Abstract
Aims/hypothesis
Secondary type 1 diabetes prevention trials require selection of participants with impending diabetes. HLA-A and -B alleles have been reported to promote disease progression. We investigated whether typing for HLA-B*18 and -B*39 may complement screening for HLA-DQ8, -DQ2 and -A*24 and autoantibodies (Abs) against islet antigen-2 (IA-2) and zinc transporter 8 (ZnT8) for predicting rapid progression to hyperglycaemia.
Methods
A registry-based group of 288 persistently autoantibody-positive (Ab+) offspring/siblings (aged 0–39 years) of known patients (Ab+ against insulin, GAD, IA-2 and/or ZnT8) were typed for HLA-DQ, -A and -B and monitored from the first Ab+ sample for development of diabetes within 5 years.
Results
Unlike HLA-B*39, HLA-B*18 was associated with accelerated disease progression, but only in HLA-DQ2 carriers (p < 0.006). In contrast, HLA-A*24 promoted progression preferentially in the presence of HLA-DQ8 (p < 0.002). In HLA-DQ2- and/or HLA-DQ8-positive relatives (n = 246), HLA-B*18 predicted impending diabetes (p = 0.015) in addition to HLA-A*24, HLA-DQ2/DQ8 and positivity for IA-2A or ZnT8A (p ≤ 0.004). HLA-B*18 interacted significantly with HLA-DQ2/DQ8 and HLA-A*24 in the presence of IA-2 and/or ZnT8 autoantibodies (p ≤ 0.009). Additional testing for HLA-B*18 and -A*24 significantly improved screening sensitivity for rapid progressors, from 38% to 53%, among relatives at high Ab-inferred risk carrying at least one genetic risk factor. Screening for HLA-B*18 increased sensitivity for progressors, from 17% to 28%, among individuals carrying ≥3 risk markers conferring >85% 5 year risk.
Conclusions/interpretation
These results reinforce the importance of HLA class I alleles in disease progression and quantify their added value for preparing prevention trials.
Keywords: Autoantibodies, HLA-A, HLA-B, HLA class I, HLA class II, HLA-DQ, Prediction, Prevention, Risk assessment, Type 1 diabetes
INTRODUCTION
HLA-A*24 was recently shown to complement HLA-DQ2/DQ8 and islet antigen-2 (IA-2) and zinc transporter 8 (ZnT8) autoantibodies (IA-2A and ZnT8A, respectively) for identifying first-degree relatives (FDRs) with impending diabetes [1] who may qualify for participation in immunointervention trials [2]. HLA-B alleles were also reported to promote disease progression in risk groups for type 1 diabetes, some studies identifying B*18 as an accelerator [3], others implicating B*39 [4]. There is, however, agreement on the need to adjust for closely linked HLA class II haplotypes when looking for class I effects [4, 5]. HLA-A*24 was proposed to interact preferentially with HLA-DQ8 or DQ8/DQ2 [6], HLA-B*18 with HLA-DQ2 [7] and HLA-B*39 with HLA-DR3/DR4 [4].
While the mechanisms behind the disease-accelerating role of HLA class I alleles remain hypothetical, the added value of typing for HLA-B*18 and -B*39 on the selection of participants for immunointervention trials remains largely unknown. Therefore, we investigated, first, whether HLA-B*18 and -B*39 are associated with accelerated progression to diabetes in a registry-based group of persistently autoantibody-positive (Ab+) FDRs younger than 40 years and stratified according to HLA-DQ risk haplotype and, second, whether they represent additional predictors of impending diabetes that may usefully assist IA-2A, ZnT8A, HLA-DQ2/DQ8 and HLA-A*24 in identifying high risk individuals.
