Abstract
Given that the leading clinical conditions associated with Acute kidney injury (AKI), namely, sepsis, major surgery, heart failure and hypovolemia, are all associated with shock, it is tempting to attribute all AKI to ischemia on the basis of macro-hemodynamic changes. However, an increasing body of evidence has suggested that in many patients, AKI can occur in the absence of overt signs of global renal hypoperfusion. Indeed, sepsis-induced AKI can occur in the setting of normal or even increased renal blood flow. Accordingly, renal injury may not be entirely explained solely on the basis of the classic paradigm of hypoperfusion, and thus other mechanisms must come into play. Herein, we put forward a “unifying theory” to explain the interplay between inflammation and oxidative stress, microvascular dysfunction, and the adaptive response of the tubular epithelial cell to the septic insult. We propose that this response is mostly adaptive in origin, that it is driven by mitochondria and that it ultimately results in and explains the clinical phenotype of sepsis induced AKI.
Keywords: Acute kidney injury, sepsis, microcirculation, mitochondria, inflammation, cell cycle
Introduction
Close and careful clinical observation of patients is the very backbone of medical inductive and deductive reasoning, fundamental to establish the paradigms that govern our understanding of health and disease. Such paradigms, once established are deeply rooted in the psyche, and are thus rarely disputed or modified. The paradigm that explains the pathophysiology of acute kidney injury (AKI) is not an exception. Given that the leading clinical conditions associated with AKI, namely, sepsis, major surgery, heart failure and hypovolemia1 are all associated with shock, it is tempting to attribute all AKI to ischemia on the basis of macro-hemodynamic changes. However, an increasing body of evidence suggests that AKI can occur in the absence of overt signs of hypoperfusion, either global or regional. For instance, a large-scale study that included more than 1800 patients with confirmed diagnosis of pneumonia found that AKI was common among patients with non-severe pneumonia, including those who were never admitted to the ICU nor had any evidence of hemodynamic instability.2 Despite the limitation of being unable to address whether transient periods of hypotension occurred, the fact that these patients never required escalation of care suggests that AKI can occur in the absence of overt signs of shock and more importantly, in the population that the medical community would not classify as “at risk”.2 In addition, despite wide controversy about the role of global renal blood flow (RBF), animal models and human studies have shown that the occurrence of sepsis-induced AKI is not exclusive of decreased RBF states, and that on the contrary, can develop in the setting of increased RBF.3,4 Not only can AKI occur in the absence of macro-hemoynamic signs of hypoperfusion, it seems that whole body warm ischemia may not be sufficient to cause it either. Of interest, only a very small proportion of patients after cardiac arrest, a natural “model” of warm ischemia, develop AKI, and those who do, are patients who develop cardiogenic shock in the post-arrest period.5 These results are reminiscent of animal studies that failed to induce AKI in the setting of hemorrhagic shock.6,7 Finally, in vitro studies using cell cultures, in which hemodynamics are no longer relevant, have shown that many of the cardinal features of sepsis-induced AKI can be reproduced in human epithelial tubular cells by exposing them to plasma from septic patients.8 These data provide evidence that at least in some patients, renal injury cannot be explained solely on the basis of the classic paradigm of hypoperfusion, and that other mechanisms must come into play. This review will focus on the potential mechanisms that could explain the occurrence of AKI in the absence of tissue hypoperfusion.
One of the limitations in advancing the understanding of sepsis-induced AKI is the lack of pathologic specimens available in this patient population, given that, as a consequence of the inherent risks, biopsies are almost never performed. Clinical studies based on physiologic data, and few recent post-mortem reports have started to define what sepsis-induced AKI actually looks like and how it differs from other types of kidney injury. Histologically, sepsis-induced AKI has been typified by patchy, heterogeneous tubular cell injury with apical vacuolization, but with absence of tubular necrosis or even extensive apoptosis.9 Importantly, all these features develop in the context of renal vasodilatation and normal or increased RBF10–15, and frame the clinical phenotype characterized by a profound decrease in glomerular filtration rate (GFR), creatinine clearance and the development of uremia.
While cell death (either via necrosis or apoptosis) is not a prominent feature of sepsis-induced AKI, a consistent observation in septic humans and animals, regardless of disease stage, severity, or organ examined appears to be the presence of three main alterations: inflammation16,17, diffuse microcirculatory flow abnormalities18 and, cell bioenergetic adaptive responses to injury.19 The study and understanding of these three domains in terms of the relationship between them, their relative contribution to the development of tubular injury and AKI, may provide a roadmap to unravel the mechanisms by which sepsis causes AKI and perhaps, organ injury in general and may facilitate the development of more targeted therapies. These three domains frame a unifying theory that we are proposing.
A possible unifying theory
Functionally, sepsis-induced AKI manifests as a dramatic decline in GFR and variable tubular dysfunction. However, the histologic footprint does not entirely explain this functional phenotype. It is characterized by the presence of non-specific, patchy areas of tubular cell vacuolization, and a remarkable absence of apoptosis or necrosis20. Current evidence suggests that the origin of most cases of AKI is multifaceted rather than the result of an individual insult, and that several, concurrent mechanisms may be at work. These mechanisms include inflammation, profound, heterogeneous distortion of microvascular flow at the peritubular and glomerular levels and stimulation of mitochondrial quality control processes and cell cycle arrest. Given that these three major players occur early in the course of sepsis, we propose the following theory to explain the pathophysiology of sepsis-induced AKI.
We conceptualize sepsis-induced AKI as the early clinical and biochemical manifestation of an adaptive response of the tubular cells to an injurious, inflammatory danger signal. We submit that the interplay of inflammation and microvascular dysfunction characterize and amplify this signal, and that in response, mitochondria within tubular cells orchestrate a complete metabolic downregulation and reprioritization which favors individual cell survival processes (such as maintenance of membrane potential and cell cycle arrest), at the expense of “kidney function” (i.e. tubular absorption and secretion of solutes).
The “danger” signal: amplification by microvascular dysfunction and impact on the tubular epithelial cell
During sepsis, inflammatory mediators derived from pathogens and activated immune cells (i.e. LPS, cytokines, etc. also known as Damage or Pathogen Associated Molecular Patterns or DAMPs/PAMPs) which prime, signal, alert and guide the immune system to fight infection, also mediate host cellular injury. DAMPs and PAMPs can be recognized not only by cells of the immune system, but also epithelial and parenchymal cells, through Pattern Recognition Receptors or PRRs. These include Toll-like receptors (TLR), NOD-like receptors and RIG-I-like receptors.21 The fact that the kidney receives 20% of the cardiac output and filters about 120–150 ml of plasma every minute places it on the front line to be exposed to such mediators. DAMPs and PAMPs can exert their effects on the renal tubular cells either via the peritubular microcirculation or they can be filtered at the glomerulus (Fig. 1). It has been recently demonstrated that these molecules can be recognized by tubular cells through TLR-4 (and TLR-2) dependent pathways (Fig. 2).22 Despite that potentially all nephrons in the kidney could be exposed to these mediators, only patches of tubular cells seem to display signs of distress to this “danger signal”.23 This patchy appearance closely resembles the heterogeneous distribution of flow due to microvascular dysfunction, and we speculate that these two events may be causally related. We further venture that microvascular dysfunction may play a key role in amplifying the “danger signal” in specific areas of low flow, exposing neighboring tubular epithelial cells and causing damage in the observed patchy distribution.
