Abstract
Background
Kidney disease remains a prevalent problem in HIV care. The contribution of highly active antiretroviral therapy (HAART), HIV disease factors and traditional factors needs further evaluation.
Methods
A cross-sectional study of all patients seen at an HIV outpatient clinic during 2005 was performed. All data were collected from medical record review. Multivariate regression modelling was used to identify independent predictors of lower glomerular filtration rate (eGFR) and chronic renal failure (CRF) from factors significant in univariate analysis. eGFR was calculated using the simplified modification of diet in renal disease equation. Results were compared with those for persons from the National Health and Nutrition Examination Survey (NHANES) matched for age, race and gender.
Results
Of 845 HIV-infected persons, 64% were men and 34% were Caucasian, and the mean age was 39.8 years. Thirty per cent of the patients had proteinuria and 43% had eGFR<90 mL/min/1.73 m2. Persons on HAART (63%) had a lower mean eGFR than those not on HAART (92.0 vs. 101.6). In multivariate analyses, significant predictors of eGFR decline were diagnoses of hypertension, hyperlipidaemia, proteinuria, use of tenofovir or stavudine, and lower viral load. Compared with those in NHANES, HIV-infected persons had a lower mean eGFR (94.9 vs. 104.2) and a higher prevalence of CRF (8% vs. 2%).
Conclusion
In this cohort, the prevalence of CRF is low, but remains higher than that of the general population. Clinicians should routinely screen for early asymptomatic kidney disease to address risk factors that can be treated.
Keywords: AIDS, chronic kidney disease, highly active antiretroviral therapy, HIV
Introduction
The use of combination, highly active antiretroviral therapy (HAART) since the mid-1990s has resulted in significant and sustained reductions in morbidity and mortality from HIV infection, including significant declines in HIV-associated nephropathy (HIVAN) [1]. At the same time, however, a variety of HAART-related renal side effects have been noted, including proteinuria and renal tubular damage, interstitial nephritis, nephrolithiasis, and overall declines in glomerular filtration rate (GFR) [2]. Kidney function has been estimated to be abnormal in up to 30% of HIV-infected patients [3]. In addition, other metabolic complications such as type 2 diabetes and hypertension may also contribute to renal dysfunction over time.
The nucleotide analogue tenofovir has become a prominent component of combination antiretroviral therapy due to its tolerability, convenient dosing, and potency when used with two other active antiretroviral agents, yet concerns regarding its renal tubular toxicity remain. While several case reports and cohort studies have noted varying degrees of tenofovir-associated renal tubular dysfunction or small declines in GFR [4–8], there has been little characterization of renal function overall within large HIV-infected cohorts. Analyses focusing on HIV-associated renal dysfunction in the context of HAART use in general (rather than on persons treated with a specific antiretroviral drug) have found significant benefits of HAART, with overall declines in rates of renal failure and end-stage renal disease [8,9]. In addition, a recent large European cohort study examining chronic renal failure (CRF) in HIV-infected patients found a low overall prevalence of CRF (<5%) [10]. However, the majority of patients were Caucasian and it is unclear if these results can be generalized to a more diverse population or if they are representative of an increase in CRF prevalence over expected rates for a similar HIV-negative population. As life expectancy for HIV-infected persons continues to improve with more potent therapies, it remains critical to identify persons with early mild/moderate renal insufficiency in order to provide the greatest opportunity to modify risk for progression to CRF.
Currently over one million persons in the USA have been diagnosed with HIV/AIDS and can be expected to receive antiretroviral therapy at some point during the course of their disease. Given recent data discouraging the use of HAART interruptions [11], it is expected that the majority of persons who start HAART may continue medications indefinitely and continue to live with HIV infection as a chronic disease. Consequently, treatment guidelines have been developed for the screening and management of chronic kidney disease in HIV-infected persons [3]. Recommendations include routine urinalysis for proteinuria and calculated creatinine clearance or eGFR, as well as careful attention to comorbidities that may contribute to elevated risk of kidney disease. In order to gain better understanding of the prevalence and risk factors for renal dysfunction among HIV-infected patients, all medical records of a large HIV-infected outpatient population were reviewed to (1) describe the overall prevalence of varying degrees of reduced eGFR within this population; (2) determine risk factors for reduced eGFR and chronic kidney disease; and (3) compare the prevalence of reduced eGFR and its associated risk factors with those in a contemporary US cohort matched for age, gender and race.
