Abstract
The purpose of this study was to use a retrospective nonlinear distortion correction technique and evaluate the changes in DTI metrics in areas of interest in and around GBM tumors. A total of 24 histologically confirmed GBM patients with pre-operative 20-direction DTI scans were examined. Variability in apparent diffusion coefficient (ADC) and fractional anisotropy (FA) in normal tissue before and after distortion correction were examined. Changes in mean, median and variance of ADC and FA in contrast enhancing and T2/FLAIR ROIs were also examined with and without distortion correction. Results suggest the intra-subject standard deviations of ADC and FA decreased in normal tissue after the application of distortion correction (P<0.0001). FA mean and median values decreased after distortion correction in both T1+C and T2 ROIs (P<0.017), while ADC mean and median values did not significantly change except for the median ADC in T1+C ROIs (P=0.0054). The intra-subject standard deviation of ADC and FA values in tumor ROIs changed significantly with distortion correction, and Bland-Altman analysis indicated that the bias and the standard deviation of the bias of these intra-subject standard deviations were larger than those of the mean and median terms. Additionally, the means of the two curves of a double Gaussian fit to the histogram of ADC values from T1+C ROIs, ADCL (mean of lower Gaussian) as well as ADCH (mean of the higher Gaussian) were found to change significantly with distortion correction (P=0.0045 for ADCL and P=0.0370 for ADCH). Nonlinear distortion correction better aligns neuro-anatomical structures between DTI and anatomical scans, and significantly alters the measurement of values within tumor ROIs for GBM patients.
Keywords: glioblastoma, diffusion tensor imaging, DTI, ADC histogram analysis, distortion correction, MRI
Introduction
Glioblastoma multiforme (GBM) is the most aggressive form of primary brain tumor with low median survival time of 14.6 months using radiotherapy and temozolomide [1]. Infiltration along white matter tracts and subsequent proliferation of tumor cells result in both local and distal recurrence, altering the extracellular matrix and local biological environment [2, 3]. Diffusion tensor imaging (DTI) is a magnetic resonance imaging technique that can be used to probe sub-voxel extracellular microstructure and potentially elicit insight regarding proliferation and infiltration behavior in GBMs. Although controversial, the apparent diffusion coefficient (ADC), a quantity that describes the magnitude of water diffusion, has shown sensitivity to tumor cellularity [4–6], aggressiveness [7, 8], and response to therapies [9, 10]. Fractional anisotropy (FA), a quantitative measure of diffusion anisotropy derived from DTI, has shown sensitivity to tumor infiltration and proliferation [11–14], and is used routinely for preoperative delineation of tumors for resection [15]. However, acquisition of diffusion MR images is prone to geometric distortions from differences in magnetic susceptibility at tissue boundaries [16]. These geometric distortions may lead to inaccurate stereotactic placement of tumor and resulting in imprecise quantitative measurements as well as resection boundaries.
Retrospectively, geometric distortion correction of DTI data can be performed using nonlinear registration of the reference (b=0) image of the DTI dataset with geometric distortions to an anatomical image, and then applying this transformation to rest of the raw DTI data [17, 18]. In the current study we tested whether retrospective, nonlinear distortion correction of DTI data would result in improved anatomical alignment of DTI to anatomical images, reduced variability in FA and ADC measurements within tumor tissue, and altered DTI measurements of tumor characteristics when compared to standard DTI measurement techniques.
Materials and Methods
Patient Population
Patients were retrospectively selected from our neuro-oncology database. All patients signed appropriate institute review board approved consent forms, and image acquisition and processing was performed in conformance with the Health Insurance Portability and Accountability Act (HIPAA) regulations. A total of n=24 subjects were selected based on the following criteria: 1) newly diagnosed (no previous treatments) and pathologically confirmed GBM, 2) uniformly collected high quality pre-operative, 20 direction DTI scans, and 3) high quality anatomical T2 and T1 post-contrast scans. Patient scan dates spanned from August 8th, 2009, to December 11th, 2011. Patient age at time of scan was 64±13 years (mean±standard deviation). At the time of last assessment (October 8th 2012), 14 of the 24 patients were deceased.
