Abstract
OBJECTIVE
The goal was to determine the effect of training in newborn care and resuscitation on 7-day (early) neonatal mortality rates for very low birth weight (VLBW) infants. The study was designed to test the hypothesis that these training programs would reduce neonatal mortality rates for VLBW infants.
METHODS
Local instructors trained birth attendants from 96 rural communities in 6 developing countries in protocol and data collection, the World Health Organization Essential Newborn Care (ENC) course, and a modified version of the American Academy of Pediatrics Neonatal Resuscitation Program (NRP), by using a train-the-trainer model. To test the impact of ENC training, data on infants of 500 to 1499 g were collected by using a before/after, active baseline, controlled study design. A cluster-randomized, controlled trial design was used to test the impact of the NRP.
RESULTS
A total of 1096 VLBW (500–1499 g) infants were enrolled, and 98.5% of live-born infants were monitored to 7 days. All-cause, 7-day neonatal mortality, stillbirth, and perinatal mortality rates were not affected by ENC or NRP training.
CONCLUSIONS
Neither ENC nor NRP training of birth attendants decreased 7-day neonatal, stillbirth, or perinatal mortality rates for VLBW infants born at home or at first-level facilities. Encouragement of delivery in a facility where a higher level of care is available may be preferable when delivery of a VLBW infant is expected.
Keywords: neonatal mortality, perinatal mortality, stillbirth, developing countries, health care systems, very low birth weight, prematurity
Approximately 50% of the 6 million perinatal deaths throughout the world each year are early neonatal deaths (occurring in the first 7 days after birth).1 Early neonatal deaths account for ~75% of all deaths that occur within the first 28 days after birth.1,2 A major decrease in early neonatal deaths is needed to achieve United Nations Millennium Declaration Development Goal 4, which is to reduce the 1990 child (<5 years of age) mortality rate by two-thirds.3 Stillbirths are prevalent but sometimes are difficult to distinguish from early neonatal deaths. Therefore, examining both stillbirths and early neonatal deaths is important when evaluating programs that may affect perinatal mortality rates.4
Birth asphyxia, low birth weight/prematurity, and infection are major causes of perinatal deaths. Low-cost interventions, including neonatal resuscitation training5 and other interventions introduced as a package of neonatal care,6,7 have been tested, but data are limited. Studies showed that training in the World Health Organization Essential Newborn Care (ENC) course8,9 improved midwives’ skills and knowledge10 and reduced early neonatal mortality rates among low-risk women who delivered in Zambian clinics.11 Perinatal mortality rates may be decreased through training of community birth attendants.12
We showed recently that, for ≥1500 g infants born in rural communities, training in the ENC course decreased perinatal mortality rates for births conducted by birth attendants and decreased overall stillbirth rates independent of the presence of a birth attendant.13 By design, infants of <1500 g (very low birth weight [VLBW]) were not included in that study, because the study goals were to evaluate the effect of the limited interventions included in the ENC course on infants of >1500 g. VLBW infants are at high risk of death, and a package of simple neonatal care interventions provided mostly at home may not improve the outcomes of VLBW infants in the absence of advanced medical care. The current study was designed to test the hypothesis that training of birth attendants in the World Health Organization ENC course, followed by training in a modified version of the American Academy of Pediatrics Neonatal Resuscitation Program (NRP), would each reduce all-cause, 7-day (early) neonatal mortality rates for VLBW infants (<1500 g) in rural communities in developing countries.
METHODS
Population
This study was part of the First Breath Trial, a trial of training birth attendants in the provision of neonatal care.13 Infants of <1500 g were not part of the main trial because advanced medical care for <1500 g infants was generally not available in the communities. However, the personnel who participated in the trial provided care for and collected data on <1500 g infants in a manner identical to that used for ≥1500 g infants, because they were not aware that these infants were not included in the First Breath Trial outcomes. Informed consent was obtained from the mothers. The consent rate was >99% in the 4 study subgroups. A waiver of the requirement for consent was approved in 1 country.
