech, and finger movements in stuttering and nonstuttering adults. Journal of Speech, Language, & Hearing Research. 2003;46:215–32. doi: 10.1044/1092-4388(2003/017). [DOI] [PubMed] [Google Scholar]
- Max L, Gracco V. Coordination of oral and laryngeal movements in the perceptually fluent speech of adults who stutter. Journal of Speech Language and Hearing Research. 2005;48:524–542. doi: 10.1044/1092-4388(2005/036). [DOI] [PubMed] [Google Scholar]
- Max L, Yudman EM. Accuracy and variability of isochronous rhythmic timing across motor systems in stuttering versus nonstuttering individuals. Journal of Speech, Language and Hearing Research. 2003;46:146–163. doi: 10.1044/1092-4388(2003/012). [DOI] [PubMed] [Google Scholar]
- McAuley J, Jones M, Holub S, Johnston H, Miller N. The time of our lives: Life span development of timing and event tracking. Journal of Experimental Psychology: General. 2006;135:348–367. doi: 10.1037/0096-3445.135.3.348. [DOI] [PubMed] [Google Scholar]
- McClean M, Runyan CM. Variations in the relative speeds of orofacial structures with stuttering severity. Journal of Speech, Language, & Hearing Research. 2000;43:1524–31. doi: 10.1044/jslhr.4306.1524. [DOI] [PubMed] [Google Scholar]
- McClean M, Tasko S, Runyan C. Orofacial movements associated with fluent speech in persons who stutter. Journal of Speech and Hearing Research. 2004;47:294–303. doi: 10.1044/1092-4388(2004/024). [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychology. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paden E, Yairi E, Ambrose N. Early childhood stuttering II: Initial status of phonological abilities. Journal of Speech, Language, & Hearing Research. 1999;42:1113–1124. doi: 10.1044/jslhr.4205.1113. [DOI] [PubMed] [Google Scholar]
- Riley G, Riley J. Motoric and linguistic variables among children who stutter: A factor analysis. Journal of Speech and Hearing Disorders. 1980;45:504–514. doi: 10.1044/jshd.4504.504. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, Fazio F. Localization of grasp representations in humans by PET: 1. Observation versus execution. Experimental Brain Research. 1996;111:246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Robbins J, Klee T. Clinical assessment of oropharyngeal motor development in young children. Journal of Speech and Hearing Disorders. 1987;52:271–277. doi: 10.1044/jshd.5203.271. [DOI] [PubMed] [Google Scholar]
- Robertson S, Zelaznik H, Lantero D, Gadacz K, Spencer R, Doffin J. Correlations for timing consistency among tapping and drawing tasks: Evidence against a single timing process for motor control. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1316–1330. doi: 10.1037//0096-1523.25.5.1316. [DOI] [PubMed] [Google Scholar]
- Serrien DJ. The neural dynamics of timed motor tasks: evidence from a synchronization-continuation paradigm. European Journal of Nneuroscience. 2008;27:1553–1560. doi: 10.1111/j.1460-9568.2008.06110.x. [DOI] [PubMed] [Google Scholar]
- Smith A. Factors in the etiology of stuttering. American Speech-Language-Hearing Association Reports, Research Needs in Stuttering: Roadblocks and Future Directions. 1990;18:39–47. [Google Scholar]
- Smith A. Stuttering: A unified approach to a multifactorial, dynamic disorder. In: Ratner N, Healey C, editors. Research and Treatment of Fluency Disorders: Bridging the Gap. Mahwah, NJ: Erlbaum; 1999. pp. 27–44. [Google Scholar]
- Smith A, Kleinow J. Kinematic correlates of speaking rate changes in stuttering and normally fluent adults. Journal of Speech, Language and Hearing Research. 2000;43:521–536. doi: 10.1044/jslhr.4302.521. [DOI] [PubMed] [Google Scholar]
- Smith A, McFarland DH, Weber CM. Interactions between speech and finger movements: An exploration of the dynamic pattern perspective. Journal of Speech and Hearing Research. 1986;29:471–480. doi: 10.1044/jshr.2904.471. [DOI] [PubMed] [Google Scholar]
- Smits-Bandstra S, De Nil L, Rochon E. The transition to increased automaticity during finger sequence learning in adult males who stutter. Journal of Fluency Disorders. 2006;31:22–42. doi: 10.1016/j.jfludis.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- Starkweather CW, Gottwald SR, Halfond MM. Stuttering Prevention: A Clinical Method. Englewood Cliffs, NJ: Prentice-Hall; 1993. [Google Scholar]
- Tingley BM, Allen GD. Development of speech timing control in children. Child Development. 1975;46:186–194. [Google Scholar]
- Van Riper C. The Nature of Stuttering. Englewood Cliffs, NJ: Prentice-Hall; 1982. [Google Scholar]
- Wall M, Myers F. Clinical Management of Childhood Stuttering. 2. Austin, TX: Pro-Ed; 1995. [Google Scholar]
- Ward D. Intrinsic and extrinsic timing in stutterers’ speech: Data and implications. Language and Speech. 1997;40:289–310. doi: 10.1177/002383099704000305. [DOI] [PubMed] [Google Scholar]
- Watkins R, Yairi E, Ambrose N. Early childhood stuttering III: Initial status of expressive language abilities. Journal of Speech, Language, & Hearing Research. 1999;42:1025–1035. doi: 10.1044/jslhr.4205.1125. [DOI] [PubMed] [Google Scholar]
- Webster WG. Neurolopsychologial models of stuttering I. Representation of sequential response mechanisms. Neuropsychologia. 1985;23:263–267. doi: 10.1016/0028-3932(85)90110-1. [DOI] [PubMed] [Google Scholar]
- Webster WG. Response sequence organization and reproduction by stutterers. Neurpsychologia. 1986;24:813–821. doi: 10.1016/0028-3932(86)90080-1. [DOI] [PubMed] [Google Scholar]
- Westphal G. An experimental study of certain motor abilities of stutterers. Child Development. 1933;4:214–221. [Google Scholar]
- Wing AM, Kristofferson AB. Response delays and the timing of discrete motor performance. Perception & Psychophysics. 1973;14:5–12. [Google Scholar]
- Yairi E, Ambrose N. Early childhood stuttering I: Persistency and recovery rates. Journal of Speech, Language, & Hearing Research. 1999;42:1097–1112. doi: 10.1044/jslhr.4205.1097. [DOI] [PubMed] [Google Scholar]
- Zelaznik HN, Smith A, Franz EA. Motor performance of stutterers and nonstutterers on timing and force control tasks. Journal of Motor Behavior. 1994;26:340–347. doi: 10.1080/00222895.1994.9941690. [DOI] [PubMed] [Google Scholar]
- Zelaznik HN, Smith A, Franz EA, Ho M. Differences in bimanual coordination associated with stuttering. Acta Pschologica. 1997;96:229–243. doi: 10.1016/s0001-6918(97)00014-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann G. Articulatory dynamics of fluent utterances of stutterers and nonstutterers. Journal of Speech and Hearing Research. 1980;23:95–107. doi: 10.1044/jshr.2301.95. [DOI] [PubMed] [Google Scholar]