MRI
All images were collected on either a 1.5T Siemens Avanto or 3-T Siemens Tim Trio (Siemens Medical, Erlangen, Germany) MR system using sequences supplied by the scanner manufacturer. A total of 6 subjects had images collected on a 1.5T scanner and 18 had images collected on a 3T scanner. Anatomical T2 and gadopentetate dimeglumine enhanced (Magnevist; Berlex; 0.1 mmol/kg) axially acquired 3D T1-weighted volumetric images were acquired. DTI scans were obtained with the following scan parameters: TE/TR=84–100ms/9400–12900ms, NEX=1, slice thickness=2mm with no interslice gap, matrix size=128×128, and FOV=25.6 cm giving a 2×2×2 mm3 voxel size with one b=0 mm2/s and 20 b=1000 mm2/s images in non-collinear directions.
Non-Corrected DTI Estimation
DTI data was re-constructed using FDT, FMRIB’s Diffusion Toolbox in FSL [19] (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT). First, the data was corrected for eddy currents and bulk motion using FSL. The 3×3 2nd order diffusion tensor field, ADC, and FA were then estimated using FSL. DTI metrics were resampled to the resolution of the anatomical T2 images using tri-linear interpolation implemented in the Analysis of Functional NeuroImages (AFNI) software package (http://afni.nimh.nih.gov/afni/). The resulting DTI-derived maps were then used for subsequent analyses.
Distortion-Corrected DTI Estimation
Distortion corrected DTI data were calculated using FSL’s linear (FLIRT) and nonlinear (FNIRT) registration tools. First, eddy current correction was performed using FDT’s eddy current correction. Then, the non-diffusion-weighted b=0 s/mm2 image was linearly registered to the anatomical T2 image using FLIRT with a 12-degree of freedom transformation and mutual information cost function. Next, nonlinear registration was used to warp the registered b=0 s/mm2 image to the anatomical T2-weighted image using a free-form deformation algorithm with a B-spline basis function available using FNIRT [20, 21]. Linear registration matrices and nonlinear warp fields were then applied to the subsequent DTI data series, producing “corrected” ADC and FA maps.
Regions of Interest
Contrast enhancement on post-contrast T1-weighted images (T1+C) and T2 hyperintense regions of interest (ROIs) were examined in the current study. First, anatomical post-contrast 3D T1-weighted images were aligned and resampled to the resolution of T2-weighted images. Then, T1+C or T2 hyperintense ROIs were selected using a semi-automated process of: 1) manually isolating the general region of interest to areas of suspected tumor, 2) applying a case-by-case threshold to isolate T1+C or T2 lesions, and then 3) manually editing the resulting masks to exclude any errors.
Normal-appearing tissue ROIs were selected by applying FMRIB’s automated segmentation tool, FAST [22] (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FAST) to T1-weighted images to segment cerebrospinal fluid (CSF), gray matter (GM) and white matter (WM) using a hidden Markov random field model along with an Expectation-Maximization algorithm and excluding any regions of tumor. Normal-appearing tissue ROIs were then visually checked for accurate segmentation (Figure 1B and 1H).
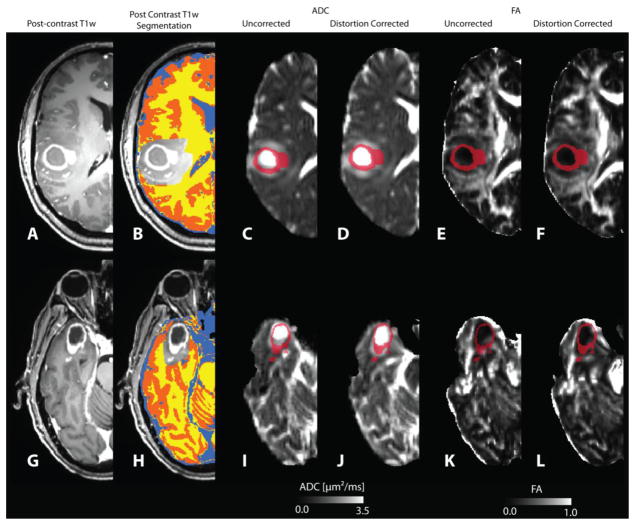
Figure 1.
Alignment of contrast enhancing lesion (T1+C) region of interest (ROI) with uncorrected and distortion-corrected ADC and FA maps for two representative patients. A, G) Post-contrast T1-weighted images. B, H) T1-based tissue segmentation for isolating white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF). Location of T1+C ROIs on uncorrected ADC maps (C,I), distortion-corrected (D,J) ADC maps, uncorrected FA maps (E,K), and distortion-corrected (F.L) FA maps.