METHODS
Study population
Persistently Ab+ FDRs (positive for insulin autoantibodies [IAA], GAD autoantibodies [GADA], IA-2A and/or ZnT8A; n = 288 [134 offspring and 154 siblings]) of type 1 diabetes probands were identified and followed by the Belgian Diabetes Registry as reported [1]. At entry, and during follow-up, blood was sampled and a short questionnaire completed after informed consent. Inclusion criteria are shown in electronic supplementary material (ESM) Fig. 1. At baseline (first Ab+ sample), the median age (interquartile range [IQR]) of FDRs (156 males and 132 females) was 12 (6–19) years. Of these FDRs, 68% (n = 197) were positive at recruitment and 32% (n = 91) seroconverted to persistent Ab positivity after a median (IQR) follow-up of 36 (24–72) months. The progression rate to diabetes from the first Ab+ sample was similar in initially Ab+ relatives and in seroconverters [8]. During a median (IQR) total follow-up time from the first Ab+ sample of 71 (36–120) months, 33% (n = 96) out of 288 relatives progressed to type 1 diabetes whereas 67% (n = 192) did not. Since we focused on rapid progression to diabetes, the follow-up after the first Ab+ sample was truncated at 60 months, thereby reducing the proportion of progressors to 17% (n = 50). After truncation, the median (IQR) follow-up time was 60 (36–60) months (73% of FDRs completed the 5 year follow-up). Diabetes was diagnosed according to ADA criteria [9] and ascertained as reported [1]. The 288 FDRs were typed for HLA-B*18 and HLA-B*39 status and the results were analysed for the entire group with and without stratification for HLA-DQ2 or HLA-DQ8. Further analysis was performed in the subgroup of 246 relatives (85%; ESM Fig. 1) carrying HLA-DQ2 and/or -DQ8. Their baseline characteristics (ESM Table 1) closely resembled those of the entire group [1]. The study protocol was approved by the ethics committees of the Belgian Diabetes Registry and participating university hospitals and was carried out according to The Helsinki Declaration as revised in 2008 (www.wma.net/en/30publications/10policies/b3/, accessed 18 April 2013).
Analytical methods
HLA-DQ and -A*24 [1] and autoantibodies [8] were previously determined. HLA-B*39 and HLA-B*18 were typed by a PCR sequence-specific oligonucleotide dot-blot method as reported for HLA-A*24 [1, 10], using group-specific primers (ESM Table 2). The probe panels identified all 102 HLA-B*39 alleles except HLA-B*39:01:11/30/33/34/36/43 and all 103 HLA-B*18 alleles except HLA-B*18:29/72 (www.ebi.ac.uk/ipd/imgt/hla/probe.html, release 3.10, accessed 18 April 2013).
Statistical analyses
McNemar and χ2 tests (Fisher’s exact test when appropriate) were used to compare proportions between groups in paired and independent samples, respectively, Mann–Whitney U test for differences between groups for continuous data and Kaplan–Meier survival analysis and logrank test for differences in diabetes-free survival. Multivariate Cox regression analysis was used to assess the independent contribution to 5 year diabetes risk of potential factors identified in univariate analysis (p < 0.10). All statistical analyses were performed using IBM SPSS 20.0 (IBM Corporation, Armonk, NY, USA) or GraphPad Prism 5 (GraphPad, San Diego, CA, USA) software. Significance was defined as p < 0.05 or p < 0.05/k (Bonferroni correction).