Study Sites
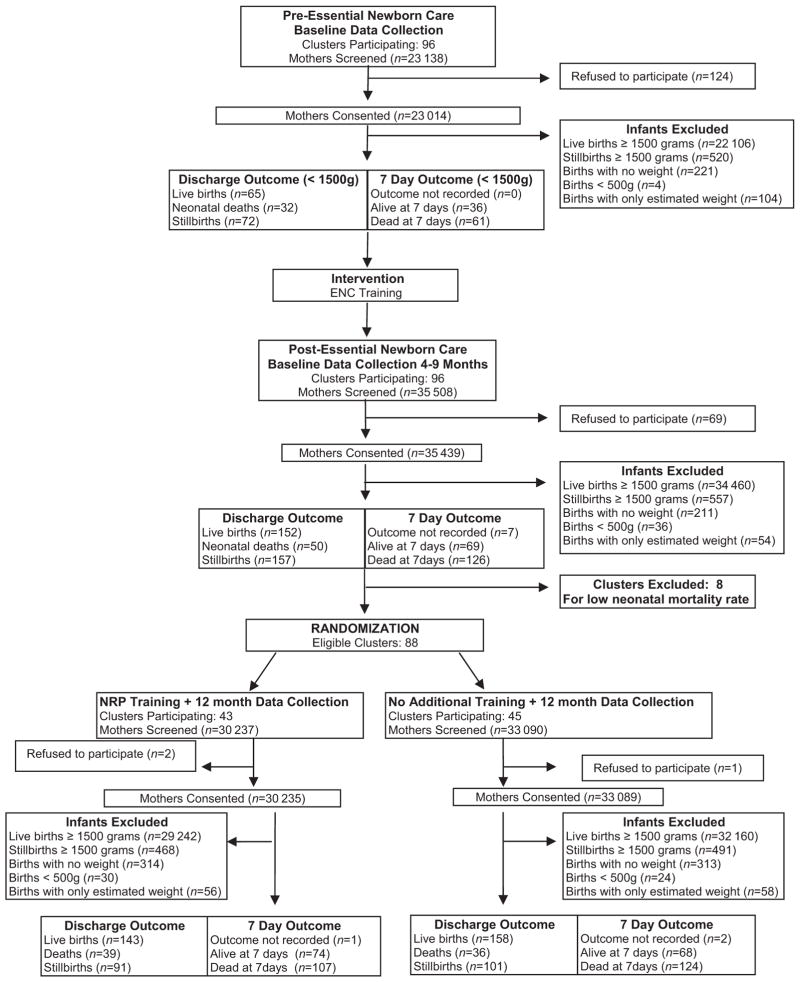
The effect of ENC training was assessed in a before/after study conducted in 96 rural communities in 7 sites of the Global Network for Women’s and Children’s Health Research (in Argentina, Democratic Republic of Congo, Guatemala, India [2 sites], Pakistan, and Zambia) between March 2005 and February 2007 (Fig 1). An active baseline design was used to minimize biases attributable to historical or otherwise inadequate baseline data.14 The active baseline design requires the full protocol, except for the intervention, to be initiated before baseline data collection is started. The cluster-randomized trial of NRP training was conducted in 88 communities (in all locations except Argentina) between July 2006 and August 2008. The sample size for this VLBW infant study was determined by the period of data collection for the First Breath Trial.13 Most communities had poor health systems, with high rates of home births assisted by traditional birth attendants. Because of the intent-to-treat design, all births in the geographic area, including deliveries at all clinics, hospitals, and homes within these communities, regardless of a birth attendant’s presence, were included. Maternal and neonatal data were collected for all births, including stillbirths. The study was reviewed and approved by the institutional review boards of participating sites in the developing countries and in the United States.
FIGURE 1.
Study flowchart.
Procedures and Interventions
The procedures and interventions were the same as those used for the First Breath Trial.13 Briefly, during 3 separate 3-day training periods, a train-the-trainer educational program was used to train all birth attendants in the communities in the study procedures and in the 2 courses. These courses were tested in a clinic-based study in Zambia11 and were modified for the First Breath Trial for community-based birth attendants, specifically with training material and data collection forms for illiterate birth attendants.13
Before baseline data collection began, experienced trainers trained 2 master trainers at each site in all study procedures, including explanation of the protocol, data collection, differentiation between stillbirth and early neonatal death (use of stethoscopes to detect heart rate and Apgar scoring), clinical assessments (fetal heart rate monitoring, signs of life at delivery, and Apgar scoring), and adult education/training techniques. The master trainers subsequently trained ≥1 research staff member (either a physician or nurse) as a community coordinator in each community. The community coordinators, in turn, trained the birth attendants (including traditional birth attendants, nurses, midwives, and physicians) in each community and distributed weight scales (UNICEF spring Salter Scales [UNICEF model 145555], Copenhagen, Denmark, from the United Nations Children’s Fund), stethoscopes, and delivery kits.
