Abstract
We evaluated the efficacy of attractive toxic sugar baits (ATSB) in the laboratory and field with the low-risk active ingredient dinotefuran against mosquito populations. Preliminary laboratory assays indicated that dinotefuran in solution with the sugar baits was ingested and resulted in high mortality of female Culex quinquefasciatus Say and Aedes aegypti Linnaeus. Field studies demonstrated >70% reduction of mosquito populations at 3 wk post-ATSB application. Nontarget feeding of seven insect orders—Hymenoptera, Lepidoptera, Coleoptera, Diptera, Hemiptera, Orthoptera, and Neuroptera—was evaluated in the field after application of attractive sugar baits (ASB) on vegetation by dissecting the guts and searching for food dye with a dissecting microscope. Nontargets were found stained with ASB 0.9% of the time when the application was applied on green nonflowering vegetation. Only two families were significantly impacted by the ASB application: Culicidae (mosquitoes) and Chironomidae (nonbiting midges) of the order Diptera. Pollinators of the other insect orders were not significantly impacted. No mortality was observed in the laboratory studies with predatory nontargets, wolf spiders or ground beetles, after feeding for 3 d on mosquitoes engorged on ATSB applied to vegetation. Overall, this novel control strategy had little impact on nontarget organisms, including pollinators and beneficial insects, and was effective at controlling mosquito populations, further supporting the development of ATSB for commercial use.
Keywords: integrated vector control, pollinator, environmental impact, beneficial insect, mosquito
Vector control programs face a myriad of issues, from legislative to economics, that are restricting programs worldwide. This prompted the World Health Organization (WHO) to urge vector control programs to implement integrated vector management (IVM) strategies that focus on environmentally friendly, cost-effective methods that are sustainable (WHO 2004, 2012).
A new method that appears to meet these goals of the WHO IVM program is the attractive toxic sugar bait (ATSB) technology. This novel form of vector control targets the sugar feeding behavior of male and female mosquitoes in nature (Müller and Schlein 2006,2008; Schlein and Müller 2008; Müller et al. 2008, 2010a,b,; Beier et al. 2012). ATSB methods developed and field-tested in the Middle East, the United States, and Africa demonstrate how they literally decimated local populations of anopheline and culicine mosquito species (Müller and Schlein 2006, 2008; Müller et al. 2008, 2010 a; Gu et al. 2011; Beier et al. 2012). Similar successful ATSB field trials also controlled Culex quinquefasciatus Say (Müller et al. 2010a) and Aedes albopictus Skuse (Qualls et al. 2012, Naranjo et al. 2013) from storm drains, cisterns, wells, and residential backyards in Florida.
Although ATSB methods are highly effective, technologically simple, and low-cost, they have not been evaluated against nontarget arthropod populations. Unlike most insecticides used in adult mosquito control that are applied as broadcast sprays, ATSB solutions can either be applied to spots of vegetation or suspended in removable bait stations that attract mosquitoes from a large area (attract and kill). After locating the ATSB, mosquitoes ingest the toxic solutions and are killed. Because ATSB targets the sugar-seeking behavior of mosquitoes and uses a safe oral toxin such as boric acid, it circumvents problems traditionally associated with the indiscriminate use of contact insecticides (Enayati and Hemingway 2010). The bait method is suitable to be combined with any type of gut active low-risk toxin, even with some exempt materials, which makes it a potential valuable tool to fight rising resistance against conventional contact pesticides (Allan 2011). One low-risk toxin that has proven to be effective for Anopheles gambiae Meigen control is dinotefuran (Corbel et al. 2004). The purpose of this study was to evaluate the low-risk active ingredient dinotefuran as a potential toxin for use in ATSB applications. In addition, we evaluated the potential impact of this novel control method on nontarget organisms in Morocco.