RESULTS
HLA-B*18 and HLA-A*24 as predictors of progression to diabetes with distinct HLA-DQ associations
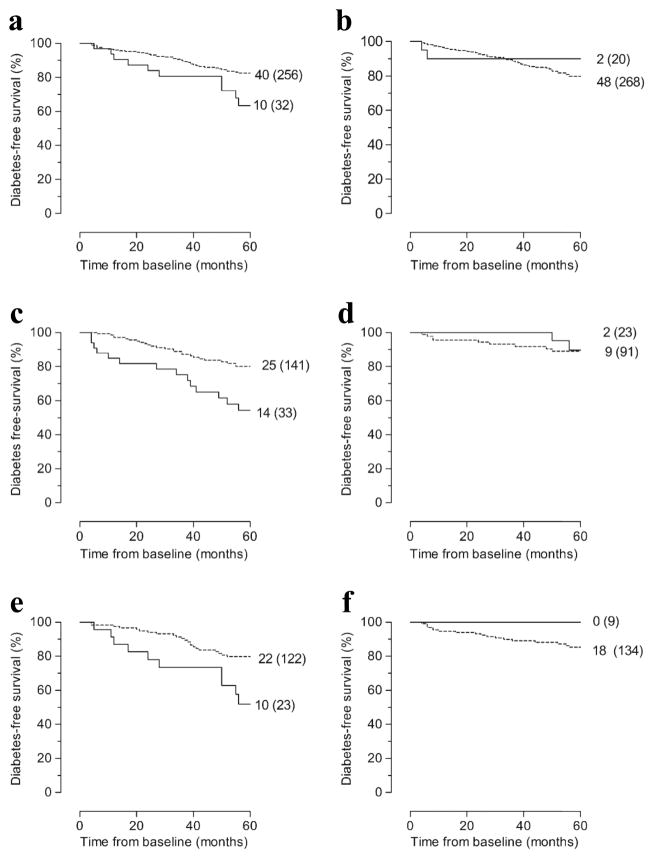
Within 5 years, 50 relatives (17%; 25 male and 25 female relatives; 18 offspring and 32 siblings) developed diabetes after a median (IQR) follow-up time of 29 (12–42) months. Baseline characteristics (autoantibody, HLA-DQ2, -DQ8 and -A*24 status) of progressors and non-progressors were reported previously [1]. Of 288 persistently Ab+ FDRs, 32 (11%) carried HLA-B*18 (progressors, 20%; non-progressors, 9%) and 20 (7%) carried HLA-B*39 (progressors, 4%; non-progressors, 8%). Kaplan–Meier analysis showed slightly more rapid progression to diabetes in relatives with HLA-B*18 than in those without (p < 0.021; Fig. 1a; ESM Table 3). In contrast, HLA-B*39 status had no effect on progression rate (p = 0.377; Fig. 1b). Cox regression analysis maintained IA-2A and/or ZnT8A, HLA-DQ2/DQ8 and HLA-A*24 as predictors of diabetes within 5 years [1] (Table 1). In addition, HLA-B*18, but not B*39, reached borderline significance (p = 0.058; Table 1).
Figure 1. Diabetes-free survival according to HLA-B*18, HLA-B*39 or HLA-A*24 status in 288 persistently Ab+ FDRs with or without stratification according to the presence or absence of HLA-DQ8 or HLA-DQ2.
(a) Whole group (n = 288) with (solid line) vs without (dashed line) HLA-B*18 (p = 0.021). (b) Whole group (n = 288) with (solid line) vs without (dashed line) HLA-B*39 (p = 0.377). (c) HLA-DQ8-positive (n = 174) relatives with (solid line) vs without (dashed line) HLA-A*24 (p = 0.002). (d) HLA-DQ8-negative relatives (n = 114) with (solid line) vs without (dashed line) HLA-A*24 (p = 0.750). (e) HLA-DQ2-positive (n = 145) relatives with (solid line) vs without (dashed line) HLA-B*18 (p = 0.005). (f) HLA-DQ2-negative relatives (n = 143) with (solid line) vs without (dashed line) HLA-B*18 (p = 0.277). The numbers given for each arm are number of events (total number at study entry). For the number of relatives under follow-up at different time points, see ESM Table 3. p by logrank test.
Table 1.
Cox regression analysis for 5 year progression to diabetes in Ab+ first-degree relativesa
| Variable | Whole group (n = 288) | HLA-DQ8 and/or HLA-DQ2 carriers (n = 246) | ||||
|---|---|---|---|---|---|---|
| p valueb | HR (95% CI)b | HR (95% CI)c | p valueb | HR (95% CI)b | HR (95% CI)c | |
| HLA-A*24 | 0.024 | 2.0 (1.1, 3.6) | 2.2 (1.2, 4.1)d | 0.006 | 2.3 (1.3, 4.3) | 2.5 (1.3, 4.5)d |
| HLA-B*18 | 0.025 | 2.2 (1.1, 4.4) | 2.0 (1.0, 4.0)e | 0.012 | 2.4 (1.2, 4.9) | 2.4 (1.2, 4.9)d |
| HLA-B*39 | 0.386 | 0.5 (0.1, 2.2) | NT | 0.257 | 0.3 (0.0, 2.3) | NT |
| HLA-DQ2 | 0.048 | 1.8 (1.0, 3.2) | 1.2 (0.5, 2.8) | 0.170 | 1.5 (0.8, 2.8) | NT |
| HLA-DQ8 | 0.007 | 2.5 (1.3, 4.9) | 1.3 (0.4, 4.5) | 0.036 | 2.2 (1.0, 4.8) | 1.3 (0.5,3.3) |
| HLA-DQ2/DQ8 | <0.001 | 3.1 (1.8, 5.4) | 2.6 (1.5, 4.6)f | <0.001 | 2.9 (1.6, 5.1) | 2.7 (1.5, 4.8)f |