Methods
Design
This was a retrospective, cross-sectional study of all patients at least 18 years of age who completed two or more visits to the Washington University Outpatient Infectious Diseases Clinic during the year 2005. Data collected on each patient included sociodemographics, body mass index, blood pressure, current and past medication and medical history, and most recent fasting laboratory data (as documented in the medical record) obtained as part of routine care. For patients with multiple visits/fasting laboratory values, the most recent were used for the study. Laboratory data, including urinalysis and measurements of lipids and serum creatinine, were obtained using the same commercial assay (Quest Diagnostics®, Madison, NJ, USA) for all patients. Persons with significant changes in serum creatinine levels were excluded to minimize the impact of acute renal injury. Clinical and laboratory data, including traditional risk factors for kidney disease, were then compared with those obtained from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 data files [12]. The study was reviewed and approved by the Washington University Human Studies Committee.
Definitions
According to the National Kidney Foundation, mild kidney dysfunction (stage 2) is defined as GFR from 60 to 89 mL/min/1.73 m2, moderate dysfunction (stage 3) as GFR from 30 to 59 mL/min/1.73 m2, severe dysfunction (stage 4) as GFR from 15 to 29 mL/min/1.73 m2, and kidney failure (stage 5) as GFR <15 mL/min/1.73 m2 or on dialysis [13]. For this study, we defined kidney dysfunction as eGFR<90 mL/min/1.73 m2 and CRF was defined as eGFR<60 mL/min/1.73 m2. eGFR used in data analyses was calculated using the simplified modification of diet in renal disease (MDRD) equation:
Use of a nephrotoxic agent was defined as current or prior history of taking any of the following medications: nonsteroidal anti-inflammatory drugs, adefovir, cidofovir, gancyclovir/valgancyclovir, trimethoprim-sulfa, amino-glycosides or a chemotherapeutic agent. Hypertension was defined as blood pressure ≥ 140/90 mmHg or currently on medication for hypertension. HAART was defined as the use of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a nonnucleoside reverse transcriptase inhibitor (NNRTI), two NRTIs plus a protease inhibitor (PI), or an NNRTI plus a PI.
Statistical analysis
Data were analysed using χ2 or Fisher's exact test for categorical variables. Continuous variables were compared using Student's t-test or the Mann–Whitney U-test for normally and nonnormally distributed variables, respectively. Bivariate correlation testing was also used to test the strength of association between eGFR and other clinical variables. Significant variables were then entered stepwise into a linear regression model in order to determine the best multivariate model. Variables were assessed for collinearity and adjusted appropriately. Binary logistic regression was performed to determine the risk of any (mild or greater) renal dysfunction based on an eGFR cut-off of <90 mL/min/1.73 m2 and to determine the risk of CRF. HIV RNA levels were log-transformed for analysis. All P-values were two-tailed. For comparisons between HIV-infected patients and seronegative controls, HIV-infected patients were randomly matched to subjects from the NHANES 2003–2004 data files, which are freely accessible on the Centers for Disease Control website [12]. Subjects from NHANES were randomly chosen from those who matched our patients according to age, gender and race. For matched patients chosen from NHANES, HIV testing is only given to those <50 years of age, and thus we could only be certain that NHANES subjects <50 years old were HIV-negative. However, the number of patients >50 years old in the HIV-infected cohort was low (16%). Data were analysed with the use of the SPSS software package version 14.0 (SPSS, Chicago, IL).