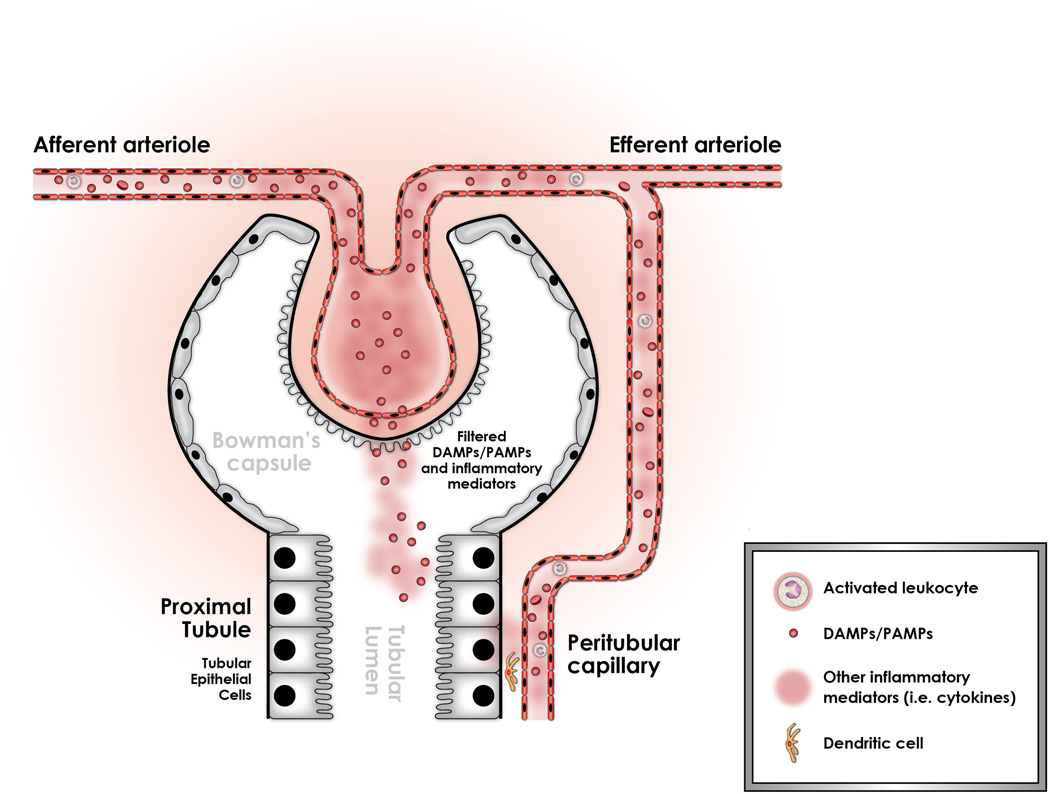
Figure 1.
Sepsis is associated with the release of damage and pathogen associated molecular patterns (DAMPs and PAMPs) into the circulation. These inflammatory mediators are derived from bacterial products as well as from the immune cells which respond to infection. Together, they constitute an alarm “danger signal” that can be recognized by and can potentially injure the tubular epithelial cell. It has been shown recently that these mediators can readily gain access to the tubular space through glomerular filtration. Specifically, LPS has been shown to be filtered through the capsule of bowman, and into the tubular fluid. Once in the tubular space, LPS can directly interact with the tubular epithelial cell which can recognize it through a TLR-4 – dependent mechanism. Alternatively, there is indirect data suggesting that inflammatory mediators released by activated leukocytes in the peritubular capillaries can stimulate the tubular epithelial cell. It is unknown however, if this stimulation occurs by direct migration of these DAMPs through the endothelial and epithelial layers, or if they exert their actions through cellular interactions activating the endothelium, stimulating dendritic cells and ultimately, triggering a response in the tubular epithelial cell.
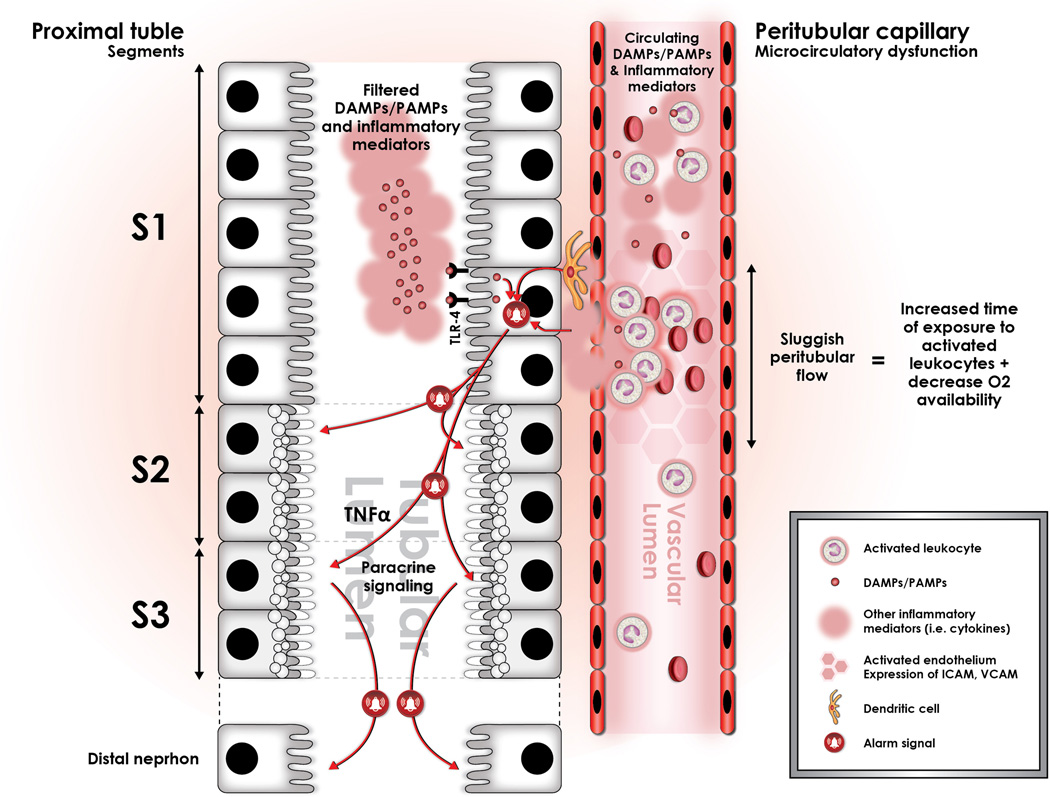
Figure 2.