ADC Histogram Analysis
ADC histogram analysis was performed using techniques described elsewhere [23, 24]. Briefly, ADC values using both techniques (corrected and non-corrected) were extracted from contrast-enhancing image voxels. Then, a histogram was formed using bin spacing of 0.05 um2/ms and a double Gaussian mixed model was fit to the histogram data using nonlinear regression in GraphPad Prism version 4.0c (GraphPad Software, Inc., La Jolla, CA). The double Gaussian model was defined as: p(ADC)=f·N(μADCL,σADCL)+(1−f)·N(μADCH,σADCH), where p(ADC) is the probability of obtaining a particular value of ADC in the histogram, f is the relative proportion of voxels represented by the lower histogram, N(μ,σ) represents a normal (Gaussian) distribution with mean μ and standard deviation σ, ADCL represents the mean of the lower and ADCH represents the mean of the larger of the two Gaussian distributions. The accuracy of model fits was manually examined to exclude erroneous results. In some cases regression was re-run with different initial conditions until convergence was obtained.
Hypothesis Testing
To test whether nonlinear distortion-correction would improve alignment between DTI data and anatomical images we compared intra-subject standard deviations of FA and ADC values in each tissue type before and after distortion-correction using Wilcoxon matched-pairs signed rank tests, postulating that the variability in ADC and FA would be reduced because of better alignment. To test whether measurements of tumor FA and ADC change after distortion-correction in regions severely affected by geometric distortions we compared the uncorrected and distortion-corrected mean, median and standard deviation within T2 and T1+C ROIs for both FA and ADC using a Wilcoxon matched-pairs signed rank tests. For ADC histogram analysis, we compared uncorrected and distortion-corrected estimates of ADCL and ADCH via Wilcoxon matched-pairs signed rank tests, as well as performing Bland-Altman tests. We also compared the differences between uncorrected and distortion corrected values extracted from each different ROI when adjusted for field strength (1.5T and 3T) using Bonferroni corrected Mann-Whitney tests to determine the effect field strength on distortion correction.
Results
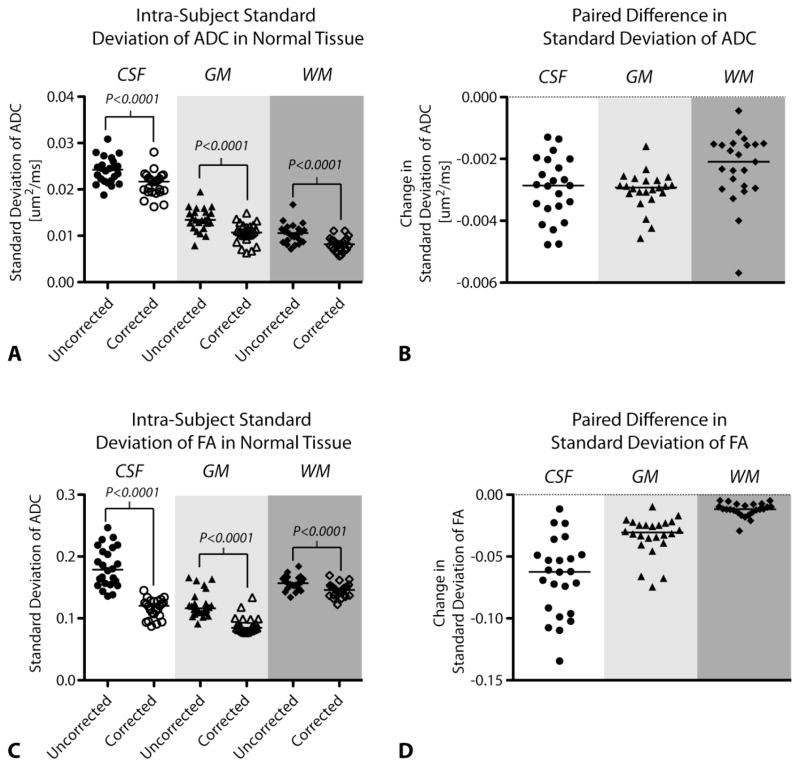
In general, distortion-correction tended to better align DTI data to neuroanatomical structures in the T2 and high-resolution post contrast T1-weighted images as defined in regions near the ventricles, white matter tracts, peritumoral edematous regions, and contrast-enhancing tumor regions. In most cases distortion correction either improved alignment or maintained adequate alignment if original DTI data were relatively distortion free. Without distortion correction, contrast-enhancing regions of interest were often misaligned and included necrotic tissue (high ADC, low FA) and adjacent normal-appearing tissue (Figure 1). This qualitative observation was verified by comparing the standard deviation of FA (Figure 2A) and ADC (Figure 2B) within normal-appearing tissue types. Results indicated a decrease in FA and ADC variability in all normal-appearing tissue ROIs after application of nonlinear distortion correction (Wilcoxon Matched-Pairs Signed Rank Test, P<0.0001 for all comparisons), as demonstrated by the paired differences in measurements shown in Figures 2C–D.