| Ageg>12 vs≤12 years | 0.026 | 0.5 (0.3, 0.9) | 0.6 (0.3, 1.1) | 0.010 | 0.4 (0.2, 0.8) | 0.5 (0.3, 1.0)e |
| Offspring of diabetic mother | 0.042 | 0.4 (0.2, 1.0) | 0.8 (0.3, 2.3) | 0.061 | 0.4 (0.2, 1.0) | 1.0 (0.4, 2.8) |
| IA-2A and/or ZnT8A positive | <0.001 | 6.9 (3.7, 13.0) | 6.7 (3.6, 12.7)f | <0.001 | 5.6 (3.0, 10.7) | 5.8 (3.0, 11.0)f |
Except indicated as NT (not tested) all variables shown were included in multivariate models
Persistently positive for IAA, GADA, IA-2A and/or ZnT8A;
Univariate analysis (enter method);
Multivariate analysis (forward stepwise method) for variables with p < 0.10 in univariate analysis;
p < 0.02;
p < 0.06;
p < 0.002;
Median = 12 years
HLA-A*24 carriers progressed more rapidly to diabetes in the presence of HLA-DQ8 (p < 0.003; Fig. 1c; ESM Table 3) but not in its absence (Fig. 1d). In contrast, positivity for HLA-B*18 was associated with more rapid progression to diabetes in HLA-DQ2-positive relatives (p < 0.006; Fig. 1e; ESM Table 3) but not in HLA-DQ2-negative ones (Fig. 1f). Multivariate Cox regression analysis confirmed HLA-A*24 and HLA-B*18 as predictors of impending diabetes, the effect of HLA-A*24 being mainly observed in HLA-DQ8 carriers (p = 0.002; ESM Table 4) and that of HLA-B*18 being limited to carriers of HLA-DQ2 (p = 0.004; ESM Table 4). HLA-B*39 did not predict diabetes regardless of HLA-DQ status.
Added value of HLA-B*18 and -A*24 in predicting 5 year diabetes risk in carriers of HLA-DQ8 and/or HLA-DQ2
Since HLA-B*18 and -A*24 were only predictive in the presence of HLA-DQ2 and/or HLA-DQ8 we investigated the contribution made by HLA-B*18 towards the identification of rapid progressors in carriers of ≥1 susceptible HLA-DQ haplotype. This group comprised 246 of the 288 (85%) persistently Ab+ relatives and 47 of the 50 (94%) rapid progressors (ESM Table 1). These 47 progressors (19% of 246) developed diabetes after 33 (17–43) months (median [IQR]). The baseline characteristics (ESM Table 1) of progressors (n = 47) vs non-progressors (n = 199) were similar to those of the entire group (n = 288) [1]. In summary, compared with non-progressors, progressors tended to carry more HLA-DQ2/DQ8 (p < 0.001), HLA-A*24 (p < 0.005) and HLA-B*18 (p = 0.024) but not HLA-B*39 (p = 0.355) (ESM Table 1). In these relatives at HLA-DQ-inferred risk, the presence of HLA-B*18 was associated with a more rapid progression (p = 0.010 vs absence; Fig. 2a), as was also the case for the presence of IA-2A and/or ZnT8A, HLA-DQ2/DQ8 or HLA-A*24 [1] (not shown). Progression rate gradually increased according to number of these four markers (0 to ≥3) present at baseline (overall p < 0.001; Fig. 2b). Multivariate Cox regression analysis confirmed the presence of HLA-B*18 (p = 0.015), HLA-A*24 (p = 0.004) and HLA-DQ2/DQ8 (p = 0.001) and positivity for IA-2A and/or ZnT8A (p < 0.001) as predictors of diabetes (Table 1). A significant interaction between HLA-B*18 and HLA-DQ2/DQ8 was revealed (p = 0.009), while the reported strong interaction of HLA-A*24 with IA-2A and/or ZnT8A positivity [1] was maintained (p < 0.001; ESM Table 5). Borderline significance was reached for younger age at the first Ab+ sample (Table 1).
Figure 2.