Sepsis induces profound alterations in microcirculatory flow in the entire organism, and the kidney is not the exception. This alteration is characterized by a significant increment in the heterogeneity of flow, as well as an increase in the proportion of capillaries with sluggish or stop flow (represented in the figure by darker hexagons in the peritubular capillary). We have conceptualized that these areas of sluggish peritubular flow increase the transit time of activated, cytokine spilling leukocytes, and that this may set the stage for an amplification of the “danger signal” in such areas. These areas of sluggish flow have been shown to co-localize with expression of oxidative stress in the tubular epithelial cells, suggesting causation. In addition, immuno-histologic studies have shown that oxidative stress is localized to the apex of the tubular epithelial cell, and that it is associated to the formation of apical vacuoles as represented hereby in the figure. Importantly, this may explain the mechanism by which apical vacuoles are formed during sepsis-induced AKI and also the histologic phenotype. In addition, filtered LPS is recognized by S1 tubular epithelial cells through TLR-4 and is internalized via endocytosis. This event has been shown to trigger an oxidative outburst, not in the S1 segment cells, but rather in the S2 segment cells. This seems to be associated with the expression in S1, but not in S2 epithelial cells of Heme-oxigenase 1 (HO-1) and Sirt1, both highly protective against oxidative damage.22,80 In addition, expression of TNF receptors in the S2 segment tubular cells has led to the hypothesis that S1 cells may signal distal segments in a paracrine fashion through secretion of TNF-alpha. Finally, there is also data suggesting that this paracrine signal may also include mediators of cell cycle arrest, namely TIMP-2 and IGFBP-7.
Microvascular Dysfunction
Sepsis causes a profound alteration in microvascular blood flow distribution throughout the body. This abnormality is characterized by increasing heterogeneity of flow. Anatomically, a significant decrease in capillary density occurs. Functionally, there is a decrease in the proportion of capillaries with “nutritive” or continuous blood flow, along with a concomitant increment in the proportion of capillaries with intermittent or no flow.24–26 The renal microcirculation is altered in a similar fashion, as has been recently described in different models of sepsis-induced AKI. These alterations have been predominantly characterized by a decrease in vessels with continuous flow 17,27,28, and a concomitant increase in vessels with intermittent or no flow.
Although not the focus of this review, it is important to underscore two important processes. The first is that such a microcirculatory alteration may create areas of hypoperfusion and hypoxia29,30 in parallel to the mechanisms that we will describe, and that these loci of hypoxia may contribute to the inflammatory process and to the adaptive metabolic down-regulation of the renal tubular cell through a process known as oxygen conformance.31 The second is the potential role of nitric oxide (NO) in the genesis of microvascular dysfunction and in the pathophysiology of AKI. Although it is known that sepsis elicits a global increment in production of NO32, the expression of one of the most important catalyzers of its production, inducible Nitric Oxide Synthase (iNOS) is heterogeneous.32 Therefore it is reasonable to consider that heterogeneous expression of iNOS may result in heterogeneous regional concentrations of NO, which could potentially lead to pockets of vascular beds deprived of NO even in the setting of elevated systemic levels.33 This is important as it directly relates to the heterogeneous pattern that has been described in sepsis-induced microvascular dysfunction, and furthermore, may relate to possible pathophysiologic phenomena like shunting and hypoxia.33 Nevertheless, the relationship between NO, microvascular dysfunction and AKI may not be as straight forward, as sepsis may also cause an iNOS-dependent decrease in endothelial derived NOS activity (eNOS), which would also result in impaired microvascular homeostasis.34,35 Finally, selective inhibition of iNOS can not only restore the renal microcirculatory derangements brought about by sepsis, but it’s also associated with decreased histologic and functional manifestations of renal injury, suggesting that microcirculatory abnormalities may be in the mechanistic pathway of sepsis-induced AKI.27
Amplification of the signal: association between sluggish flow and tubular oxidative stress
Sepsis-induced microvascular dysfunction produces areas of sluggish peritubular flow, which seems to be central to the amplification of the inflammatory signal. In support of this, Holthoff et al. showed that red blood cell (RBC) velocity is severely decreased 6 hours after cecal ligation and puncture (CLP) in these dysfunctional capillaries.28 Just as with the RBCs, activated leukocytes passing through these areas of sluggish microvascular flow also decrease their velocities and increase their transit time as demonstrated by Goddard et al. in myocardial capillaries during a porcine model of endotoxemia.36 In addition, there is evidence of up-regulation of inflammatory molecules, such as ICAM-1 and VCAM-137,38 in these peritubular capillaries that would contribute to prolonged leukocyte transit and increased signaling with kidney dendritic cells. (Fig. 2) This prolonged transit may directly translate into a greater time of exposure of the endothelium and neighboring tubular epithelial cells to activated, cytokine secreting leukocytes and to other PAMPs and DAMPs that ultimately amplify the inflammatory signal and cause greater oxidative stress. The tubular epithelial cells exposed to this amplified signal then act as primary targets for this alarm, respond to it by undergoing oxidative stress, vacuolization, and adaptation to the microtubular environment, and ultimately signal other tubular cells to shut down in a paracrine fashion (see below). Importantly, this provides an explanation for why, only a few heterogeneous groups of tubular epithelial cells demonstrate the typical histopathologic changes. (Fig. 2)
Oxidative stress, a hallmark of sepsis-induced tubular injury, is an early event that it is spatially associated with these areas of sluggish flow. Already within 4 hours after CLP reactive oxygen (ROS) and nitrogen (RNS) species concentrations increase, predominantly localized to tubules bordered with no capillary blood flow, suggesting sluggish/stop flow may not only be an epiphenomenon, but rather part of the causation pathway.16,17 Furthermore, using intravital microscopy and epi-illumination to detect surface contour, oxidative stress has been localized to apical vacuoles.23 This is important, because one of the very few histopathologic features that are uniformly identified in animal and human sepsis-induced AKI has been apical epithelial tubular cell vacuolization. In addition, the temporal association between these phenomena suggest a mechanistic link to how this may develop as peritubular capillary dysfunction and the expression of ICAM-1 and VCAM-1, epithelial cell oxidative stress and “renal failure” (as measured by functional assays BUN and creatinine) occurred at 2, 4 and 10 hours, respectively.
Although hypoxia may contribute to tubular injury, inflammation and induce an adaptive response23,39,40 we theorize that this is not the only mechanism, and that DAMP-induced inflammation and oxidative stress through TLR-4 activation may be at least as important.
The response to danger: Tubular metabolic down-regulation and reprioritization of cellular functions
The tubular cell response to this rarefied peritubular microenvironment seems to be adaptive in origin. The bland histology and the surprising paucity of apoptosis and necrosis in septic kidneys supports this notion, and has led to the understanding that sepsis-induced AKI does not follow the same injury pattern as ischemia-reperfusion and hemorrhage, and that it is not characterized by acute tubular necrosis.9 On the contrary, the tubular epithelial cell appears to limit processes that can otherwise activate apoptotic and necrotic signaling pathways, notably, energy imbalance and DNA damage. Accordingly, we propose that the initial insult to the tubular epithelial cell is framed by inflammation and oxidative stress, and that this triggers an adaptive response characterized by suppressing energy turnover, down-regulating metabolism through prioritization of energy consumption19,41–43, and undergoing cell cycle arrest (Fig. 3).44 We submit that this response, orchestrated by mitochondria (see below), limits further injury by maintaining energy balance and preventing further DNA damage, and is central to providing the cell with an opportunity to regain function once danger has abated.
