Abstract
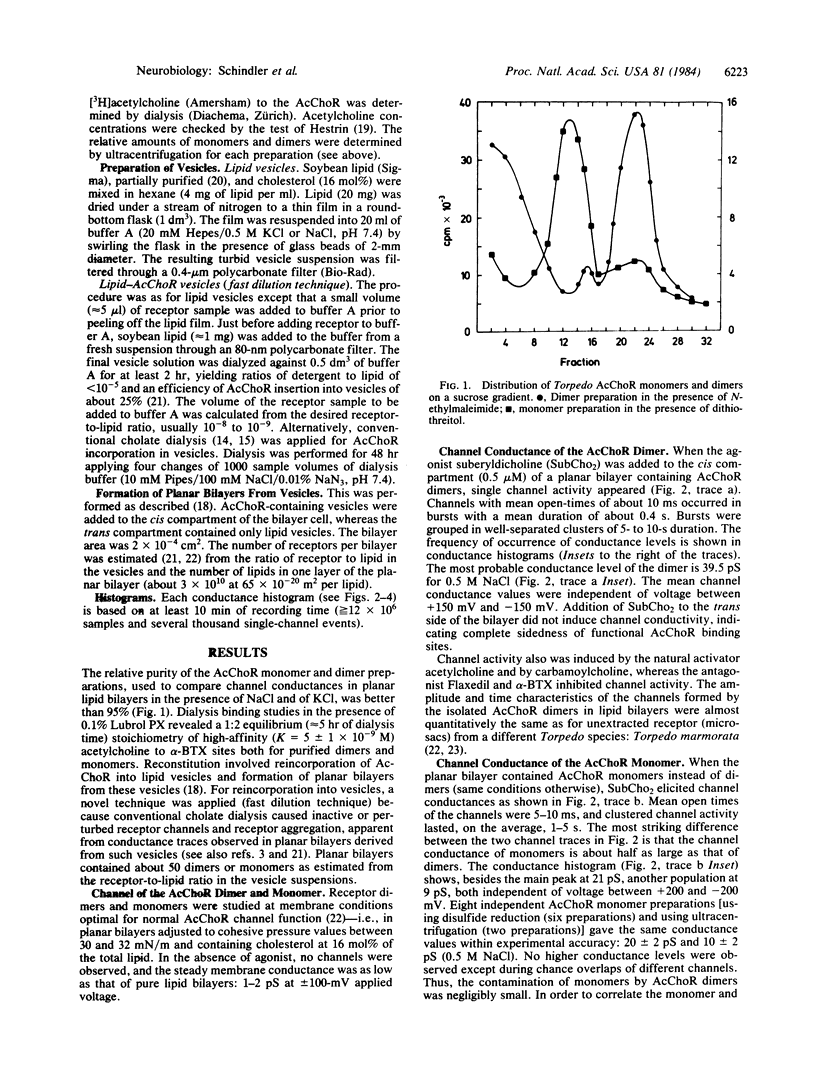
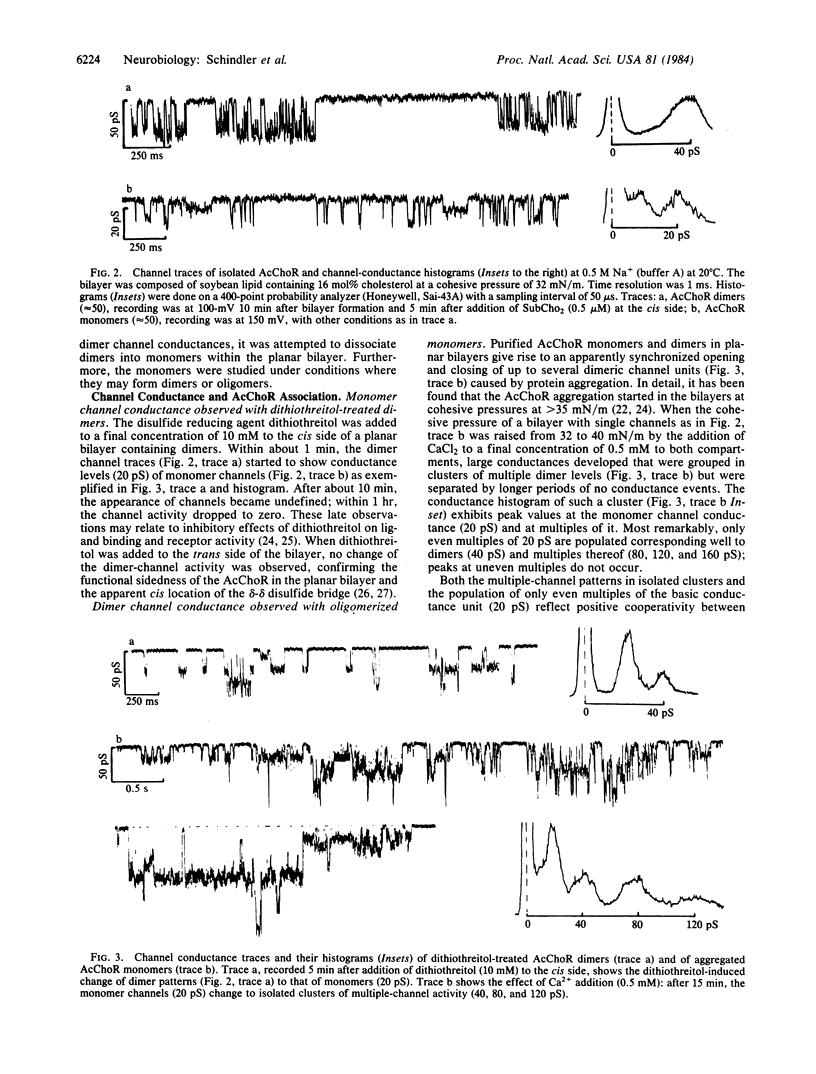
It is demonstrated that the monomeric and dimeric structures of the nicotinic acetylcholine receptor of Torpedo californica electric tissue, reconstituted in planar lipid bilayers, are functionally different. The native dimer D of Mr 500,000 (heavy-form) exhibits a "single" channel conductance about twice as large as that of the monomer M of Mr 250,000 (light form). Under conditions where monomers aggregate, the conductance changes from the level of the monomer M to that of dimers M2. The dimer conductances (D and M2) seem to result from synchronous opening and closing of the two channels in the dimer, giving the impression of "single channel" activity. This channel cooperativity is apparently mediated by noncovalent interactions between the two monomers, since it requires no disulfide linkage between monomers. Both the monomers M and the dimers D and M2 show at least one substate of lower conductivity. The relative population of the two conductance levels depends on the ion type (Na+ and K+), indicating ion-specific channel states. Since the channel conductance of isolated dimers resembles those obtained from unextracted microsacs, the dimer with two synchronized channels appears to be the in vivo predominant gating unit. In the linear association of dimers, observed in the native membrane, channel synchronization may extend to more than two channels as suggested by oligomeric channel cooperativity in associations of monomers and dimers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anholt R., Lindstrom J., Montal M. Functional equivalence of monomeric and dimeric forms of purified acetylcholine receptors from Torpedo californica in reconstituted lipid vesicles. Eur J Biochem. 1980 Aug;109(2):481–487. doi: 10.1111/j.1432-1033.1980.tb04819.x. [DOI] [PubMed] [Google Scholar]
- Bernhardt J., Neumann E. Single channel gating events in tracer flux experiments. III. acetylcholine receptor-controlled Li+ efflux from sealed Torpedo marmorata membrane fragments. Biophys Chem. 1982 Jul;15(4):327–341. doi: 10.1016/0301-4622(82)80016-1. [DOI] [PubMed] [Google Scholar]