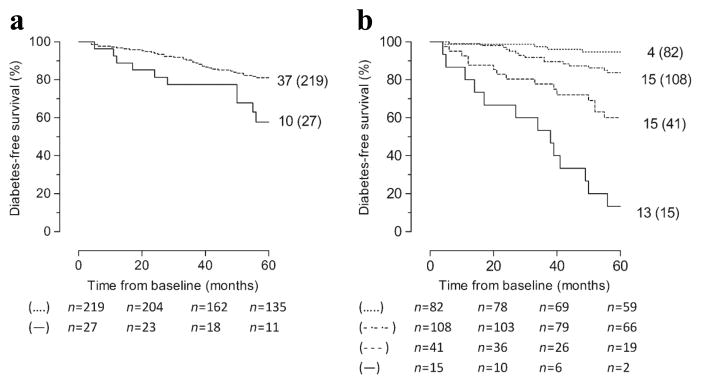
Diabetes-free survival in 246 persistently Ab+ FDRs carrying HLA-DQ2 and/or HLA-DQ8 according to (a) presence (solid line) vs absence (dashed line) of HLA-B*18 (p = 0.010) or (b) presence of at least three of four markers (solid line) vs two of four markers (dashed line) vs one of four markers (dotted/dashed line) vs absence of all four markers (dotted line) (overall p < 0.001). Markers are independent predictors of 5 year diabetes risk in HLA-DQ2- and/or HLA-DQ8-positive relatives (positive for IA-2A and/or ZnT8A, HLA-DQ2/DQ8, HLA-A*24 and HLA-B*18). The numbers given for each arm are number of events (total number at study entry). p by logrank test.
In the 246 persistently Ab+ FDRs the overall 5 year progression increased from 21% to 37–42% in the presence of any of the four predictors (IA-2A and/or ZnT8A, HLA-DQ2/DQ8, -A*24 or -B*18) (ESM Table 6) at the expense of a decreased sensitivity (21–72%) for detecting individuals with impending diabetes (ESM Table 6). The presence of HLA-A*24 and/or HLA-B*18 in addition to HLA-DQ2/DQ8+ and/or IA-2A and/or ZnT8A increased the sensitivity for identifying rapid progressors among individuals with ≥1 risk marker and ≥30% 5 year diabetes risk from 85% to 91% (p < 0.001; three additional individuals; ESM Table 6). Moreover, screening for HLA-B*18, in addition to IA-2A and/or ZnT8A, HLA-DQ2/DQ8 and HLA-A*24 significantly increased the sensitivity of detecting rapid progressors from 38% (18 of 47) to 53% (25 of 47) (p < 0.001; ESM Table 6) among relatives at high Ab-inferred risk and carrying at least one genetic susceptibility factor, conferring a ≥60% 5 year risk. The presence of any three markers among IA-2A and/or ZnT8A, HLA-DQ2/DQ8, HLA-A*24 and HLA-B*18 identified 15 relatives with an 87% 5 year progression rate. Hence additional screening for HLA-B*18 increased the sensitivity of detecting relatives carrying three markers and with >85% progression rate from 17% (8 of 47) to 28% (13 of 47) (p = 0.016; ESM Table 6).
DISCUSSION
In a representative Belgian group of persistently Ab+ FDRs, the association of HLA-B*18, but not of HLA-B*39, with more rapid progression from autoimmunity to type 1 diabetes [3] was confirmed and shown to occur preferentially in the presence of HLA-DQ2 [7], as opposed to the preferential accelerating effects of HLA-A*24 in carriers of HLA-DQ8 [6]. The identification of HLA-B*18 as a predictor of impending diabetes—complementing the established markers IA-2A and/or ZnT8A, HLA-DQ2/DQ8 and HLA-A*24 [1], in Ab+ FDRs carrying at least one HLA-DQ susceptibility haplotype—constitutes the main finding of the present study. Screening for HLA-B*18 in addition to testing for IA-2A, ZnT8A, HLA-DQ2/DQ8 and HLA-A*24 significantly increased the number of rapid progressors identified among individuals positive for IA-2A and/or ZnT8A and carrying at least one genetic risk marker and overall >60% 5 year risk by 39%. Likewise, it expanded by 63% the number of FDRs identified as having impending diabetes among carriers of three risk markers conferring an overall >85% 5 year risk. The complementarity of testing for HLA-B*18 and -A*24 is further underscored by their distinct interaction with HLA-DQ2/DQ8 and IA-2A and/or ZnT8A, respectively. These findings increase the importance of HLA class I alleles in identifying rapid progressors and facilitate the constitution of homogeneous groups of participants in future immunointervention studies. The strengths and weaknesses of our approach have been addressed in a previous paper [1]. Briefly, strengths include the representativeness of the FDRs, their broad age range and completeness of the data set for all variables. Follow-up from birth is lacking but is not relevant in the context of identifying selection criteria for adolescent and adult participants in immunointervention trials. While the distinctive predictor abilities of HLA-B*18 and -A*24 are significant, their contribution to the identification of rapid progressors remains modest in absolute numbers. Their inclusion in prediction studies should be weighed against the efficacy of other predictors (e.g. metabolic markers) [11, 12].