Figure 2.
Standard deviation of ADC and FA in cerebrospinal fluid (CSF), gray matter (GM) and white matter (WM) in uncorrected and distortion-corrected DTI data. Scatter plots of intra-subject standard deviation (SD) values (A,B) showing a decrease in the SD of FA and ADC values after distortion-correction. Paired difference plots (C,D) showing that for each subject, the SD of FA and ADC values in tissue decrease after distortion correction. Line shows median value of group.
Within contrast enhancing tumor ROIs (T1+C), the mean, median, and standard deviation of FA were significantly reduced when examining distortion-corrected compared with uncorrected FA maps (Table 1) Results also showed significant differences in median and standard deviation of ADC values within T1+C ROIs (Table 1). Similar to T1+C ROIs, median, mean, and standard deviation of FA values within T2 hyperintense lesions were also significantly reduced after distortion correction (Table 1); however, only standard deviation in ADC were significantly different between distortion-corrected and uncorrected ADC values within T2 hyperintense ROIs (Table 1).
Table 1.
Bland Altman analyses and Wicoxon matched-pairs signed rank tests for FA and ADC values extracted from T1+C and T2 tumor ROIs. Significance defined as P<0.017 after Bonferroni correction.
| T1+C ROI | Bland Altman Analysis | Wilcoxon matched-pairs signed rank test | |||
|---|---|---|---|---|---|
| Bias (%) | SD of Bias (%) | P Value | Significant? | ||
| FA | Median | −6.772 | 7.307 | 0.0006 | Yes |
| Mean | −6.276 | 6.035 | 0.0001 | Yes | |
| SD | −8.517 | 13.70 | 0.0019 | Yes | |
| ADC | Median | 1.640 | 3.182 | 0.0054 | Yes |
| Mean | 0.07559 | 2.950 | 0.7642 | No | |
| SD | −19.77 | 19.85 | <0.0001 | Yes | |
| T2 ROI | |||||
| FA | Median | −4.889 | 5.101 | <0.0001 | Yes |
| Mean | −6.478 | 5.378 | <0.0001 | Yes | |
| SD | −10.96 | 8.707 | <0.0001 | Yes | |
| ADC | Median | 0.5702 | 2.257 | 0.2472 | No |
| Mean | 0.4717 | 2.109 | 0.3105 | No | |
| SD | −13.43 | 6.194 | <0.0001 | Yes | |
Bland Altman analysis revealed that ADC values tended to increase (positive bias), FA values tended to decrease (negative bias), and the standard deviation of these values tended to decrease (negative bias) after distortion-correction (Table 1). The increase in ADC median and mean values was relatively small within both T1+C (1.6% and 0.08%, respectively) and T2 hyperintense ROIs (0.57% and 0.47%, respectively), suggesting distortion-correction may only result in subtle changes in mean or median ADC measurement. The decrease in FA was more dramatic, showing median and mean FA within T1+C ROIs decreasing 6.8% and 6.3%, respectively, and median and mean FA within T2 hyperintense ROIs decreasing 4.9% and 6.5%, respectively. Although median and mean DTI measurements were only modestly affected by nonlinear distortion correction, standard deviation in ADC and FA, within both T1+C and T2 hyperintense ROIs, demonstrated substantial decreases in variability. For example, standard deviation of ADC measurements within T1+C and T2 hyperintense ROIs reduced approximately 20% and 13%, respectively. Similarly, standard deviation of FA measurements in T1+C and T2 hyperintense ROIs reduced approximately 8.5% and 11%, respectively. When comparing the difference between uncorrected and distortion corrected values in subjects scanned on either a 1.5T or 3T scanner Bonferroni corrected Mann-Whitney tests suggested significant differences in intra-subject standard deviation of ADC in cerebrospinal fluid (P=0.0069), but no significant differences in other parameters. Together, these results suggest ADC and FA values, as well as the variability in these measures, are significantly altered as a result of distortion correction.