Results
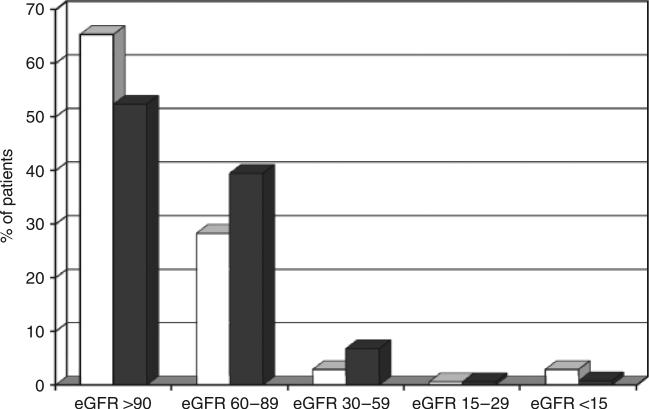
A total of 845 HIV-infected patients (63.7% men and 36.3% women) met the inclusion criteria for study analysis. Of these, 122 were excluded because they had fewer than two clinic visits in 12 months or did not have laboratory values available for analysis. Sixty-four per cent of the patients were African-American and 63% were currently receiving HAART. Forty-three per cent of patients had renal dysfunction (eGFR<90 mL/min/1.73 m2), based on eGFR estimation using the MDRD equation, but only 7.5% had CRF and fewer than 1% had biopsy-proven HIVAN. A similar proportion of patients (6.4%) had CRF as calculated using the Cockcroft–Gault equation. Additional patient characteristics are shown in Table 1. HIV-infected patients with renal dysfunction were more likely to be male, older, of Caucasian race, to have hypertension, proteinuria, higher total cholesterol and higher triglycerides, and to be taking HAART, with lower mean HIV RNA levels compared with patients with normal renal function (all P≤0.05). There was no association between lower eGFR and any prior exposure to nephrotoxic agents. When the relationship between HAART use and renal dysfunction was further explored, nearly 50% of patients on HAART were found to have some degree of renal dysfunction (eGFR<90 mL/min/1.73 m2) compared with only 35% of those not on HAART, mainly at mild/moderate levels of renal dysfunction (P<0.05; see Fig. 1).
Table 1.
Patient characteristics according to renal function as measured using the glomerular filtration rate (eGFR)
| Characteristic | eGFR ≥ 90 mL/min/1.73 m2 (N = 483) | eGFR < 90 mL/min/1.73 m2 (N = 362) | P-value |
|---|---|---|---|
| Age (years) (mean ± SEM) | 37.0 ± 0.5 | 43.5 ± 0.5 | < 0.01 |
| Gender | |||
| Male | 294 (60.9) | 244 (67.4) | 0.05 |
| Female | 189 (39.1) | 118 (32.6) | |
| Race | |||
| Caucasian | 123 (25.5) | 163 (45.0) | < 0.01 |
| African-American | 349 (72.3) | 192 (53.0) | |
| Hispanic | 11 (2.3) | 7 (1.9) | |
| Body mass index (kg/m2) (mean ± SEM) | 27.4 ± 0.3 | 27.5 ± 0.4 | 0.82 |
| Injecting drug use | 20 (4.1) | 21 (5.8) | 0.33 |
| CD4 count (cells/μL) (mean ± SEM) | |||
| Current | 419 ± 13 | 447 ± 16 | 0.16 |
| Nadir | 225 ± 10 | 198 ± 10 | 0.06 |
| On HAART | 279 (57.8) | 253 (69.9) | < 0.01 |
| HIV RNA < 400 copies/mL | 210 (75.3) | 200 (80.2) | 0.30 |
| Presence of proteinuria (n = 534) | 78 (25.2) | 80 (35.7) | < 0.01 |
| Diagnosis of hypertension | 123 (25.5) | 156 (43.1) | < 0.01 |
| Diagnosis of diabetes | 22 (4.6) | 27 (7.5) | 0.10 |
| Chronic hepatitis B | 21 (4.3) | 20 (5.5) | 0.52 |
| Chronic hepatitis C | 50 (10.4) | 49 (13.5) | 0.16 |
| Diagnosis of opportunistic infection | |||
| Current | 25 (5.2) | 20 (5.5) | 0.22 |
| Prior history | 119 (24.6) | 108 (29.8) | |
| Fasting total cholesterol (mg/dL) (mean ± SEM) | 175.5 ± 2.2 | 186.4 ± 3.1 | < 0.01 |
| Fasting LDL (mg/dL) (mean ± SEM) | 101.0 ± 2.2 | 106.1 ± 3.1 | 0.17 |
| Fasting HDL (mg/dL) (mean ± SEM) | 47.9 ± 0.9 | 47.2 ± 1.1 | 0.65 |
| Fasting TG (mg/dL) (mean ± SEM) | 149.6 ± 6.5 | 183.0 ± 8.4 | < 0.01 |
Values are n (%), unless otherwise indicated.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; SEM, standard error of the mean; TG, triglycerides.
Fig. 1.