The present study does not allow one to decide whether disease acceleration is caused by HLA-A and -B alleles themselves or whether haplotypic factors are involved. However, the observed preferential effect of HLA-B*18 in carriers of HLA-DQ2 is compatible with reports that HLA-B*18 is often found in a haplotype combined with DRB1*03-DQB1*02 conferring a higher risk than B*08-DRB1*03-DQB1*02, suggesting that specific interactions with HLA-DR/DQ might be involved [7]
Our results on the positive association of HLA-B*18 and -A*24 with more rapid progression to diabetes are in accordance with the findings of some previous reports [3, 13]. In contrast, Lipponenet al documented an HLA-DR3/DR4-restricted acceleration of the disease process in the presence of HLA-B*39 but not of HLA-B*18 or -A*24 [4]. These discrepancies might relate to regional differences in genetic background, familial history, gene–environment interactions or age at inclusion of the cohorts followed [1, 3–7, 13]. The lack of a significant accelerating effect of HLA-B*39 in the present study might relate to the low prevalence of the allele (7%) in our cohort of Ab+ relatives and in 594 healthy controls (7%; E. Mbunwe, unpublished). The association of HLA-B*39 with type 1 diabetes (11% in 1,670 patients vs 7% in controls; p < 0.01) was also less strong than for HLA-B*18 (17% in patients vs 10% in controls, p < 0.001) (E. Mbunwe, unpublished). Since HLA-B*39 has been associated with early age at diagnosis [14], the lack of accelerating effect of this allele in our study might in part derive from the overall older age at first Ab+ sample (median age, 12 years) compared with the individuals studied by Lipponen et al (median age, 1.8 years) [4].
Taken together, results from our group and others [1, 3–7, 13] underscore the importance of HLA class I alleles in disease progression from autoimmunity to overt diabetes, compatible with the role of HLA class I molecules in presentation of antigenic epitopes to cytotoxic CD8+ cells [15–20]. The present study has demonstrated the added value of HLA-B and HLA-A typing in selection strategies for participants in secondary prevention trials. Further investigations should try to correlate the presence of disease accelerators with changes in functional beta cell mass as assessed by standardised beta cell function tests to better understand the preclinical phase of type 1 diabetes and to further streamline screening strategies [11, 12, 18]. Our results suggest that various pathways may converge to cause beta cell loss and that screening strategies for high-risk individuals need to take regional differences in genetic susceptibility into account. The association of both HLA-A*24 and IA-2A and/or ZnT8A with rapid progression to diabetes might be reconciled with reports on lower humoral responses to IA-2 or ZnT8 at [19] or after [20] diagnosis of type 1 diabetes in HLA-A*24 carriers hypothesising more pronounced loss of antigenic stimulus or less opportunity of inter- and intra-molecular spreading of autoimmune response in HLA-A*24-positive individuals [19].