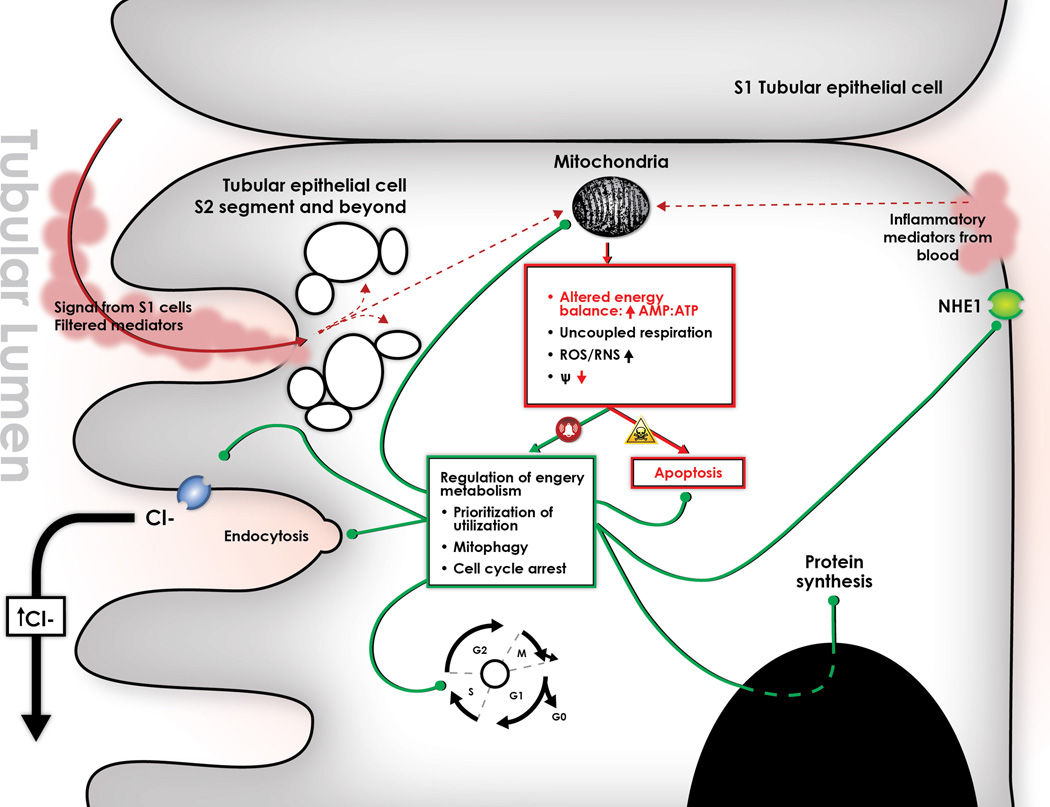
Figure 3.
Paracrine stimulation from S1 segment tubular epithelial cells produces an oxidative outburst in the S2 and S3 segment tubular epithelial cells, which is histologically appreciated by the generation of apical vacuoles. This oxidative outburst can potentially alter mitochondrial function by uncoupling respiration, which in turn leads to energetic imbalance, radical oxygen and nitrogen species (ROS/RNS) production, and loss of mitochondrial membrane potential. All of these alterations should activate apoptosis, and yet this is not seen during sepsis-induced AKI. Thus we hypothesize that the tubular epithelial cell coordinates a response to this “danger signal” that avoids triggering apoptosis and allows the cell to survive at least for a limited period of time. We submit that this response is orchestrated by mitochondria, and is centered on regulating energy metabolism by different pathways: 1. Re-prioritizes energy utilization, which inhibits electrolyte transport through cytoplasmic membranes and blocks protein synthesis; 2. Induces mitophagy, a process by which dysfunctional mitochondria are engulfed by autophagosomes, and their components are lysed and recycled as a source of energy; 3. Induces cell cycle arrest. The cell cycle is a normal process by which the cell prepares to undergo mitosis. There seems to be specific check-points along this cycle in which the cell “evaluates” whether or not there is sufficient energy to proceed to the next stage. Presumably, in the setting of energy imbalance (such as sepsis), the cell is unable to overcome such check-points and releases mediators that arrest the cycle to avoid undertaking a potentially lethal endeavor. Such mediators (TIMP-2 and IGFBP-7) have been validated as the best predictors of risk of AKI in critically ill patients70, and we submit that they may be involved in first, arresting the tubular epithelial cell cycle, and second, the paracrine signaling to distal tubular cells. Finally, we hypothesize that the link between tubular injury and the dramatic decline in glomerular filtration rate is the activation of tubuloglomerular feedback. As the tubular cell down-regulates apical ionic transport, chloride accumulates in the tubular lumen. This increases the chloride load delivered to the Macula Densa, triggering Tubuloglomerular feedback (TGF). The constriction of the afferent arteriole by this mechanism decreases glomerular filtration rate, and thus reproduces the clinical phenotype of sepsis-induced AKI.
Oxidative stress, inflammation and the trigger of the adaptive response
There is evidence to suggest that sepsis-induced oxidative stress is not only related to histopathologic findings, but also to tubular dysfunction. Gupta et al. showed that in the presence of LPS, proximal tubules of mice have a delayed uptake of low molecular weight dextran, a sign of reduced endocytic capacity.45 Furthermore, Good et al. have shown in an LPS-induced rodent sepsis model, that LPS inhibits the Na+/H+ exchanger 1 (NHE1), and thus blocks bicarbonate re-absorption in the medullary thick ascending limb of the loop of Henle.46
In addition, this oxidative stress response seems to be the result of an organized interaction between DAMPs and PAMPs and the tubular epithelial cell rather than a random event. DAMPs and PAMPs originating from remote sites of injury or infection can gain access to the renal tubules by glomerular filtration or by proximity to the peritubular capillaries.47 Furthermore, sepsis induces renal-wide expression of otherwise constitutively expressed TLR-448, and DAMPs/PAMPs are actively recognized by tubular epithelial cells through TLR-4.22 Kalackeche et al. has elegantly shown that TLR-4-dependent LPS recognition in the tubular epithelial cells occurs in the S1 segment of the proximal tubule22, that assembly of LPS with TLR-4 in the tubular epithelial cell produces internalization of LPS through fluid-filled endocytosis, and that this triggers an organized oxidative outburst in epithelial cells of the adjacent tubular segments (S2 and S3) but not in the S1 segment (Fig. 2).22 These findings have led Kalakeche et al. to suggest that the S1 segment of the proximal tubule acts as a “sensor of danger”, that activates a series of events resulting in oxidative stress within distal tubular segments (S2, S3) and that could potentially explain tubular dysfunction in the setting of sepsis. We further hypothesize that this oxidative outburst is the trigger for the adaptive response of the tubular epithelial cell, which is characterized by reprioritizing energy expenditure, down-regulating metabolism, and undergoing cell cycle arrest. (Fig. 3)
The adaptive response of the tubular epithelial cell to sepsis-induced injury
Apoptosis is the principal mechanism of programmed cell death in multicellular organisms.49 It can be triggered by a myriad of stimuli including DNA damage, energy failure, growth factor deprivation, and endoplasmic reticulum stress49, all of which also occur as a consequence of sepsis. Yet, tubular cell apoptosis is largely absent in patients with sepsis-induced AKI. It is likely that the scarcity of apoptosis is mainly orchestrated by mitochondria, as these organelles are central in the process of triggering the programmed cell death machinery.50 Importantly, mitochondria influence three key processes that could potentially lead to apoptosis: 1. Energy homeostasis and prioritization of energy consumption; 2. Maintenance of cellular organelle function through quality control processes (general autophagy and mitophagy); and 3. Cell cycle and DNA replication. We submit that these processes are not only fundamental aspects of the adaptive response of the tubular epithelial cell, but also explain, at least in part, the sepsis-induced AKI phenotype.