- Boheim G., Hanke W., Barrantes F. J., Eibl H., Sakmann B., Fels G., Maelicke A. Agonist-activated ionic channels in acetylcholine receptor reconstituted into planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3586–3590. doi: 10.1073/pnas.78.6.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartaud J., Benedetti E. L., Cohen J. B., Meunier J. C., Changeux J. P. Presence of a lattice structure in membrane fragments rich in nicotinic receptor protein from the electric organ of Torpedo marmorata. FEBS Lett. 1973 Jun 15;33(1):109–113. doi: 10.1016/0014-5793(73)80171-1. [DOI] [PubMed] [Google Scholar]
- Cartaud J., Popot J. L., Changeux J. P. Light and heavy forms of the acetylcholine receptor from Torpedo marmorata electric organ: morphological identification using reconstituted vesicles. FEBS Lett. 1980 Dec 1;121(2):327–332. doi: 10.1016/0014-5793(80)80374-7. [DOI] [PubMed] [Google Scholar]
- Chang H. W., Bock E. Molecular forms of acetylcholine receptor. Effects of calcium ions and a sulfhydryl reagent on the occurrence of oligomers. Biochemistry. 1977 Oct 4;16(20):4513–4520. doi: 10.1021/bi00639a028. [DOI] [PubMed] [Google Scholar]
- Epstein M., Racker E. Reconstitution of carbamylcholine-dependent sodium ion flux and desensitization of the acetylcholine receptor from Torpedo californica. J Biol Chem. 1978 Oct 10;253(19):6660–6662. [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., McLaughlin M., Karlin A. Disulfide bond cross-linked dimer in acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1977 Dec 7;79(3):692–699. doi: 10.1016/0006-291x(77)91167-6. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Salpeter S. R. Organization of acetylcholine receptors in quick-frozen, deep-etched, and rotary-replicated Torpedo postsynaptic membrane. J Cell Biol. 1979 Jul;82(1):150–173. doi: 10.1083/jcb.82.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Bartels E. Effects of blocking sulfhydryl groups and of reducing disulfide bonds on the acetylcholine-activated permeability system of the electroplax. Biochim Biophys Acta. 1966 Nov 8;126(3):525–535. doi: 10.1016/0926-6585(66)90010-0. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Anholt R., Einarson B., Engel A., Osame M., Montal M. Purification of acetylcholine receptors, reconstitution into lipid vesicles, and study of agonist-induced cation channel regulation. J Biol Chem. 1980 Sep 10;255(17):8340–8350. [PubMed] [Google Scholar]