Level of kidney function as measured using the glomerular filtration rate (eGFR; mL/min/1.73 m2) in an HIV-infected cohort according to current highly active antiretroviral therapy (HAART) status (open bars, not on HAART; shaded bars, on HAART).
When the use of specific antiretrovirals was examined, a significant negative correlation was found between eGFR and current use of several different antiretrovirals (tenofovir, ritonavir, stavudine, indinavir, emtricitabine and efavirenz; P≤0.05 for all), with the strongest correlation with tenofovir (r = –0.128, P<0.001). Among patients on tenofovir, 58% were also on low-dose ritonavir and 33% on efavirenz. There was a significant negative correlation between eGFR and tenofovir in combination with either of these other antiretrovirals (P<0.05 for both). However, there was no association between tenofovir and the presence of proteinuria (there was a significant association with ritonavir; P<0.001). Overall in multivariate analyses using eGFR as a continuous outcome variable, significant predictors of lower eGFR were greater age, white race, the presence of proteinuria, and the use of tenofovir or stavudine (adjusted r2 = 0.22, P<0.05 for all). When variables were excluded in the MDRD equation (age, race and gender), significant predictors of eGFR decline were a diagnosis of hypertension, higher total cholesterol, the presence of proteinuria, the use of tenofovir or stavudine, and lower HIV RNA (adjusted r2 = 0.11, P<0.05 for all). Separate analyses were also performed with eGFR as a dichotomous variable in order to determine the odds ratios (ORs) of any renal dysfunction based on an eGFR cut-off of <90 mL/min/1.73 m2 and to determine the risk of CRF (Table 2).
Table 2.
Predictors of renal dysfunction (multivariate analysis)
| Characteristic | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Predictors of eGFR < 90 mL/min/1.73 m2 | |||
| Age | 1.05 (per year increase) | 1.03–1.07 | < 0.01 |
| African-American race | 0.34 | 0.22–0.51 | < 0.01 |
| Presence of proteinuria | 2.06 | 1.34–3.17 | < 0.01 |
| Diagnosis of hypertension | 1.78 | 1.17–2.70 | 0.01 |
| Current tenofovir use | 1.56 | 1.04–2.33 | 0.03 |
| Predictors of chronic renal failure (eGFR < 60 mL/min/1.73 m2) | |||
| Nadir CD4 cell count | 1.01* | 1.00–1.01 | 0.03 |
| Presence of proteinuria | 4.96 | 1.84–13.35 | < 0.01 |
| Diagnosis of hypertension | 6.81 | 2.39–19.41 | < 0.01 |
Per cell decrease.
eGFR, glomerular filtration rate.
Race/ethnicity and HIV-infected patients
African-Americans in particular may be at higher risk for renal disease and, as the majority of patients were African-American, we further explored racial differences in renal function given the initial unexpected findings in univariate analyses. Although African-Americans were more likely to have proteinuria than Caucasians [OR = 1.79; confidence interval (CI) 1.18–2.69], they were also less likely to be on HAART (OR 5 0.44; CI 0.32–0.61), and were younger (P<0.05) compared with Caucasians. There were no significant differences in the prevalence of renal dysfunction between men and women when stratified by race.
The HIV-infected cohort vs. the NHANES cohort
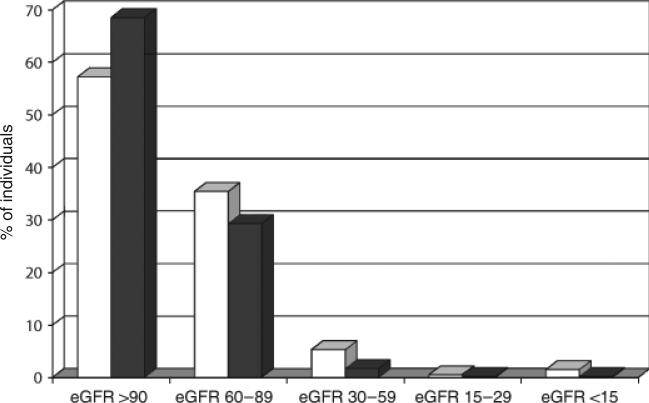
The prevalence of renal dysfunction was increased in the HIV-infected patients compared with the controls from NHANES (42.8% vs. 31.5%; P<0.001). There were greater proportions of HIV-infected persons at lower levels of kidney function compared to NHANES (Table 3 and Fig. 2), and HIV-infected patients had overall higher mean creatinine levels (1.13 vs. 0.95 mg/dL; P<0.01). In addition, HIV-infected persons were more likely to have other risk factors for kidney disease, including diagnosis of hypertension, chronic hepatitis and any history of injecting drug use (IDU) (Table 3).