Reprioritization of energy consumption
Atkinson was the first to propose that ATP consuming processes should have a hierarchy of response dependent on the level of energy charge (ATP) or energy supply51, and Buttgereit and Brand provided evidence of this for the first time in stimulated thymocytes.52 As predicted by Atkinson, they showed that certain pathways such as macromolecular synthesis, and transmembrane sodium and chloride cycling were more sensitive to changes in energy supply. Buck and Hochachka showed that a similar process occurs when cells are exposed to hypoxia. In turtle hepatocytes, hypoxia induced a hierarchical down-regulation of major “energy sinks” such as protein synthesis53, and were capable of suppressing energy consumption 10-fold. Importantly, processes that were necessary for cell survival, such as maintenance of cytosolic membrane potential and integrity (Na/K ATPase) were the least suppressed. Thus, ATP supply by mitochondria actively regulates energy turnover, avoiding energy expenditure in “non-essential” pathways, and allowing the cell to prioritize energy consuming in essential processes such as maintenance of ion gradients.54 This is a highly conserved mechanism across species that seems to frame the core strategy of cellular response to threatening circumstances. It also provides the conceptual ground to suggest that cellular metabolic down-regulation and reprioritization of energy consumption are pillars of the tubular epithelial response to sepsis, and furthermore, explains why organ function is sacrificed in benefit of individual cell survival.45,46
Mitophagy
Autophagy (and the specialized process of mitochondrial removal called mitophagy), is an evolutionarily conserved, quality control mechanism, by which eukaryotic cells remove and digest dysfunctional organelles from the cytoplasm.55,56 During sepsis, TLR-4 mediated inflammation57, oxidative stress58,59 and alterations in the electron transport chain that “uncouple” respiration from ATP production and depolarize the mitochondrial membrane, are potent triggers of mitophagy.56 This early mitochondrial uncoupling characterized by an increment in O2 consumption (VO2) is not to be confused with the adaptive response it triggers, which is framed by the activation of mitophagy, and is characterized by a decrement in VO2 and conservation of energy.
In the kidney, mitophagy is activated as early as 3 hours after CLP-induced sepsis60, suggesting it is part of the early response of tubular epithelial cells to injury. Importantly, the time-dependent decline in mitophagy has been associated with proximal tubular dysfunction with decreased sodium transport and creatinine clearance, and increased BUN and creatinine.60 Finally, insufficient activation of mitophagy has been associated with worse outcome in critically ill patients, and it has been postulated to contribute to cell and organ dysfunction.61
On the other hand, stimulation of autophagy has been shown to be effective at protecting organ function. Gunst et al. showed in critically ill rabbits that treatment with Rapamycin (a potent inductor of mitophagy) was associated with protection of renal function.61 Similarly, Hsiao et al. showed that pre-incubation of NRK-52E cells (proximal tubule epithelial cell line) with Rapamycin prevented TNF-alpha-induced cell death, whereas inhibition of autophagy exaggerated it. Furthermore, they demonstrated in CLP-induced septic rats that a decline in autophagy was associated with increase BUN and creatinine, and a decline in proximal tubular sodium transport.60
As a protective response, mitophagy offers several advantages, namely removal of dysfunctional mitochondria, with subsequent decrement in ROS/RNS production and energy conservation, as “non-essential” energy consumption from uncoupled respiration is reduced, and lipids and proteins are recycled for ulterior use as a source of energy. Importantly, these benefits may limit oxidative stress damage and intercept pro-apoptotic signals at the mitochondrial level impeding triggering of apoptosis.62 Finally, there is also evidence that cross-talk between autophagy and apoptosis does occur, as they both share common factors, interconnections and regulatory steps.63–65
It is unknown however, what mitophagy-induced maintenance of renal function really means. The adaptive response, framed by metabolic down-regulation and prioritization of energy consumption would most likely decrease tubular and renal function and not promote it, just as hibernation promotes function laesa. Indeed, increased or preserved renal function in the setting of stress may result harmful in the long run. Yet, animal and human data associate acute stimulation of autophagy with preserved renal function, and its faulty activation or decline with worse outcome. It is possible that the interplay of autophagy and tubular cell function vary with time, and that persistence of the initial protective response may ultimately be deleterious in the subacute or chronic phases.
Cell cycle arrest
There is a growing body of evidence indicating that mitochondria are intimately involved in the regulation of the cell cycle.56 The ability of mitochondria to move within the cell, change shape and coalesce in different ways has recently emerged as an important feature, which may affect the cell cycle.66 Briefly, the cell cycle is the progression of cells through a number of steps in preparation for mitosis (G0, G1, S, G2, M). This preparation portrays several checkpoints in which the cell seems to evaluate whether it is prepared or not to advance to the next phase. Of particular interest to renal tubular injury in sepsis and the involvement of mitochondrial regulation is the G1-S checkpoint. Growing cells appear to have a mixture of tubular and fragmented mitochondria, whereas cells at the G1-S margin display a single, tubular network of mitochondria. This mesh seems to be transient and specific to the G1-S transition, and mitochondria in this structure seem to act as syncytia, with electrical coupling and unusual hyperpolarization.67 These findings fit well with prior studies showing an increase in O2 consumption during the G1-S transition of the cell cycle.68 This also relates to that the finding that a reduction in ATP production induced by specific ETC mutations produces cell cycle arrest at the G1-S checkpoint.69 Together these data indicate that the formation of this giant tubular network is necessary to meet the energy requirement needed to synthetize all the components for adequate cell division. It also suggests that the G1-S border is an important checkpoint of the cycle, and that the inability to meet such energy requirements triggers pathways that lead to cycle arrest preventing the cell from undertaking a process that could represent a lethal energy imbalance.66 Yang et al. recently showed in a rodent model of CLP-induced sepsis that G1-S cell cycle arrest was associated with kidney injury, and that recovery of renal function paralleled cell cycle progression 48 hours after CLP.44 These findings have become even more clinically relevant as Tissue Inhibitor of Metalloproteinases-2 (TIMP-2) and Insulin-like growth factor binding protein-7 (IGFBP-7), two markers involved in G1-S cycle arrest, have been identified as the most sensitive and specific markers to predict risk of development of AKI in critically ill patients.70 We speculate that the renal cell cycle arrest in the epithelial tubular cell may provide an advantage by avoiding replication for the following reasons: 1. Limiting cell replication conserves energy levels and prevents the cell from undergoing an energetically overtaxing endeavor that could potentially trigger apoptosis; 2. Limiting replication diminishes the probability of DNA damage which reduces the chances of triggering apoptosis, and also, conserves energy by sparing the cell from repairing damaged DNA.