In conclusion, HLA-A*24 and HLA-B*18, but not HLA-B*39, significantly contributed to prediction of 5 year progression to diabetes in Ab+ FDRs of patients with type 1 diabetes in Belgium. The effects of HLA-B*18 are HLA-DQ2 selective whereas HLA-A*24 is preferentially effective in the presence of HLA-DQ8.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the expert technical assistance of co-workers at the central unit of the Belgian Diabetes Registry (P. Goubert, C. Groven) and the reference laboratory of the Belgian Diabetes Registry (BDR) (V. Baeten, T. De Mesmaeker, H. Dewinter, N. Diependaele, S. Exterbille, T. Glorieux, T. Haulet, A. Ivens, D. Kesler, F. Lebleu, M. Van Molle, S. Vanderstraeten, K. Verhaeghen and A. Walgrave from the Department of Clinical Chemistry and Radio-immunology, University Hospital Brussels Free University-UZ Brussel, Brussels, Belgium; and G. De Block, E. Quartier, G. Schoonjans from the Brussels Free University-VUB, Brussels). The authors also thank the various university teams of co-workers for their excellent assistance in collecting samples and organising the fieldwork: L. Van Gaal, C. De Block, R. Braspenning, J. Michiels, J. Van Elven and J. Vertommen from the University Hospital Antwerp, Antwerp, Belgium; B. Keymeulen, K. Decochez, E. Vandemeulebroucke, U. Van de Velde from the University Hospital Brussels Free University-UZ Brussel, Brussels, Belgium; J. M. Kaufman, J. Ruige, A. Hutse, A. Rawoens and N. Steyaert from the University Hospital Ghent, Ghent, Belgium; and C. Mathieu, P. Gillard, M. Carpentier, M. Robijn, K. Rouffe, A. Schoonis and H. Morobé from the University Hospital Leuven, Leuven, Belgium. The authors sincerely thank all members of the BDR who contributed to the recruitment of relatives for the present study (list of names: see ESM Appendix).
FUNDING
The present work was supported by grants from the JDRF, Center Grant 4-2005-1327, the European Union (FP-7 project no. 241833), the Belgian Fund for Scientific Research (FWO Vlaanderen projects G.0319.01, G.0514.04, G.0311.07, G.0374.08 and G.0868.11; senior clinical research fellowships to I. Weets, K. Decochez and B. Keymeulen), the Research Council of the Brussels Free University (research fellowship to E. Mbunwe) and the Willy Gepts Fund (projects 3–2005 and 3/22-2007; University Hospital Brussels-UZ Brussel). J. C. Hutton received funding from DERC (NIH P30 DK57516), NIH R01 DK052068 and JDRF 4-2007-1056. The BDR was sponsored by the Belgian National Lottery, the ministries of Public Health of the Flemish and French Communities of Belgium, Hippo & Friends, WeightWatchers, Ortho-Clinical Diagnostics, Novo Nordisk Pharma, Lifescan, Roche Diagnostics, Bayer and Eli Lilly.
ABBREVIATIONS
- Ab
Autoantibody
- Ab+
Autoantibody-positive
- BDR
Belgian Diabetes Registry
- FDRs
First-degree relatives
- GADA
GAD autoantibodies
- IAA
Insulin autoantibodies
- IA-2
Islet antigen-2
- IA-2A
IA-2 autoantibodies
- IQR
Interquartile range
- ZnT8
Zinc transporter 8
- ZnT8A
ZnT8 autoantibodies
Footnotes
DEDICATION
We dedicate this paper to the memory of J. C. Hutton who passed away on December 18, 2012. He contributed to the design of the study, the discussion of the results and the first version of the manuscript.
DUALITY OF INTEREST
The authors declare that there is no duality of interest associated with this manuscript.