Materials and Methods
Mosquito Laboratory Evaluations
Initial laboratory studies were conducted at United States Department of Agriculture, Center for Medical and Veterinary Entomology Laboratory, Gainesville, FL, to determine efficacy of the toxicant against mosquitoes before use in the field study. Assays were conducted following Allan (2011) and consisted of placing 10 mosquitoes in disposable plastic cups (100 ml) covered with fabric screen. Five-day-old, sugar-starved females of Cx. quinquefasciatus and Aedes aegypti L. from laboratory colonies were used. Sections of cotton dental wick (1 cm in length) (Unipack Medical Corp., Commerce, CA) were saturated with the 10% sucrose solution (ATSB) with different concentrations of dinotefuran (Safari 20 SG, Valent USA Corporation, Walnut Creek, CA). Mortality was observed hourly up to 4 h and then at 24 h. Mosquitoes were considered dead if they were unable to stand and had no wing movement. For each dose, five assay cups of adult mosquitoes were tested with each dose and replicated on three different days. Controls consisted of wicks saturated with the bait solution with no pesticide (attractive sugar bait [ASB] controls). Food coloring was added to treatment and control solutions of some replicates to verify that mosquitoes were ingesting solutions. Droplets of excreted material containing dye were counted for sucrose controls and all of the insecticide doses examined.
Mosquito Field Evaluations
Mosquito field studies were conducted by using the low-risk pesticide dinotefuran at 100 mg/liter. The treatment site consisted of a 400-m-long ditch that was overgrown by cactus (Opuntia ficus-indica (Linnaeus) Miller; Cactaceae), bearing ripe fruit. The control site was a 350-m-long ditch situated between a farm and nearby wasteland. The ATSB and ASB solutions were applied by using a backpack sprayer to cover the vegetation in the treatment areas until it was wet with bait solution and just before run off. The applications were made following existing Environmental Protection Agency (EPA) guidelines. The test substance was applied to the site at the rate, frequency, and method specified on the label (EPA 2012a). Mosquito populations were monitored with six ultraviolet (UV) tray traps per site five times a week before ATSB application and twice a week for the next 3 wk.
Nontarget Evaluations
Predatory invertebrates were studied in semifield conditions in Morocco. Predatory invertebrates, wolf spiders (Lycosidae) and ground beetles (Carabidae), were collected in the field and transferred individually to 20- by 20- by 12-cm plastic trays (with a layer of 1 cm of local sandy soil, and some dry leaves). The trays were closed with gauze and kept on a table in the shade of a large sun umbrella. The predators were fed in the trays with micro forceps with ATSB-engorged (based on dye stains in the gut visible through the integument), wingless, but living, mosquitoes. Feeding was verified by visual observation of the food dye, and survival was evaluated twice at 12-h intervals for three consecutive days.
Field studies evaluating feeding on vegetation treated with ASB by nontarget insects were conducted by dissecting and examining guts for food dye under a dissecting microscope. The insect orders included Hymenoptera (with focus on Aculeata, including honey bee (Apis mellifera), wild bees, and wasps), Lepidoptera (Rhopalocera, all families of Macroheterocera and Microlepidoptera), Coleoptera (Carabidae, Tenebrionidae, Scarabaeidae, Cerambycidae, Chrysomelidae, other beetles), Diptera (Brachycera and Chironomidae), Hemiptera (Cicadomorpha and Heteroptera), Orthoptera (Caelifera and Ensifera), and Neuroptera (Myrmeleontiformiaand Hemerobiiformia).
Nontarget field studies were conducted next to a stagnant ditch (≈1 m in width) partially overgrown with reeds (Phragmites sp. and Arundo sp.; Graminaea) and some intermittent herbaceous vegetation. ASB used for the nontarget studies were prepared from industrial-grade ASB concentrate (Westham Ltd, Tel Aviv, Israel) by diluting the concentrate 1:4 in tap water and adding a red food dye (1:200; Azorubine, Stern, Natanya, Israel). The vegetation was then treated (0.5-by 0.5-m plot or 0.5-m strip intervals) by using abackpack sprayer (Solo, model 425, Newport News, VA) while moving the nozzle up and down to cover both the under and upper side of the foliage. In the absence of specific EPA guidelines, we designed experiments to approximate use of the product under field conditions. Testing was performed under field trail conditions with ATSB foliar spray application on nonflowering plants per product label instructions (EPA 2012 a,b,c).