Table 3.
Comparison between HIV-infected patients and National Health and Nutrition Examination Survey (NHANES) controls (HIV-uninfected)
| Characteristic | HIV-infected patients (N = 845) | NHANES controls (N = 845) | P-value |
|---|---|---|---|
| Age (years) (mean ± SEM) | 39.8 ± 0.4 | 39.8 ± 0.4 | 1.00 |
| Gender | |||
| Male | 538 (63.7) | 538 (63.7) | 1.00 |
| Female | 307 (36.3) | 307 (36.3) | |
| Race | |||
| Caucasian | 286 (33.8) | 286 (33.8) | 1.00 |
| African-American | 541 (64.0) | 541 (64.0) | |
| Hispanic | 18 (2.1) | 18 (2.1) | |
| eGFR (mL/min/1.73 m2) (mean ± SEM) | 94.9 ± 0.9 | 104.2 ± 0.9 | < 0.01 |
| Creatinine (mg/dL) (mean ± SEM) | 1.13 ± 0.04 | 0.95 ± 0.02 | < 0.01 |
| Level of kidney function | |||
| eGFR > 90 mL/min/1.73 m2 | 483 (57.2) | 579 (68.5) | < 0.01 |
| eGFR 60–89 mL/min/1.73 m2 | 299 (35.4) | 248 (29.3) | < 0.01 |
| eGFR 30–59 mL/min/1.73 m2 | 45 (5.3) | 14 (1.7) | < 0.01 |
| eGFR 15–29 mL/min/1.73 m2 | 5 (0.6) | 2 (0.2) | 0.33 |
| eGFR < 15 mL/min/1.73 m2 | 13 (1.5) | 2 (0.2) | < 0.01 |
| Body mass index (kg/m2) (mean ± SEM) | 27.4 ± 0.2 | 29.0 ± 0.3 | < 0.01 |
| Injecting drug use | 41 (4.9) | 17 (2.0) | 0.02 |
| Diagnosis of hypertension | 279 (33.0) | 211 (24.9) | < 0.01 |
| Diagnosis of diabetes | 49 (5.8) | 47 (5.6) | 0.10 |
| Chronic hepatitis B | 41 (4.9) | 6 (0.7) | < 0.01 |
| Chronic hepatitis C | 99 (11.7) | 46 (5.4) | < 0.01 |
| Fasting total cholesterol (mg/dL) (mean ± SEM) | 180.3 ± 1.8 | 197.7 ± 1.5 | < 0.01 |
| Fasting LDL (mg/dL) (mean ± SEM) | 103.2 ± 1.8 | 120.7 ± 1.9 | < 0.01 |
| Fasting HDL (mg/dL) (mean ± SEM) | 47.6 ± 0.7 | 52.0 ± 0.5 | < 0.01 |
| Fasting TG (mg/dL) (mean ± SEM) | 164.4 ± 5.2 | 130.3 ± 3.9 | < 0.01 |
Values are n (%), unless otherwise indicated.
eGFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SEM, standard error of the mean; TG, triglycerides.
Fig. 2.
Comparison of level of kidney function, as measured using the glomerular filtration rate (eGFR; mL/min/1.73 m2), for HIV-infected patients (open bars) vs. individuals from the National Health and Nutrition Examination Survey (NHANES; shaded bars).
Discussion
In a large, predominantly African-American HIV-infected outpatient population, we found a high prevalence of mild/moderate renal dysfunction and proteinuria despite a very low prevalence of CRF. In addition, the prevalence of renal dysfunction was significantly higher than that in a well-matched control population. Although HIV-infected persons were more likely to have other risk factors for renal disease such as chronic hepatitis and history of IDU, the results of multivariate analyses suggest that HAART-related and certain traditional risk factors (i.e. age and hypertension) were more significant predictors of overall decline in renal function.