CONTRIBUTION STATEMENT
All authors contributed to the design of the study, acquisition of data, statistical analysis, discussion and/or revision of the manuscript. All authors approved the final version. Electronic supplementary material
References
- 1.Mbunwe E, van der Auwera BJ, Vermeulen I, et al. HLA-A*24 is an independent predictor of 5-year progression to diabetes in autoantibody-positive first-degree relatives of type 1 diabetic patients. Diabetes. 2013;62:1345–1350. doi: 10.2337/db12-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Herrath M, Peakman M, Roep B. Progress in immune-based therapies for type 1 diabetes. Clin Exp Immunol. 2013;172:186–202. doi: 10.1111/cei.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tait BD, Colman PG, Morahan G, et al. HLA genes associated with autoimmunity and progression to disease in type 1 diabetes. Tissue Antigens. 2003;61:146–153. doi: 10.1034/j.1399-0039.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 4.Lipponen K, Gombos Z, Kiviniemi M, et al. Effect of HLA class I and class II alleles on progression from autoantibody positivity to overt type 1 diabetes in children with risk-associated class II genotypes. Diabetes. 2010;59:3253–3256. doi: 10.2337/db10-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nejentsev S, Howson JM, Walker NM, et al. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450:887–892. doi: 10.1038/nature06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fennessy M, Metcalfe K, Hitman GA, et al. A gene in the HLA class I region contributes to susceptibility to IDDM in the Finnish population. Diabetologia. 1994;37:937–944. doi: 10.1007/BF00400951. [DOI] [PubMed] [Google Scholar]
- 7.Valdes AM, Wapelhorst B, Concannon P, Erlich HA, Thomson G, Noble JA. Extended DR3-D6S273-HLA-B haplotypes are associated with increased susceptibility to type 1 diabetes in US Caucasians. Tissue Antigens. 2005;65:115–119. doi: 10.1111/j.1399-0039.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen I, Weets I, Costa O, et al. An important minority of prediabetic first-degree relatives of type 1 diabetic patients derives from seroconversion to persistent autoantibody positivity after 10 years of age. Diabetologia. 2012;55:413–420. doi: 10.1007/s00125-011-2376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 10.Middleton D. PCR-SSOP class I and class II (DRBI) In: Hahn AB, Land GA, Strothman RM, editors. ASHI laboratory manual. 4. American Society for Histocompatibility and Immunogenetics; Lenexa: 2000. pp. V.C.2.1–23. [Google Scholar]
- 11.Greenbaum CJ, Buckingham B, Chase HP, Krischer J. Metabolic tests to determine risk for type 1 diabetes in clinical trials. Diabetes Metab Res Rev. 2011;27:584–589. doi: 10.1002/dmrr.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu P, Beam CA, Cuthbertson D, Sosenko JM, Skyler JS, Krischer JP. Prognostic accuracy of immunologic and metabolic markers for type 1 diabetes in a high-risk population: receiver operating characteristic analysis. Diabetes Care. 2012;35:1975–1980. doi: 10.2337/dc12-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honeyman MC, Harrison LC, Drummond B, Colman PG, Tait BD. Analysis of families at risk for insulin-dependent diabetes mellitus reveals that HLA antigens influence progression to clinical disease. Mol Med. 1995;1:576–582. [PMC free article] [PubMed] [Google Scholar]
- 14.Valdes AM, Erlich HA, Noble JA. Human leukocyte antigen class I B and C loci contribute to type 1 diabetes (T1D) susceptibility and age at T1D onset. Hum Immunol. 2005;66:301–313. doi: 10.1016/j.humimm.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronenberg D, Knight RR, Estorninho M, et al. Circulating preproinsulin signal peptide-specific CD8 T cells restricted by the susceptibility molecule HLA-A24 are expanded at onset of type 1 diabetes and kill beta-cells. Diabetes. 2012;61:1752–1759. doi: 10.2337/db11-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marron MP, Graser RT, Chapman HD, Serreze DV. Functional evidence for the mediation of diabetogenic T cell responses by HLA-A2.1 MHC class I molecules through transgenic expression in NOD mice. Proc Natl Acad Sci U S A. 2002;99:13753–13758. doi: 10.1073/pnas.212221199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 18.Vandemeulebroucke E, Keymeulen B, Decochez K, et al. Hyperglycaemic clamp test for diabetes risk assessment in IA-2-antibody-positive relatives of type 1 diabetic patients. 2010 doi: 10.1007/s00125-009-1569-3. [DOI] [PubMed] [Google Scholar]
- 19.Long AE, Gillespie KM, Aitken RJ, Goode JC, Bingley PJ, Williams AJ. Humoral responses to islet antigen-2 and zinc transporter 8 are attenuated in patients carrying HLA-A*24 alleles at the onset of type 1 diabetes. Diabetes. 2013 doi: 10.2337/db12-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakanishi K, Kobayashi T, Murase T, Naruse T, Nose Y, Inoko H. Human leukocyte antigen-A24 and -DQA1*0301 in Japanese insulin-dependent diabetes mellitus: independent contributions to susceptibility to the disease and additive contributions to acceleration of beta-cell destruction. J Clin Endocrinol Metab. 1999;84:3721–3725. doi: 10.1210/jcem.84.10.6045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.