Field experiments with nontargets were conducted with the assumption that all insects feeding directly on ATSB-treated foliage would eventually die. Before death, they exhibited behavioral changes, which made it difficult to collect these insects affected by ATSB in experimental areas in amounts comparable with un-treated control sites. It was decided to use nontoxic, but color-stained, sugar baits (ASB) to explore attraction and feeding of both target and nontarget insects as the best method to obtain representative results from the field. The food dye stains the guts of insects that fed on the bait for at least 24 h. The percentage of stained insects after the first day of ASB application can therefore be seen as a potential maximal daily feeding/killing rate (Schlein and Müller 2008).
Nontarget insects were monitored 1 d/night after ASB application at the treated site with 50 yellow plates (yellow disposable plastic plates, 25 cm in diameter, filled with water and a drop of triton-x as detergent), four Malaise traps (2 and 6 m; model 2875D, BioQuip, Rancho Dominguez, CA), two large ultraviolet (UV) light traps (generator-powered 250-ML light bulb mounted in front of a 2- by 5-m white linen sheet), six UV-tray traps (Müller et al. 2011), 50 pitfall traps (500-ml plastic cups buried to the rim in the ground, baited with 10 ml vinegar) (Leather 2005), sweep-nets (BioQuip) (two collectors), and entomological hand nets (BioQuip) (two collectors) (Muller et al. 2005, 2006). Collected insects were immediately killed in collecting jars (BioQuip) with ethyl acetate and stored in a freezer (−20°C) before processing.
Because of the large number of nontargets that were collected, aliquots from each collecting method were used to determine the percentage of stained insects. Because of the volume of the collections of morphospecies, species that are distinct based on morphological characteristics were identified instead of identifying each specimen to species level.
Statistical Analysis
The counts of trapped and stained insects of each species had a Poisson distribution. Therefore, we used a generalized linear model to compare the number of stained insects of each species. We used the total number of each species as an offset to produce proportions of stained insects for each species. Because there was marked overdispersion, a negative binomial link was used. The results were reported as the percent and SE of stained insects for each species. Planned comparisons of each species with mosquitoes were also reported. Laboratory data were arcsine transformed and comparisons between means made with paired t-tests (P < 0.05). The 0.05 significance level was used to determine statistical significance. SAS 9.3 (SAS Institute, Inc., Cary, NC) was used for all analyses.
Results
Laboratory Evaluation
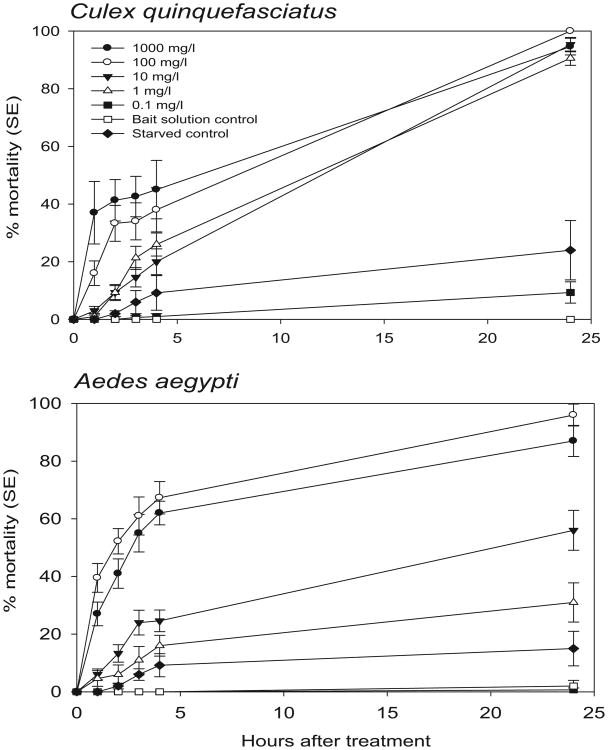
Assays indicated that dinotefuran in solution with the sugar baits was ingested and provided high mortality of female Cx. quinquefasciatus and Ae. aegypti (Fig. 1). Bait solution control mortality was <3% and mortality of starved controls (no water or sugar) was <20% at 24-h postexposure. For the two highest doses of dinotefuran (100 mg/liter, 1000 mg/liter) 1 h after exposure, 16−37% of Cx. quinquefasciatus and 27–39% of Ae. aegypti were dead. Mortality >80% was achieved at 24 h by doses of 1,10, 100, and 1,000 mg/liter dinotefuran for Cx. quinquefasciatus and 100 and 1,000 mg/liter dinotefuran for Ae. aegypti. As there was no difference in efficacy between 100 mg/liter and 1,000 mg/liter dinotefuran for either species from 2 to 24 h (paired t-tests; P < 0.05), the dose of 100 mg/liter was selected for field studies. Ingestion of solutions with dye was confirmed by detection of dye in excreted droplets in cups and in dissected mosquitoes. At the higher doses, fewer dyed excreted droplets were present in cups treated with 100 and 1,000 mg/liter than with lower doses and controls (data not presented). Dye was clearly evident in mosquitoes at all doses.