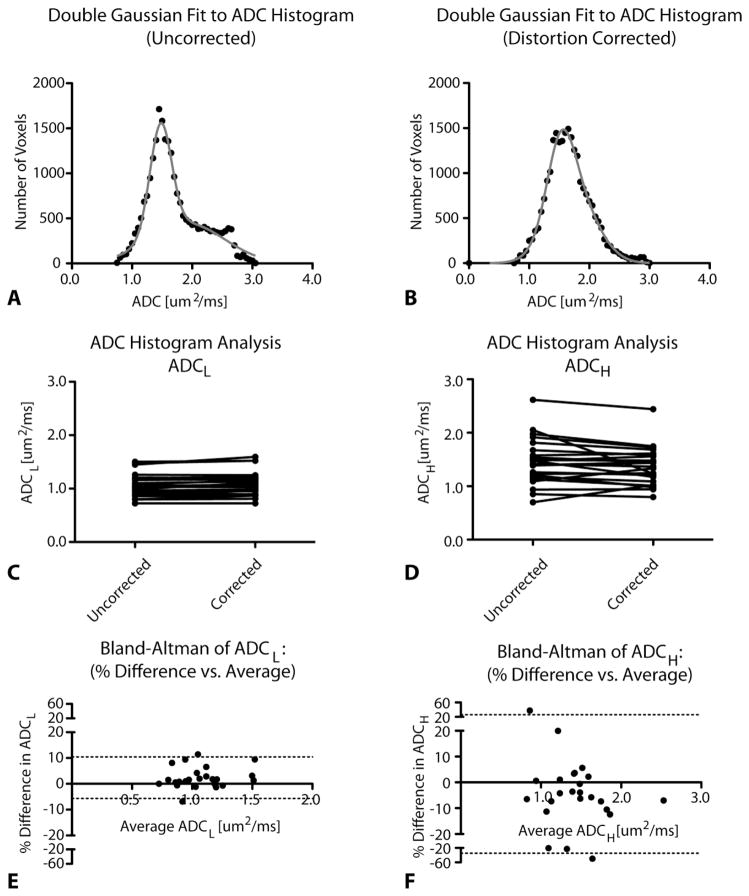
Lastly, we examined how nonlinear distortion correction of DTI data might affect ADC histogram analysis (Figure 3). Results indicated that both ADCL and ADCH appeared altered as a result of distortion correction (Figure 3C–D; Wilcoxon Matched-Pairs Signed Rank, P=0.0045 for ADCL and P=0.0370 for ADCH). ADCL tended to have a low variability (standard deviation of bias of 4.1%) across all ADCL values and positive bias of 2.4%; whereas variations in ADCH were larger (SD of bias of 15%) and tended to decrease (bias of −4.4%) after distortion correction (Figure 3E–F). Figure 3A–B illustrates an example of how ADC histograms changed as a result of distortion correction. In particular, without distortion correction the T1+C ROIs tend to have a well-defined second Gaussian distribution (ADCH), while after distortion correction this distribution was less pronounced and almost could be characterized using a single Gaussian distribution. These results appear to demonstrate that most of the information captured in the higher Gaussian distribution may be the result of misalignment due to geometric distortion of ADC maps.
Figure 3.
ADC histogram analysis results before and after distortion correction. A) Uncorrected and B) distortion-corrected ADC histograms from T1+C ROIs. C) Paired graphs showing ADCL values before and after distortion correction. D) Paired graphs showing ADCH values before and after distortion correction. E) Bland Altman graphs comparing distortion correction for ADCL. F) Bland Altman graphs comparing distortion correction for ADCH. Dashed lines represent 95% limits of agreement.
Discussion
Quantitative measurements of ADC [4, 23, 24] and FA [11–14] derived from DTI have demonstrated the ability to be useful imaging biomarkers for characterizing malignancy and predicting prognosis in patients with glioblastoma. Severe geometric distortion in these scans, however, can complicate the interpretation of these data and may misrepresent underlying microstructural features. In the current study we implemented a simple nonlinear distortion correction technique for retrospectively correcting geometric distortions associated with DTI data and demonstrated that distortion-correction may significantly alter anatomical alignment and diffusion measurements in glioblastoma. We believe this improved anatomical alignment more correctly captures the DTI signal coming from the actual tumor or peritumoral voxels, and thus samples a more homogenous tissue (tumor) and excludes contamination from voxels of other tissue types (normal tissue), though this hypothesis needs histological verification.