How are tubular injury and GFR related?
Finally, although these mechanisms may provide a clear explanation of how sepsis may induce tubular damage, they fail to link early tubular injury with the massive decline in GFR. Conversely, inflammation-mediated microvascular dysfunction, extra-glomerular shunting, and capillary dropout may serve to explain why GFR is reduced during sepsis-induced AKI, but fail to explain why tubular injury occurs or how these phenomena relate to each other. The strong association between these two phenotypic signatures of sepsis-induced AKI makes a single mechanism more likely than separate mechanisms acting independently. A likely mechanism linking both phenomena is tubuloglomerular feedback (TGF). Sepsis-induced tubular injury and dysfunction45,46,60 may interfere with Na+ reabsorption through the Na/K/2Cl co-transporter in the proximal tubule71, increase the load of NaCl delivered to the macula densa, and thus trigger TGF (Fig. 3). Activation of TGF would decrease hydrostatic pressure in the glomerulus and thus decrease GFR. Importantly, all of the adaptive changes in tubular cell function discussed above are entirely consistent with this scenario and serve to underscore the linkage between tubular cell biology and glomerular function.
Potential therapeutic targets
One of the most important advantages of understanding the mechanistic underpinnings of a disease process is the possibility it offers to find novel, and more importantly, effective therapeutic interventions. In no other disease process, affecting the critically ill, is this more true compared to sepsis. For decades therapeutic efforts have failed to significantly reduce mortality. The unifying theory presented herein provides a possible roadmap to unraveling the pathophysiology of sepsis-induced AKI, a known driver of mortality in this population. Furthermore, if proven, new avenues to attack this disease process, at different stages, may be opened. Indeed stage-specific manipulation of inflammation, microvascular dysfunction and of cellular energy regulation may provide a new way to prevent and/or treat sepsis-induced AKI, and possible other sepsis-induced organ failures. The recognition of the derangement in microcirculatory flow, for example, has triggered the investigation of therapeutic strategies to understand how to maintain or re-establish microvascular auto-regulation that would have never been conceived should this not have been recognized. For example, the use of vasodilators in the setting of sepsis is currently under investigation including use of systemic vasoactive medications,72–76 modulation of NO production, exogenous NO administration and protection of the endothelial cell in the context of inflammatory activation.30,33,35,77,78 In the same way, animal data has suggested that stimulation of evolutionarily conserved, intrinsic cellular mechanisms that regulate energy utilization and quality control processes may result in organ protection in the setting of sepsis-induced AKI.79 Indeed the conservation and promotion of autophagy has been shown to be associated with better prognosis in septic animals and humans.55,61 In summary, we believe that this unifying theory may shed light on possible future targets for intervention, destined to protecting the microvasculature and the endothelium, balancing energy utilization and modulating inflammation.
Conclusion
We have put forward a possible “unifying theory” of the pathogenesis of sepsis-induced AKI, framed by the concept that the clinical phenotype is predominantly the early expression of an adaptive response of the tubular cells to an injurious, inflammatory danger signal. We submit that the interplay of inflammation and microvascular dysfunction characterize and amplify this signal, and that in response, mitochondria within tubular cells orchestrate a complete metabolic downregulation and reprioritization of energy utilization which favors individual cell survival processes (such as mitophagy and cell cycle arrest), at the expense of “kidney function” (i.e. tubular absorption and secretion of solutes).
The concepts of the pathogenesis of sepsis-induced AKI, although still incompletely understood, have suffered profound change in recent years. It seems that modern conceptualization of the disease process has abandoned the notion of equating sepsis-induced AKI to acute tubular necrosis. Furthermore, despite that hypoxia remains rightfully embedded in the core of the pathophysiologic rationale, there has been a healthy recognition that the processes that lead to sepsis-induced AKI may perhaps be more complex than previously thought, and that involve other important processes. The work of many has provided solid foundations to this modern thinking, and has also laid the necessary knowledge and tools to better appreciate the roles of these key processes.
We emphasize that this “unifying theory”, is not a universal theory, and thus, many other potentially important processes have not been considered. Nevertheless, the data hereby presented, may provide new avenues of investigation that will hopefully lead to unraveling the mechanisms by which sepsis induces AKI, and better yet, mechanistic patterns that govern global organ dysfunction in this setting that may facilitate the development of more and better targeted future therapies.
ACKNOWLEDGMENT
We thank Dr. Michael R. Pinsky, Brian Zuckerbraun and Alonso Gomez for their valuable input in the conception of this theory and review of this manuscript.
FINANCIAL SUPPORT AND POTENTIAL CONFLICT OF INTEREST
This work was funded by a research grant from the NIH/NHLBI number 1K12HL109068-02 awarded to Hernando Gomez.
REFERENCES
- 1.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005 Aug 17;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. Mar;77(6):527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006 Jun;69(11):1996–2002. doi: 10.1038/sj.ki.5000440. [DOI] [PubMed] [Google Scholar]
- 4.Langenberg C, Bellomo R, May C, Wan L, Egi M, Morgera S. Renal blood flow in sepsis. Crit Care. 2005 Aug;9(4):R363–R374. doi: 10.1186/cc3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chua HR, Glassford N, Bellomo R. Acute kidney injury after cardiac arrest. Resuscitation. 2012 Jun;83(6):721–727. doi: 10.1016/j.resuscitation.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Zager RA. Partial aortic ligation: a hypoperfusion model of ischemic acute renal failure and a comparison with renal artery occlusion. The Journal of laboratory and clinical medicine. 1987 Oct;110(4):396–405. [PubMed] [Google Scholar]
- 7.Cerchiari EL, Safar P, Klein E, Diven W. Visceral, hematologic and bacteriologic changes and neurologic outcome after cardiac arrest in dogs. The visceral post-resuscitation syndrome. Resuscitation. 1993 Apr;25(2):119–136. doi: 10.1016/0300-9572(93)90090-d. [DOI] [PubMed] [Google Scholar]
- 8.Mariano F, Cantaluppi V, Stella M, et al. Circulating plasma factors induce tubular and glomerular alterations in septic burns patients. Crit Care. 2008;12(2):R42. doi: 10.1186/cc6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen S, Heyman SN. Difficulties in understanding human "acute tubular necrosis": limited data and flawed animal models. Kidney international. 2001 Oct;60(4):1220–1224. doi: 10.1046/j.1523-1755.2001.00930.x. [DOI] [PubMed] [Google Scholar]