Fig. 1.
Mortality of female Culex quinquefasciatus and Aedes aegypti provided with attractive sugar bait solutions combined with different doses of dinotefuran in laboratory bioassays.
Field Evaluation
In total, four mosquito species were collected at the control and test site. The dominant mosquito species (>95%) atboth sites was Culex perexiguus Theobald, followed by Culex theileri Theobald (2.0%), Aedes caspius (Pallas) (1.5%), and Culex pipiens L. (1.0%). Mosquito populations were reduced significantly, >70%, at the treatment site up to 3 wk postapplication, compared with the control site (F = 23.65; df1,2 = 3, 112; P < 0.01). Overall densities of mosquitoes from the pretreatment period (1–7 d) decreased more than 2.1-fold (8–21 d) compared with more than a 1-fold increase at the control site that did not receive ATSB treatment. Mosquito densities averaged 75.1 ± 13.5 before ATSB application at the control site and 120.5 ± 18.8 after ATSB application at the treatment site. At the treatment site, mosquito densities averaged 108.6 ± 20.3 pretreatment and 53.2 ± 10.4 post-ATSB treatment. Figure 2 shows the reduction of male and female Cx. perexiguus after the ATSB treatment, although the ATSB treatment affected the populations of all four species equally well.
Fig. 2.

Number (mean ± SE) of male and female Cx. perexiguus captured by light traps pre- and postattractive toxic sugar bait application at (A) control site without attractive toxic sugar bait application and (B) treatment site with attractive toxic sugar bait application. (Figure in color online.)
Nontarget Evaluations
No mortality was observed in either Lycosidae (0/28) or Carabidae (0/30) after feeding for 3 days on mosquitoes engorged on ATSB applied to vegetation. There was no control mortality of the untreated (starved) control spiders (0/25) and beetles (0/35).
The potential impact on nontarget insects of ATSB applied on green nonflowering vegetation was low for all nontarget groups, as only 0.9% of the individual insects were stained with the red dye from the ASB solutions. Nonbiting midges and mosquitoes were stained significantly more than the other nontarget orders collected (F = 6.33; df1,2 = 7,18; P = 0.01; Table 1). Of the sampled nonbiting midges, 29% were stained, indicating a strong impact of the ATSB on these populations when used. Mosquitoes were also collected stained 35.8% of the time during the non-target evaluation.
Table 1. Percentage (SE) of stained insects in each order compared with mosquitoes (control group).
| Species | n | Percent stained | SE | P valuea |
|---|---|---|---|---|
| Mosquitoes | 1,000 | 35.8 | 29.3 | |
| Coleoptera | 2,690 | 0.6 | 0.3 | 0.001 |
| Dipterab | 3,000 | 15.0 | 8.7 | 0.399 |
| Hemiptera | 800 | 0.8 | 0.6 | 0.003 |
| Hymenoptera | 2,300 | 1.3 | 0.7 | 0.003 |
| Lepidoptera | 3,130 | 0.6 | 0.2 | <0.001 |
| Neuroptera | 600 | 0.3 | 0.2 | 0.001 |
| Orthoptera | 920 | 1.0 | 0.7 | 0.003 |
Comparison of insects of all orders with the control group (mosquitoes).