Results from the current study showed modest reductions in the average FA within tumor regions, while average ADC changes appeared subtler. This systematic decrease in FA values, but not in ADC values, was also reported by Kim et al [25] and was attributed to geometric distortions in uncorrected DTI data resulting in artificially increasing the FA. While average ADC appeared to be relatively robust and only exhibit small changes after distortion correction, variance in both ADC and FA were dramatically reduced. This reduction in variability in DTI parameters suggests nonlinear distortion correction may result in selection of more homogeneous tissue within ROIs, whereas uncorrected ROIs may contain a variety of tissue types from misalignment of DTI data to the underlying anatomical data.
Additionally, our results showed that ADCL changed significantly with geometric distortions in the DTI data, and ADCH measurements changed even more drastically with the presence of distortion. Interestingly, Pope et al [23, 24] found that pre-treatment ADCL values, but not ADCH values, within contrast-enhancing ROIs could predict response to bevacizumab, a potent anti-angiogenic agent. Results from the current study suggest the lack of predictability in ADCH measurements may in part be due to the presence of geometric distortions in the underlying DWI data; however, future studies aimed at testing whether distortion correction can improve the predictability of ADC histogram analysis in glioblastoma are warranted.
While a few parameters are known to affect the degree of distortion in DTI scans, such as field strength and position of certain structures, we only observed a significant difference in the intra-subject standard deviation of ADC values in CSF when accounting for fields strength; however, this effect may be more pronounced when examining a larger cohort of patients. Similarly, we expect the degree of distortion to be highly dependent o the location of the tumor within the brain. Since the purpose of the current study was to examine the effects of distortion correction on DTI measurements and not to quantify the magnitude of distortion on diffusion images across the topography of the brain, the small sample size and nonuniform distribution of tumors throughout the brain did not make this type of analysis possible in the current study. The effect of tumor location on the magnitude of distortion remains an important unanswered question that requires further investigation.
A few potential limitations in the current study should be addressed. In the current study we chose to use the FNIRT nonlinear registration tool; however, a variety of other distortion-correction algorithms have been developed. Another potential limitation is the relatively small sample size in the current study; however, the use of 24 patients for purposes of demonstrating improved quantitation using nonlinear distortion correction appeared to be adequate.
Although nonlinear distortion correction of diffusion MRI data is relatively well accepted in the MR imaging community, it is not typically incorporated into the clinical evaluation of brain tumors. Our data suggests a relatively simple step of posthoc nonlinear distortion correction of diffusion MRI data increases the alignment between DTI and structural MRI scans, allowing us to improve our ability to more accurately measure diffusion MR parameters, and furthering our understanding of their application neuro-oncology clinical practice.
Acknowledgments
Funding: NIH/NCI R21CA167354 (BME); UCLA Institute for Molecular Medicine Seed Grant (BME); UCLA Radiology Exploratory Research Grant (BME); University of California Cancer Research Coordinating Committee Grant (BME); ACRIN Young Investigator Initiative Grant (BME); Art of the Brain (TFC); Ziering Family Foundation in memory of Sigi Ziering (TFC); Singleton Family Foundation (TFC); and Clarance Klein Fund for Neuro-Oncology (TFC).