Use of HAART has been associated with many of the significant predictors of lower eGFR in this study, including hyperlipidaemia, hypertension (which may occur as part of a constellation of other HAART-related metabolic and morphological complications) and renal tubular dysfunction [2, 14,15]. In this regard, the finding of lower HIV RNA as a significant predictor of decline in eGFR was a likely surrogate for adherence to HAART. The overall prevalence of CRF was nonetheless extremely low, supporting a change in the epidemiology of renal disease with fewer causes of nephropathy attributable to advanced HIV infection.
Notably, we found that multiple antiretrovirals were significantly correlated with declining renal function. This is not surprising as HAART generally consists of the combination of three or more antiretrovirals and the combination of tenofovir/emtricitabine/efavirenz as a once-daily regimen is now in greater widespread use. Recent studies have also found an association between declining kidney function and the use of tenofovir in combination with other drugs such as ritonavir, didanosine or amprenavir [10,16]. In the present study, we did not find any specific tenofovir combination to be a significant predictor of lower eGFR in multivariate analyses; rather, only current use of tenofovir itself or stavudine was significantly associated with lower eGFR. As these drugs may be conveniently dosed for persons with chronic kidney disease or on haemodialysis, we examined the possibility of a selection bias for the use of these drugs in persons with advanced kidney disease. However, only 39 and 10% of persons with established CRF were on tenofovir and stavudine, respectively. Use of HAART or specific antiretrovirals (tenofovir and stavudine) was overall predictive of mild/moderate decreases in eGFR rather than CRF. While tenofovir has been associated with Fanconi’s syndrome [4,5], we found no evidence of a significant association with either tenofovir or stavudine and the presence of proteinuria. Other sensitive indicators of early tubular dysfunction, such as the presence of microalbuminuria or glycosuria, were not measured as standard-of-care in this population, but may be worthy of study as potential earlier predictors of eGFR decline.
The underlying mechanism whereby HIV medications, specifically tenofovir, induce renal toxicity remains unclear. There are specific human organic anion transporters (hOAT1 and hOAT3) in combination with multidrug resistance proteins (MRP2 and MRP4) in the proximal renal tubule that secrete tenofovir into the urine [17]. There has been concern that ritonavir-boosted PIs may increase the risk of tenofovir-induced renal toxicity by blocking this secretory pathway. While large clinical trials in treatment-naïve patients (who have normal renal function at baseline) have not reported a negative impact of tenofovir on renal function [18,19], subsequent studies have reported that tenofovir given with boosted PIs yields higher plasma levels of tenofovir and causes significant declines in renal function [20,21]. The latter of these studies also identified certain genetic polymorphisms in the transporter genes that are associated with the decreased clearance of tenofovir [21]. Additional research into these areas will increase our understanding of the renal toxicity of antiretroviral therapy, but, at present, clinicians do not have the tools available to evaluate such toxicity and must take a proactive approach to monitoring kidney function.
The association between abnormal lipid parameters, specifically elevated total cholesterol and triglyceride levels, and renal dysfunction in this analysis is intriguing. There have been several studies published that suggest that hyperlipidaemia is associated with the development of renal dysfunction and progression of renal disease [22,23]. While these data come from studies in diabetic patients, the fact that both HIV itself and HIV medications cause alterations in lipid parameters suggests a possible mechanism of renal dysfunction in HIV-infected patients. However, renal dysfunction is associated with specific lipid abnormalities (elevated triglycerides and low-density lipoprotein) caused by alterations in the transport of lipoproteins [24, 25]. While the exact mechanism is unclear, it is reasonable to propose that the pro-atherosclerotic lipid particles induce changes in the renal vasculature, with negative consequences [26,27]. With the aberrant lipid parameters often seen in HIV-infected patients, there is additional reason to aggressively address renal dysfunction.