Without mosquitoes.
Discussion
Laboratory experiments demonstrated that the low-risk pesticide dinotefuran was effective at controlling mosquitoes when applied in sugar solution. High mortality was obtained at 24 h after exposure to 100-ng/liter and 100-mg/liter doses of dinotefuran solutions. In contrast to assays involving direct application of the toxicant on the insect that result in rapid mortality, oral toxic assays involve voluntary ingestion of toxicants, often resulting in delayed mortality. Our field tests further corroborated the laboratory study when ATSB– dinotefuran application resulted in control of mosquito populations for 3 wk postapplication. Dinotefuran is a neonicotinoid that acts as agonist on the nicotinic acetylcholine receptor (Tomizawa and Yamamoto 1993) and has previously been reported to be toxic to An. gambiae (Corbel et al. 2004). Because there is no pyrethroid or carbamate cross-resistance, the addition of dinotefuran in an IVM program could be a source of resistance management (Corbel et al. 2004). The results presented here, with the addition of dinotefuran as the toxin, support previous field studies conducted in Israel (Müller et al. 2008) and Mali (Müller et al. 2010b), where mosquito populations were controlled after a spinosad and boric acid ATSB application, respectively. Recently, Xue et al. (2013) demonstrated effective control of important nuisance and vector mosquito species by using an undisclosed all natural exempt active ingredient in field trials in the United States. The addition of the industrial-grade ASB concentrate increased the residual time of the ATSB application, which is important for sustainability and incorporation into IVM strategies. Further-more, the use of the low-risk pesticide dinotefuran continues to identify the versatility of the ATSB method and its role in IVM.
In this study, we did not observe any obvious negative impact of the ATSB/ASB method observed on predatory or pollinating arthropods in the field evaluations in Morocco. Our study is the first to demonstrate that ATSB applications had very little impact on nontarget populations, specifically non-sugar-feeding predatory insects and pollinators. When the ASB was applied on nonflowering vegetation, nontarget insect populations were not attracted to the baits and did not feed on them. However, when ASB was applied to flowering vegetation, the staining rate of nontargets was considerably higher, suggesting that some non-target populations were unable to recover (G.C.M., unpublished data). Most likely, the ASB-treated green vegetation does not provide a visual attractive target for pollinators, providing an explanation for our findings. To standout from the predominant green colors of leaves and stems, plants have flowers and fruits that vary in color. These colors create optical signals that are used to attract insect pollinators (Lee 2007). As a result, ATSB applications, as long as they are applied to green, nonflowering vegetation, would have little attractancy to nontarget populations and avoid any potential unacceptable negative impact. The development of bait stations made with protective grids allowing only small biting flies to feed while excluding other insects like honey bees could be a further enhancement of the ATSB strategy to reduce nontarget effects (unpublished data of authors). These studies provide essential nontarget data that are needed for the development of clear guidelines for appropriate use of the new ATSB control method for guiding the environmentally friendly but effective treatment. Low nontarget impacts are also an important concept to consider when implementing IVM programs.
We also demonstrated that ingesting ATSB-stained insects did not result in predator mortality. As ATSB has to be ingested and is not a contact poison, predatory insects that generally do not feed on plant material have a low probability of being affected by ATSB applications.
We found that whereas nontarget arthropods were neither attracted to nor feeding on the ASB foliar application, mosquitoes had a high level of staining, indicating ingestion of the bait at levels likely sufficient for control. Mosquitoes appear to be guided more by scent than optical targets when sugar-seeking, unlike in host-seeking, which is influenced by color and shape of the object (Allan et al. 1987). Mülleretal. (2010a) demonstrated effective control in storm drain systems by comparing the number of mosquitoes feeding on the ASB solution with the numbers after an ATSB application. Qualls et al. (2012) demonstrated a high level of staining from feeding on ASB (90%) by mosquitoes emerging from cisterns and wells. Based on this level of staining, it was suggested that applications of ATSB could be successful in these storm drain systems. Other studies incorporating a dyed ASB control have demonstrated, in most cases, >50% staining rate while achieving at least that percentage of control in the ATSB treatment sites (Müller and Schlein 2006, 2008; Schlein and Müller 2008; Müller et al. 2008, 2010b; Beier et al. 2012).