- 10.Brenner M, Schaer GL, Mallory DL, Suffredini AF, Parrillo JE. Detection of renal blood flow abnormalities in septic and critically ill patients using a newly designed indwelling thermodilution renal vein catheter. Chest. 1990 Jul;98(1):170–179. doi: 10.1378/chest.98.1.170. [DOI] [PubMed] [Google Scholar]
- 11.Di Giantomasso D, Bellomo R, May CN. The haemodynamic and metabolic effects of epinephrine in experimental hyperdynamic septic shock. Intensive Care Med. 2005 Mar;31(3):454–462. doi: 10.1007/s00134-005-2580-x. [DOI] [PubMed] [Google Scholar]
- 12.Di Giantomasso D, May CN, Bellomo R. Vital organ blood flow during hyperdynamic sepsis. Chest. 2003 Sep;124(3):1053–1059. doi: 10.1378/chest.124.3.1053. [DOI] [PubMed] [Google Scholar]
- 13.Di Giantomasso D, May CN, Bellomo R. Norepinephrine and vital organ blood flow during experimental hyperdynamic sepsis. Intensive Care Med. 2003 Oct;29(10):1774–1781. doi: 10.1007/s00134-003-1736-9. [DOI] [PubMed] [Google Scholar]
- 14.Ravikant T, Lucas CE. Renal blood flow distribution in septic hyperdynamic pigs. J Surg Res. 1977 Mar;22(3):294–298. doi: 10.1016/0022-4804(77)90146-9. [DOI] [PubMed] [Google Scholar]
- 15.Wan L, Bellomo R, May CN. The effect of normal saline resuscitation on vital organ blood flow in septic sheep. Intensive Care Med. 2006 Aug;32(8):1238–1242. doi: 10.1007/s00134-006-0232-4. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Holthoff JH, Seely KA, et al. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. The American journal of pathology. 2012 Feb;180(2):505–516. doi: 10.1016/j.ajpath.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seely KA, Holthoff JH, Burns ST, et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. American journal of physiology. Renal physiology. 2011 Jul;301(1):F209–F217. doi: 10.1152/ajprenal.00687.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. American journal of respiratory and critical care medicine. 2002 Jul;166(1):98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 19.Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004 Aug 7–13;364(9433):545–548. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. The New England journal of medicine. 2003 Jan 9;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 21.Fry DE. Sepsis, systemic inflammatory response, and multiple organ dysfunction: the mystery continues. Am Surg. 2012 Jan;78(1):1–8. [PubMed] [Google Scholar]
- 22.Kalakeche R, Hato T, Rhodes G, et al. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. Journal of the American Society of Nephrology : JASN. 2011 Aug;22(8):1505–1516. doi: 10.1681/ASN.2011020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu L, Gokden N, Mayeux PR. Evidence for the role of reactive nitrogen species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. Journal of the American Society of Nephrology : JASN. 2007 Jun;18(6):1807–1815. doi: 10.1681/ASN.2006121402. [DOI] [PubMed] [Google Scholar]
- 24.De Backer D, Creteur J, Preiser J-C, Dubois M-J, Vincent J-L. Microvascular Blood Flow Is Altered in Patients with Sepsis. Vol 1662002:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 25.De Backer D, Donadello K, Taccone FS, Ospina-Tascon G, Salgado D, Vincent JL. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care. 1(1):27. doi: 10.1186/2110-5820-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdant CL, De Backer D, Bruhn A, et al. Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Crit Care Med. 2009 Nov;37(11):2875–2881. doi: 10.1097/CCM.0b013e3181b029c1. [DOI] [PubMed] [Google Scholar]
- 27.Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, Mayeux PR. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. American journal of physiology. Renal physiology. 2005 Dec;289(6):F1324–F1332. doi: 10.1152/ajprenal.00124.2005. [DOI] [PubMed] [Google Scholar]
- 28.Holthoff JH, Wang Z, Seely KA, Gokden N, Mayeux PR. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. 2012 Feb;81(4):370–378. doi: 10.1038/ki.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyson A, Bezemer R, Legrand M, Balestra G, Singer M, Ince C. Microvascular and interstitial oxygen tension in the renal cortex and medulla studied in a 4-h rat model of LPS-induced endotoxemia. Shock Augusta Ga. 2011 Jul;36(1):83–89. doi: 10.1097/SHK.0b013e3182169d5a. [DOI] [PubMed] [Google Scholar]
- 30.Almac E, Siegemund M, Demirci C, Ince C. Microcirculatory recruitment maneuvers correct tissue CO2 abnormalities in sepsis. Minerva anestesiologica. 2006 Jun;72(6):507–519. [PubMed] [Google Scholar]
- 31.Schumacker PT, Chandel N, Agusti AG. Oxygen conformance of cellular respiration in hepatocytes. The American journal of physiology. 1993 Oct;265(4 Pt 1):L395–L402. doi: 10.1152/ajplung.1993.265.4.L395. [DOI] [PubMed] [Google Scholar]
- 32.Cunha FQ, Assreuy J, Moss DW, et al. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology. 1994 Feb;81(2):211–215. [PMC free article] [PubMed] [Google Scholar]
- 33.Trzeciak S, Cinel I, Phillip Dellinger R, et al. Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. 2008 May;15(5):399–413. doi: 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chauhan SD, Seggara G, Vo PA, Macallister RJ, Hobbs AJ, Ahluwalia A. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003 Apr;17(6):773–775. doi: 10.1096/fj.02-0668fje. [DOI] [PubMed] [Google Scholar]
- 35.Heemskerk S, Masereeuw R, Russel FG, Pickkers P. Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nature reviews. Nephrology. 2009 Nov;5(11):629–640. doi: 10.1038/nrneph.2009.155. [DOI] [PubMed] [Google Scholar]
- 36.Goddard CM, Allard MF, Hogg JC, Herbertson MJ, Walley KR. Prolonged leukocyte transit time in coronary microcirculation of endotoxemic pigs. The American journal of physiology. 1995 Oct;269(4 Pt 2):H1389–H1397. doi: 10.1152/ajpheart.1995.269.4.H1389. [DOI] [PubMed] [Google Scholar]
- 37.Wu L, Tiwari MM, Messer KJ, et al. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol. 2007 Jan;292(1):F261–F268. doi: 10.1152/ajprenal.00263.2006. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Guo R, Wang Y, Cunningham PN. The role of ICAM-1 in endotoxin-induced acute renal failure. Am J Physiol Renal Physiol. 2007 Oct;293(4):F1262–F1271. doi: 10.1152/ajprenal.00445.2006. [DOI] [PubMed] [Google Scholar]
- 39.Bezemer R, Legrand M, Klijn E, et al. Real-time assessment of renal cortical microvascular perfusion heterogeneities using near-infrared laser speckle imaging. Optics express. 2010 Jul 5;18(14):15054–15061. doi: 10.1364/OE.18.015054. [DOI] [PubMed] [Google Scholar]
- 40.Legrand M, Bezemer R, Kandil A, Demirci C, Payen D, Ince C. The role of renal hypoperfusion in development of renal microcirculatory dysfunction in endotoxemic rats. Intensive Care Med. 2011 Sep;37(9):1534–1542. doi: 10.1007/s00134-011-2267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002 Jul 20;360(9328):219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 42.Brealey D, Karyampudi S, Jacques TS, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004 Mar;286(3):R491–R497. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 43.Brealey D, Singer M. Mitochondrial Dysfunction in Sepsis. Current infectious disease reports. 2003 Oct;5(5):365–371. doi: 10.1007/s11908-003-0015-9. [DOI] [PubMed] [Google Scholar]