This study has some limitations. Given the cross-sectional design of the study it is unclear whether these findings represent a possible trend towards decreasing eGFR with longer use of HAART, or if other factors (i.e. advancing age or further immune reconstitution) may outweigh any HAART-associated effects on eGFR over time. Although we excluded persons with significant changes in serum creatinine, some study subjects may have had acute renal dysfunction as an underlying cause of their reduced eGFR. Given that our study was cross-sectional, other causes of changes in serum creatinine, such as recent changes in muscle mass, may have reduced eGFR. The exclusion of persons with fluctuating serum creatinine was designed to eliminate individuals with acute changes in eGFR. We were unable to capture complete cumulative antiretroviral history for clinic patients as many had initiated HAART much earlier through a different care facility or had been intermittently lost to follow-up. Data from one previous study found that risk factors for CRF were similar for the comparison of current vs. cumulative exposure to specific antiretrovirals [10], but it is not known if this would be true for mild/moderate renal dysfunction. We are also limited by the inclusion of all nephrotoxic agents. The agents included in this analysis were available in the electronic medical database. We may have underestimated the impact of OTC (over-the-counter) medications that were not reported as well as other toxic agents that were not captured in the database. The use of eGFR between 60 and 89 mL/min/1.73 m2 may have overestimated the prevalence of renal dysfunction as we did not have urinalysis data on all of these subjects. However, there have been limited data published on HIV-infected persons with mild/moderate reductions in renal function, a group in whom intervention is warranted to prevent the progression to CRF. Finally, neither the MDRD nor the Cockcroft– Gault equation has been properly validated in the HIV-infected population. Although current guidelines cite a preference for the MDRD for kidney staging purposes in this population [3], both equations may be less accurate in populations without a high prevalence of CRF [28]. In this regard, comparison with an appropriate control group (NHANES) was particularly important. The NHANES cohort also has a low prevalence of advanced kidney disease and we were able to match patients on important parameters (age, race and gender) utilized in the MDRD equation. Other strengths of the study included the large sample size and the high percentage of African-American patients, the heterogeneity of antiretroviral regimens that could be analysed, and the analysis of predictors of early decline in renal function. To our knowledge, this study is one of the first to document a very high prevalence of early, mild asymptomatic renal dysfunction among a large urban HIV-infected outpatient population. These findings support the need to initiate screening for renal disease early in the treatment of HIV infection, including routine eGFR calculation and urinalysis.
In conclusion, despite a low prevalence of CRF among HIV-infected outpatients, there was a high prevalence of asymptomatic renal dysfunction that was significantly greater than that of a well-matched control population. Declines in estimated eGFR appeared to be attributable not only to traditional risk factors for kidney disease but also to therapy-related factors, particularly among patients with an earlier stage of renal dysfunction. As patients continue to age and remain longer on HAART, further longitudinal studies will be needed to determine whether longer-term use of certain antiretrovirals will result in continued declines in renal function over time, or whether more traditional risk factors (i.e. advancing age and hypertension) will carry greater importance.
At present, clinicians should remain vigilant in screening for evidence of subtle declines in renal function in HIV-infected patients and treat modifiable risk factors for chronic kidney disease.
Acknowledgements
Financial support. Funding was received from the National Institutes of Health (1 K23 AI06533601 to KM; 5 U01 A125903-18 to ETO; and P30 DK056341 to JF).
Footnotes
These data were presented at the 14th Conference on Retroviruses and Opportunistic Infections held in Los Angeles, California on 25–28 February 2007.
Potential conflicts of interest. ETO receives grants and research support from Abbott, GlaxoSmithKline, Merck, Tibotec, Bristol-Myers Squibb, Gilead and Bavarian Nordic. He also serves as a consultant for Abbott, GlaxoSmithKline, Tibotec, Bristol-Myers Squibb and Gilead. DN received research support from Gilead. All the remaining authors have no conflicts of interest to disclose.