Nonbiting midges and mosquitoes have similar resting and sugar-feeding behaviors, providing an explanation for the high impact on these insect families (Armitage et al. 1995). Nonbiting midges are usually regarded as a nuisance pest and have been associated with human allergies and adverse economic impact (Ali 1995). Although they are an important part of the food chain, there is no evidence that they serve any significant importance for pollination. Control of chironomid midges is often difficult and costly, and suitable control strategies have yet to be fully developed (Ali 1995). Based on the staining rate in our study, ATSB could be a new strategy used for controlling this nuisance pest.
This study demonstrates that this novel control strategy applied to foliar vegetation is effective and has low environmental impact. Moreover, with industrial-quality bait concentrates that ensure bait standardization, control could be enhanced for longer periods postapplication. Future research on nontarget populations is needed in other eco-zone and eco-systems for further optimization of this strategy. However, the low nontarget impact and increased sustainability of the ATSB method evaluated in this study further supports this as an IVM strategy that can be incorporated into vector control programs.
Acknowledgments
The research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R01AI100968. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Special thanks to the personnel of the Department of Agriculture in Marrakech, Morocco, and the local authorities for the valuable assistance. We thank the U.S. Department of Agriculture–Agriculture Research Service–Center for Medical, Agricultural and Veterinary Entolmology for supplying the Cx. quinquefasciatus and Ae. aegypti eggs used during this evaluation. We would also like to thank staff and commissioners of the Anastasia Mosquito Control District for supporting this research. Thanks are extended to Faith Umoh for technical assistance and Haze Brown, Chris Swain, and Tim Carney for supplying laboratory colony mosquitoes. Portions of this work were supported by a Deployed War-Fighter Protection Research Program Grant funded by the U.S. Department of Defense through the Armed Forces Pests Management Board the Bill & Melinda Gates Foundation; and internal grants of Institut Agronomique et Vétérinaire Hassan II and Rabat-Instituts, Morocco.
References Cited
- Ali A. A concise review of Chironomid midges (Diptera: Chironomidae) as pests and their management. J Vector Ecol. 1995;21:105–121. [Google Scholar]
- Allan SA. Laboratory evaluation of mosquito susceptibility to insecticides combined with sucrose. J Vector Ecol. 2011;36:59–67. doi: 10.1111/j.1948-7134.2011.00141.x. [DOI] [PubMed] [Google Scholar]
- Allan SA, Day JF, Edman JD. Visual ecology of biting flies. Annu Rev Entomol. 1987;32:297–316. doi: 10.1146/annurev.en.32.010187.001501. [DOI] [PubMed] [Google Scholar]
- Armitage PD, Cranston PS, Pinder LCV. The Chironomidae: biology and ecology of non-biting midges. Chapman & Hall; London, United Kingdom: 1995. [Google Scholar]
- Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favored sugar-source blossoms. Malar J. 2012;11:31. doi: 10.1186/1475-2875-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel V, Duchon S, Zaim M, Hougard JM. Dinotefuran: a potential neonicotinoid insecticide against resistant mosquitoes. J Med Entomol. 2004;41:712–717. doi: 10.1603/0022-2585-41.4.712. [DOI] [PubMed] [Google Scholar]
- Enayati A, Hemingway J. Malaria management: past, present, and future. Annu Rev Entomol. 2010;55:569–591. doi: 10.1146/annurev-ento-112408-085423. [DOI] [PubMed] [Google Scholar]
- (EPA) Environmental Protection Agency. 712-C-017: Ecological effects test guidelines: OCSPP 850.3040: Field Testing for Pollinators 2012a [Google Scholar]
- (EPA) Environmental Protection Agency. 712-C-018: ecological effects test guidelines: OCSPP 850.3030: Honey Bee Toxicity of Residues on Foliage 2012b [Google Scholar]