After the baseline data collection period, an experienced World Health Organization trainer (Ornella Lincetto, MD) taught master trainers, who subsequently trained community coordinators and birth attendants, in the ENC course (2004 edition), by using the same train-the-trainer model. The contents of this course included routine neonatal care, initiation of breathing and resuscitation (including bag-mask ventilation), thermoregulation, early and exclusive breastfeeding, kangaroo (skin-to-skin) care, small infant care, danger signs, and recognition and initial management of complications. Self-inflating bags and masks were given to all birth attendants. After the post–ENC training data collection period, the same training cascade was used, for communities assigned randomly to the intervention, by an experienced trainer (Susan Niermeyer, MD) to teach the NRP (2000 edition), including in-depth, hands-on training in basic knowledge and skills regarding initial resuscitation steps and bag-mask ventilation.
The birth attendants taught mothers how to implement the ENC practices. Birth attendants and/or community coordinators obtained consent and collected all data on standardized data forms. The community coordinators performed neurologic examinations, as described by Ellis et al,15 at 7 days, for surviving neonates who had received bag-mask resuscitation. Data were reviewed by the community coordinators during weekly visits and before local data entry and transmission to the data center. The First Breath Trial design did not provide resources for referral, transport, or other components of perinatal care.
Study Outcomes
The primary outcome for both interventions was all-cause, 7-day neonatal mortality rates. The secondary hypothesis was that training in the ENC course and subsequently in the NRP would decrease rates of 7-day deaths attributable to birth asphyxia (defined as failure to initiate and/or to sustain normal breathing at birth),16 stillbirths, fresh stillbirths (defined on the basis of the absence of maceration), perinatal deaths, and deaths according to birth weight categories, locations, and birth attendants. Secondary outcomes included Apgar scores, use of resuscitation techniques, and neurologic outcomes at 7 days.15 All outcomes were prespecified.
Data Management and Statistical Analyses
Data edits, including consistency checks, were performed as described previously.13 Multivariate logistic and proportional odds regression models with generalized estimating equations adjusting for cluster were used to determine differences in maternal and neonatal characteristics between the pre–ENC training and post–ENC training data and between the NRP intervention and control data by using SAS9.1.3 (SAS Institute, Cary, NC).17 Logistic models were used for binary data, proportional odds models with cumulative logit were used for ordered multinomial variables, and proportional odds models with generalized logit were used for nonordered multinomial variables. Adjusted relative risks (RRs) and 95% confidence intervals (CIs) from multivariate logistic regression models with generalized estimating equations adjusting for clustering are reported for the post–ENC training versus pre–ENC training periods, for comparison of the effects of ENC training, and for the NRP intervention versus control groups.
RESULTS
Study Group
A total of 1096 infants had birth weights between 500 and 1499 g (Table 1). Complete 7-day follow-up data were available for 98.5% of live births. Infants of 500 to 1499 g birth weight accounted for 0.9% of the infants enrolled in the First Breath Trial. Infants without exact birth weight data (N = 272) and infants with birth weights of <500 g (N = 94) were excluded from the study. If infants who did not have exact birth weight data but were estimated to weigh 500 to 1499 g were included, then this weight group would constitute 1.2% of the infants enrolled in the First Breath Trial. Additional analyses, including analysis of the outcomes of these infants, did not change the conclusions of the analyses (data not shown). The proportions of infants with exact birth weight data increased from the pre–ENC training period to the post–ENC training period and remained large during the NRP period.
TABLE 1.