References
- 1.Lucas GM, Eustace JA, Sozio S, et al. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy and response to highly active antiretroviral therapy. Lancet. 1998;352:783–784. doi: 10.1016/S0140-6736(98)24037-2. [DOI] [PubMed] [Google Scholar]
- 2.Röling J, Schmid H, Fischereder M, et al. HIV-associated renal diseases and highly active antiretroviral therapy-induced nephropathy. Clin Infect Dis. 2006;42:1488–1495. doi: 10.1086/503566. [DOI] [PubMed] [Google Scholar]
- 3.Gupta SK, Eustace JA, Winston JA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005;40:1559–1585. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 4.Verhelst D, Mone M, Meynard JL, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis. 2002;40:1331–1333. doi: 10.1053/ajkd.2002.36924. [DOI] [PubMed] [Google Scholar]
- 5.Peyrière H, Reynes J, Rouanet I, et al. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr Hum Retrovirol. 2004;35:269–273. doi: 10.1097/00126334-200403010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Gallant JE, Parish MA, Keruly JC, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase treatment. Clin Infect Dis. 2005;40:1194–1198. doi: 10.1086/428840. [DOI] [PubMed] [Google Scholar]
- 7.Mauss S, Berger F, Schmutz G. Antiretroviral therapy with tenofovir is associated with mild renal dysfunction. AIDS. 2005;19:4555–4562. doi: 10.1097/00002030-200501030-00012. [DOI] [PubMed] [Google Scholar]
- 8.Jones R, Stebbing J, Nelson M, et al. Renal dysfunction with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy regimens is not observed more frequently: a cohort and case-control study. J Acquir Immune Defic. 2004;37:1489–1495. doi: 10.1097/01.qai.0000138983.45235.02. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz EJ, Szczech LA, Ross MJ, et al. Highly active antiretroviral therapy and the epidemic of HIV-positive end-stage renal disease. J Am Soc Nephrol. 2005;16:2412–2420. doi: 10.1681/ASN.2005040340. [DOI] [PubMed] [Google Scholar]
- 10.Mocroft A, Kirk O, Gatell J, et al. Chronic renal failure among HIV-1-infected patients. AIDS. 2007;21:1119–1127. doi: 10.1097/QAD.0b013e3280f774ee. [DOI] [PubMed] [Google Scholar]
- 11.The Strategies for Management of Antiretroviral Therapy (SMART) Study Group CD4 count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 12. [14 February 2007];The NHANES 2003–2004 datafiles. Available at: www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/demo03_04.htm.
- 13.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 14.Gazzaruso C, Bruno R, Garzaniti A, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21:1377–1382. doi: 10.1097/01.hjh.0000059071.43904.dc. [DOI] [PubMed] [Google Scholar]
- 15.Mondy K, Tebas P. Cardiovascular risks of antiretroviral therapies. Annu Rev Med. 2007;58:141–155. doi: 10.1146/annurev.med.58.072905.180040. [DOI] [PubMed] [Google Scholar]
- 16.Crane HM, Kestenbaum B, Harrington RD, Kitahata MM. Amprenavir and didanosine are associated with declining kidney function among patients receiving tenofovir. AIDS. 2007;21:1431–1439. doi: 10.1097/QAD.0b013e3281fc9320. [DOI] [PubMed] [Google Scholar]
- 17.Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50:3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izzedine H, Hulot JS, Vittecoq D, et al. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naïve HIV-1-infected patients: data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant. 2005;20:743–746. doi: 10.1093/ndt/gfh658. [DOI] [PubMed] [Google Scholar]
- 19.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs. stavudine in combination therapy in antiretroviral-naïve patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 20.Goicoechea M, Liu S, Best B, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based vs. nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008;197:102–108. doi: 10.1086/524061. [DOI] [PubMed] [Google Scholar]
- 21.Kiser JJ, Carten ML, Aquilante CL, et al. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin Pharmacol Ther. 2008;83:265–272. doi: 10.1038/sj.clpt.6100269. [DOI] [PubMed] [Google Scholar]
- 22.Ravid M, Brosh D, Ravid-Safran D, et al. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158:998–1004. doi: 10.1001/archinte.158.9.998. [DOI] [PubMed] [Google Scholar]
- 23.Muntner P, Coresh J, Smith C, et al. Plasma lipids and risk of developing renal dysfunction: the Atherosclerosis Risk in Communities Study. Kidney Int. 2000;58:293–301. doi: 10.1046/j.1523-1755.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 24.Trevisan R, Dodesini AR, Lepore G. Lipids and renal disease. J Am Soc Nephrol. 2006;17:S145–S147. doi: 10.1681/ASN.2005121320. [DOI] [PubMed] [Google Scholar]
- 25.Vaziri ND. Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int. 2003;63:1964–1976. doi: 10.1046/j.1523-1755.2003.00941.x. [DOI] [PubMed] [Google Scholar]
- 26.Chait A, Heinecke JW. Lipoprotein modification: cellular mechanisms. Curr Opin Lipidol. 1994;5:365–370. doi: 10.1097/00041433-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Abrass CK. Cellular lipid metabolism and the role of lipids in progressive renal disease. Am J Nephrol. 2004;24:46–53. doi: 10.1159/000075925. [DOI] [PubMed] [Google Scholar]
- 28.Stevens LA, Coresh J, Greene G, Levey AS. Assessing kidney function – measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]