- 44.Yang QH, Liu DW, Long Y, Liu HZ, Chai WZ, Wang XT. Acute renal failure during sepsis: potential role of cell cycle regulation. The Journal of infection. 2009 Jun;58(6):459–464. doi: 10.1016/j.jinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Gupta A, Rhodes GJ, Berg DT, Gerlitz B, Molitoris BA, Grinnell BW. Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2. Am J Physiol Renal Physiol. 2007 Jul;293(1):F245–F254. doi: 10.1152/ajprenal.00477.2006. [DOI] [PubMed] [Google Scholar]
- 46.Good DW, George T, Watts BA., 3rd Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes. Am J Physiol Renal Physiol. 2009 Oct;297(4):F866–F874. doi: 10.1152/ajprenal.00335.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Achkar TM, Hosein M, Dagher PC. Pathways of renal injury in systemic gramnegative sepsis. European journal of clinical investigation. 2008 Oct;38 Suppl 2:39–44. doi: 10.1111/j.1365-2362.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 48.El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes GJ, Dagher PC. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol. 2006 May;290(5):F1034–F1043. doi: 10.1152/ajprenal.00414.2005. [DOI] [PubMed] [Google Scholar]
- 49.Ferraro E, Cecconi F. Autophagic and apoptotic response to stress signals in mammalian cells. Archives of biochemistry and biophysics. 2007 Jun 15;462(2):210–219. doi: 10.1016/j.abb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Green DR. The end and after: how dying cells impact the living organism. Immunity. 2011 Oct 28;35(4):441–444. doi: 10.1016/j.immuni.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Atkinson D. In: Cellular Energy Metabolism and its Regulation. Atkinson D, editor. New York: Academic Press; 1977. p. 218. [Google Scholar]
- 52.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. The Biochemical journal. 1995 Nov 15;312(Pt 1):163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buck LT, Hochachka PW, Schon A, Gnaiger E. Microcalorimetric measurement of reversible metabolic suppression induced by anoxia in isolated hepatocytes. The American journal of physiology. 1993 Nov;265(5 Pt 2):R1014–R1019. doi: 10.1152/ajpregu.1993.265.5.R1014. [DOI] [PubMed] [Google Scholar]
- 54.Carre JE, Singer M. Cellular energetic metabolism in sepsis: the need for a systems approach. Biochimica et biophysica acta. 2008 Jul-Aug;1777(7–8):763–771. doi: 10.1016/j.bbabio.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 55.Vanhorebeek I, Gunst J, Derde S, et al. Mitochondrial fusion, fission, and biogenesis in prolonged critically ill patients. The Journal of clinical endocrinology and metabolism. 2012 Jan;97(1):E59–E64. doi: 10.1210/jc.2011-1760. [DOI] [PubMed] [Google Scholar]
- 56.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011 Aug 26;333(6046):1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waltz P, Carchman EH, Young AC, et al. Lipopolysaccaride induces autophagic signaling in macrophages via a TLR4, heme oxygenase-1 dependent pathway. Autophagy. 2011 Mar;7(3):315–320. doi: 10.4161/auto.7.3.14044. [DOI] [PubMed] [Google Scholar]
- 58.Frank M, Duvezin-Caubet S, Koob S, et al. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochimica et biophysica acta. 2012 Dec;1823(12):2297–2310. doi: 10.1016/j.bbamcr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy. 2012 Oct;8(10):1462–1476. doi: 10.4161/auto.21211. [DOI] [PubMed] [Google Scholar]
- 60.Hsiao HW, Tsai KL, Wang LF, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. Mar;37(3):289–296. doi: 10.1097/SHK.0b013e318240b52a. [DOI] [PubMed] [Google Scholar]
- 61.Gunst J, Derese I, Aertgeerts A, et al. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. 2013 Jan;41(1):182–194. doi: 10.1097/CCM.0b013e3182676657. [DOI] [PubMed] [Google Scholar]
- 62.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. Aug 26;333(6046):1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008 Feb 28;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005 Oct;115(10):2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciechomska IA, Goemans CG, Tolkovsky AM. Molecular links between autophagy and apoptosis. Methods in molecular biology. 2008;445:175–193. doi: 10.1007/978-1-59745-157-4_12. [DOI] [PubMed] [Google Scholar]
- 66.Finkel T, Hwang PM. The Krebs cycle meets the cell cycle: mitochondria and the G1-S transition. Proceedings of the National Academy of Sciences of the United States of America. 2009 Jul 21;106(29):11825–11826. doi: 10.1073/pnas.0906430106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proceedings of the National Academy of Sciences of the United States of America. 2009 Jul 21;106(29):11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schieke SM, McCoy JP, Jr, Finkel T. Coordination of mitochondrial bioenergetics with G1 phase cell cycle progression. Cell cycle. 2008 Jun 15;7(12):1782–1787. doi: 10.4161/cc.7.12.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mandal S, Guptan P, Owusu-Ansah E, Banerjee U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Developmental cell. 2005 Dec;9(6):843–854. doi: 10.1016/j.devcel.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 70.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013 Feb 6;17(1):R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh P, Okusa MD. The role of tubuloglomerular feedback in the pathogenesis of acute kidney injury. Contrib Nephrol. 2011;174:12–21. doi: 10.1159/000329229. [DOI] [PubMed] [Google Scholar]
- 72.Boerma EC, Koopmans M, Konijn A, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. 2010 Jan;38(1):93–100. doi: 10.1097/CCM.0b013e3181b02fc1. [DOI] [PubMed] [Google Scholar]
- 73.De Backer D, Creteur J, Dubois MJ, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006 Feb;34(2):403–408. doi: 10.1097/01.ccm.0000198107.61493.5a. [DOI] [PubMed] [Google Scholar]
- 74.De Backer D, Verdant C, Chierego M, Koch M, Gullo A, Vincent JL. Effects of drotrecogin alfa activated on microcirculatory alterations in patients with severe sepsis. Crit Care Med. 2006 Jul;34(7):1918–1924. doi: 10.1097/01.CCM.0000220498.48773.3C. [DOI] [PubMed] [Google Scholar]
- 75.Pleiner J, Mittermayer F, Schaller G, MacAllister RJ, Wolzt M. High doses of vitamin C reverse Escherichia coli endotoxin-induced hyporeactivity to acetylcholine in the human forearm. Circulation. 2002 Sep 17;106(12):1460–1464. doi: 10.1161/01.cir.0000030184.70207.ff. [DOI] [PubMed] [Google Scholar]
- 76.Tyml K, Li F, Wilson JX. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit Care Med. 2005 Aug;33(8):1823–1828. doi: 10.1097/01.ccm.0000172548.34622.de. [DOI] [PubMed] [Google Scholar]
- 77.De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. The Journal of clinical investigation. 1995 Jul;96(1):60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldfarb RD, Cinel I. Inhaled nitric oxide therapy for sepsis: more than just lung. Crit Care Med. 2007 Jan;35(1):290–292. doi: 10.1097/01.CCM.0000251290.41866.2B. [DOI] [PubMed] [Google Scholar]
- 79.Howell GMGH, Collage RD, Loughran P, Escobar D, Zhang X, Billiar TR, Zuckerbraun BS, Rosengart MR. Augmenting Autophagy to Treat Acute Kidney Injury during Endotoxemia in Mice. PLoS One. 2013;8(7):e69520. doi: 10.1371/journal.pone.0069520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. The Journal of clinical investigation. 2011 Nov;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]