- (EPA) Environmental Protection Agency. 712-C-019: ecological effects test guidelines: OCSPP 850.3020: Honey Bee Acute Contact Toxicity Test 2012c [Google Scholar]
- Gu W, Müller GC, Schlein Y, Novak RJ, Beier JC. Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PLoS ONE. 2011;6:e15996. doi: 10.1371/journal.pone.0015996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leather SR. Insect sampling in forest ecosystems. Blackwell; Oxford, United Kingdom: 2005. [Google Scholar]
- Lee D. NatureÕs palette. University of Chicago Press; London, England, United Kingdom: 2007. [Google Scholar]
- Müller GC, Schlein Y. Sugar questing mosquitoes in arid areas gather onscarce blossoms that can be used for control. Int J Parasitol. 2006;36:1077–1080. doi: 10.1016/j.ijpara.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Müller GC, Schlein Y. Efficacy of toxic sugar baits against adult cistern-dwelling Anopheles claviger. Trans R Soc Trop Med. 2008;102:480–484. doi: 10.1016/j.trstmh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Müller GC, Kravchenko VD, Schlein Y. Die Erforschung der Israelischen Lepidopteren Fauna. In: Schoenitzer K, editor. Tiere und Kunst aus Israel. Vol. 2. Berichte der Freunde der; ZSM: 2005. pp. 30–39. [Google Scholar]
- Müller GC, Kravchenko VD, Chikatunov D, Ortal R, Orlova O, Li C, Witt T, Speidel W, Mooser J, Hausmann A. General aspects of the Israeli lighttrap network concerning Coleoptera. Esperiana. 2006;12:269–281. [Google Scholar]
- Müller GC, Kravchenko VD, Schlein Y. Decline of Anopheles sergentii and Aedes caspius populations following presentation of attractive toxic (spinosad) sugar bait stations in an oasis. J Am Mos Control Assoc. 2008;24:147–149. doi: 10.2987/8756-971X(2008)24[147:DOASAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Müller GC, Junnila A, Qualls WA, Revay EE, Kline DL, Allan S, Schlein Y, Xue RD. Control of Culex quinquefasciatus in a storm drain system in Florida using attractive toxic sugar baits. J Med Vet Entomol. 2010a;24:346–351. doi: 10.1111/j.1365-2915.2010.00876.x. [DOI] [PubMed] [Google Scholar]
- Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar J. 2010b;9:210. doi: 10.1186/1475-2875-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Revay EE, Beier JC. Simplified and improved monitoring traps for sampling sand flies. J Vector Ecol. 2011;36:454–457. doi: 10.1111/j.1948-7134.2011.00188.x. [DOI] [PubMed] [Google Scholar]
- Naranjo DP, Qualls WA, Alimi TO, Roque DD, Samson DM, Arheart KC, Beier JC, Xue RD. Evaluation of boric acid sugar baits against Aedes albopictus (Diptera:Culicidae) in tropical environments. Parasitol Res. 2013;112:1583–1587. doi: 10.1007/s00436-013-3312-8. [DOI] [PubMed] [Google Scholar]
- Qualls WA, Xue RD, Revay EE, Allan SA, Müller GC. Implications for operational control of adult mosquito production in cisterns and wells in St. Augustine, FL, using attractive sugar baits. Acta Tropica. 2012;124:158–161. doi: 10.1016/j.actatropica.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Schlein Y, Müller GC. An approach to mosquito control: using the dominant attraction of flowering Tamarix jordani trees against Culex pipiens. J Med Entomol. 2008;45:384–390. doi: 10.1603/0022-2585(2008)45[384:aatmcu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Yamamoto I. Structure-activity relationships of nicotinoids and imidacloprid analogs. Nihon Noyaku Gakkaishi. J Pestic Sci. 1993;18:91–98. [Google Scholar]
- (WHO) World Health Organization. Global strategic framework for integrated vector management. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- (WHO) World Health Organization. Handbook for integrated vector management. World Health Press, World Health Organization; Geneva, Switzerland: 2012. pp. 1–78. [Google Scholar]
- Xue RD, Müller GC, Qualls WA, Smith ML, Scott JM, Lear J, Cope SE. Attractive targeted sugar baits: field evaluations and potential for use in mosquito control. Wing Beats. 2013 Spring;:13–18. [Google Scholar]