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 3.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114:443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, Okuda T, Liang L, Ge Y, Komohara Y, Ushio Y, Takahashi M. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9:53–60. doi: 10.1002/(sici)1522-2586(199901)9:1<53::aid-jmri7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med. 2000;43:828–836. doi: 10.1002/1522-2594(200006)43:6<828::aid-mrm8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Ellingson BM, Malkin MG, Rand SD, Connelly JM, Quinsey C, LaViolette PS, Bedekar DP, Schmainda KM. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging. 2010;31:538–548. doi: 10.1002/jmri.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta RK, Cloughesy TF, Sinha U, Garakian J, Lazareff J, Rubino G, Rubino L, Becker DP, Vinters HV, Alger JR. Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J Neurooncol. 2000;50:215–226. doi: 10.1023/a:1006431120031. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RK, Sinha U, Cloughesy TF, Alger JR. Inverse correlation between choline magnetic resonance spectroscopy signal intensity and the apparent diffusion coefficient in human glioma. Magn Reson Med. 1999;41:2–7. doi: 10.1002/(sici)1522-2594(199901)41:1<2::aid-mrm2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology. 2006;239:632–649. doi: 10.1148/radiol.2393042031. [DOI] [PubMed] [Google Scholar]
- 10.Chenevert TL, Stegman LD, Taylor JM, Robertson PL, Greenberg HS, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an early surrogate marker for therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 11.Beppu T, Inoue T, Shibata Y, Yamada N, Kurose A, Ogasawara K, Ogawa A, Kabasawa H. Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surg Neurol. 2005;63:56–61. doi: 10.1016/j.surneu.2004.02.034. discussion 61. [DOI] [PubMed] [Google Scholar]
- 12.Deng Z, Yan Y, Zhong D, Yang G, Tang W, Lu F, Xie B, Liu B. Quantitative analysis of glioma cell invasion by diffusion tensor imaging. J Clin Neurosci. 2010;17:1530–1536. doi: 10.1016/j.jocn.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita M, Hashimoto N, Goto T, Kagawa N, Kishima H, Izumoto S, Tanaka H, Fujita N, Yoshimine T. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage. 2008;43:29–35. doi: 10.1016/j.neuroimage.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Price SJ, Burnet NG, Donovan T, Green HA, Pena A, Antoun NM, Pickard JD, Carpenter TA, Gillard JH. Diffusion tensor imaging of brain tumours at 3T: a potential tool for assessing white matter tract invasion? Clin Radiol. 2003;58:455–462. doi: 10.1016/s0009-9260(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 15.Tropine A, Vucurevic G, Delani P, Boor S, Hopf N, Bohl J, Stoeter P. Contribution of diffusion tensor imaging to delineation of gliomas and glioblastomas. J Magn Reson Imaging. 2004;20:905–912. doi: 10.1002/jmri.20217. [DOI] [PubMed] [Google Scholar]
- 16.Jezzard P, Clare S. Sources of distortion in functional MRI data. Hum Brain Mapp. 1999;8:80–85. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<80::AID-HBM2>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merhof D, Soza G, Stadlbauer A, Greiner G, Nimsky C. Correction of susceptibility artifacts in diffusion tensor data using non-linear registration. Med Image Anal. 2007;11:588–603. doi: 10.1016/j.media.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Bhushan C, Haldar JP, Joshi AA, Leahy RM. Correcting susceptibility-induced distortion in diffusion-weighted MRI using constrained nonrigid registration. Signal & Information Processing Association Annual Summit and Conference (APSIPA ASC), 2012 Asia-Pacific; 1–9, 2012; [PMC free article] [PubMed] [Google Scholar]
- 19.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 20.Andersson JL, Jenkinson M, Smith S, Andersson J. FMRIB Technical Report TR07JA1. Oxford (UK): FMRIB Centre; Non-linear optimisation. [Google Scholar]
- 21.Andersson JL, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford; 2007. [Google Scholar]
- 22.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. Medical Imaging, IEEE Transactions on. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 23.Pope WB, Kim HJ, Huo J, Alger J, Brown MS, Gjertson D, Sai V, Young JR, Tekchandani L, Cloughesy T, Mischel PS, Lai A, Nghiemphu P, Rahmanuddin S, Goldin J. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252:182–189. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]
- 24.Pope WB, Qiao XJ, Kim HJ, Lai A, Nghiemphu P, Xue X, Ellingson BM, Schiff D, Aregawi D, Cha S, Puduvalli VK, Wu J, Yung WK, Young GS, Vredenburgh J, Barboriak D, Abrey LE, Mikkelsen T, Jain R, Paleologos NA, Rn PL, Prados M, Goldin J, Wen PY, Cloughesy T. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol. 2012;108:491–498. doi: 10.1007/s11060-012-0847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim DJ, Park HJ, Kang KW, Shin YW, Kim JJ, Moon WJ, Chung EC, Kim IY, Kwon JS, Kim SI. How does distortion correction correlate with anisotropic indices? A diffusion tensor imaging study. Magn Reson Imaging. 2006;24:1369–1376. doi: 10.1016/j.mri.2006.07.014. [DOI] [PubMed] [Google Scholar]