- Miller D. L., Moore H. P., Hartig P. R., Raftery M. A. Fast cation flux from Torpedo californica membrane preparations: implications for a functional role for acetylcholine receptor dimers. Biochem Biophys Res Commun. 1978 Nov 29;85(2):632–640. doi: 10.1016/0006-291x(78)91209-3. [DOI] [PubMed] [Google Scholar]
- Neumann E., Bernhardt J. Ion flow gating by the acetylcholine system: kinetics of isolated receptor and esterase and of receptor-mediated ion flux. J Physiol (Paris) 1981 May;77(9):1061–1072. [PubMed] [Google Scholar]
- Nickel E., Potter L. T. Ultrastructure of isolated membranes of Torpedo electric tissue. Brain Res. 1973 Jul 27;57(2):508–517. doi: 10.1016/0006-8993(73)90158-3. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T. Primary structures of beta- and delta-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature. 1983 Jan 20;301(5897):251–255. doi: 10.1038/301251a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Schindler H. Formation of planar bilayers from artificial or native membrane vesicles. FEBS Lett. 1980 Dec 15;122(1):77–79. doi: 10.1016/0014-5793(80)80405-4. [DOI] [PubMed] [Google Scholar]
- Schindler H., Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci U S A. 1980 May;77(5):3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W., Lukas R. J., McNamee M. G. Effects of thio-group modifications on the ion permeability control and ligand binding properties of Torpedo californica acetylcholine receptor. Biochemistry. 1981 Apr 14;20(8):2191–2199. doi: 10.1021/bi00511a018. [DOI] [PubMed] [Google Scholar]
- Wise D. S., Karlin A., Schoenborn B. P. An analysis by low-angle neutron scattering of the structure of the acetylcholine receptor from Torpedo californica in detergent solution. Biophys J. 1979 Dec;28(3):473–496. doi: 10.1016/S0006-3495(79)85194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. C., Raftery M. A. Functional properties of acetylcholine receptor monomeric and dimeric forms in reconstituted membranes. Biochem Biophys Res Commun. 1981 Mar 31;99(2):436–444. doi: 10.1016/0006-291x(81)91764-2. [DOI] [PubMed] [Google Scholar]
- Zingsheim H. P., Neugebauer D. C., Frank J., Hänicke W., Barrantes F. J. Dimeric arrangement and structure of the membrane-bound acetylcholine receptor studied by electron microscopy. EMBO J. 1982;1(5):541–547. doi: 10.1002/j.1460-2075.1982.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