Demographic Characteristics of Infants Before and After Implementation of ENC Course and in Intervention and Control Groups in NRP Trial
|
n (%)
|
P |
n (%)
|
P | |||
|---|---|---|---|---|---|---|
| Before ENC Training (N = 169) | After ENC Training (N = 359) | Control (N = 295) | After NRP, Intervention (N = 273) | |||
| Birth attendanta | .024 | .13 | ||||
| Physician | 33 (19.6) | 46 (12.8) | 29 (9.8) | 13 (4.8) | ||
| Nurse/midwife | 55 (32.7) | 86 (24.0) | 88 (29.8) | 122 (44.7) | ||
| Traditional birth attendants | 63 (37.5) | 159 (44.3) | 144 (48.8) | 96 (35.2) | ||
| Family/unattended/other | 17 (10.1) | 68 (18.9) | 34 (11.5) | 42 (15.4) | ||
| Location of birthb | .008 | .99 | ||||
| Hospital | 56 (33.3) | 72 (20.1) | 47 (15.9) | 51 (18.7) | ||
| Clinic | 23 (13.7) | 59 (16.4) | 58 (19.7) | 53 (19.4) | ||
| Home with birth attendant | 15 (8.9) | 56 (15.6) | 34 (11.5) | 24 (8.8) | ||
| Home | 73 (43.5) | 171 (47.6) | 155 (52.5) | 144 (52.7) | ||
| Other | 1 (0.6) | 1 (0.3) | 1 (0.3) | 1 (0.4) | ||
| Multiple birth | 26 (15.4) | 58 (16.2) | .88 | 50 (16.9) | 48 (17.6) | .90 |
| Male | 89 (53.0) | 174 (48.5) | .35 | 155 (52.5) | 136 (49.8) | .47 |
| Apgar score of <4 at 1 min | 95 (57.6) | 179 (51.3) | .26 | 132 (45.2) | 123 (45.9) | .93 |
| Apgar of <4 at 5 min | 86 (51.5) | 168 (48.1) | .54 | 112 (38.4) | 111 (41.4) | .76 |
| Apnea at birth | 104 (62.3) | 200 (55.9) | .19 | 185 (62.7) | 146 (53.5) | .15 |
| Bag-mask ventilation | 11 (6.5) | 23 (6.4) | .96 | 75 (25.4) | 59 (21.6) | .50 |
| Birth weight | .30 | .09 | ||||
| 500–999 g | 38 (22.5) | 93 (25.9) | 40 (13.6) | 50 (18.3) | ||
| 1000–1249 g | 63 (37.3) | 139 (38.7) | 97 (32.9) | 124 (45.4) | ||
| 1250–1499 g | 68 (40.2) | 127 (35.4) | 158 (53.6) | 99 (36.3) | ||
Data on some characteristics of 1 infant in the pre–ENC training group are missing.
P = .024 for pre–ENC training versus post–ENC training and P < .001 for NRP control versus intervention for birth attendant distribution.
P = .008 for pre–ENC training versus post–ENC training for location of birth distribution.
Pre–ENC Training/Post–ENC Training Study
There were differences in birth attendants and locations of birth but not other demographic characteristics between the pre–ENC training and post–ENC training periods (Table 1). Overall, the largest proportion of births was attended by traditional birth attendants, which increased from the pre–ENC training period to the post–ENC training period (from 37.5% to 44.3%) (Table 1). All-cause, 7-day neonatal mortality rates did not change after ENC training (Table 2). There was a significant increase in the 7-day neonatal mortality rate for infants with birth weights of 1250 to 1499 g but a trend for decreased 7-day neonatal mortality rates for the 2 other birth weight groups after ENC training. The rates of stillbirths and perinatal deaths did not decrease (Tables 3 and 4). Fresh stillbirths accounted for 54% and 75% of the stillbirths during the pre–ENC training and post–ENC training periods, respectively.
TABLE 2.
Seven-Day Neonatal Mortality Rates According to Characteristics of Infants and Deliveries
|
n/N (Deaths per 1000)
|
RR (95% CI) |
n/N (Deaths per 1000)
|
RR (95% CI) | |||
|---|---|---|---|---|---|---|
| Before ENC Training | After ENC Training | Control | After NRP, Intervention | |||
| Neonatal (7-day) death | 61/97 (629) | 126/195 (646) | 1.03 (0.83–1.27) | 124/192 (646) | 107/181 (591) | 0.92 (0.77–1.09) |
| Male | 36/49 (735) | 60/92 (652) | 0.89 (0.70–1.12) | 66/102 (647) | 59/90 (656) | 1.01 (0.82–1.25) |
| Female | 25/48 (521) | 66/103 (641) | 1.23 (0.88–1.72) | 58/90 (644) | 48/91 (528) | 0.82 (0.64–1.05) |
| Birth attendant | ||||||
| All birth attendants | 55/85 (647) | 91/140 (650) | 1.00 (0.82–1.24) | 106/165 (642) | 88/143 (615) | 0.96 (0.81–1.14) |
| Physician | 12/22 (546) | 16/26 (615) | 1.13 (0.67–1.91) | 8/22 (364) | 7/8 (875) | 2.41 (1.48–3.90) |
| Nurse/midwife | 20/30 (667) | 29/43 (674) | 1.01 (0.73–1.39) | 36/57 (632) | 44/76 (579) | 0.92 (0.68–1.23) |
| Traditional birth attendants | 23/33 (697) | 46/71 (648) | 0.93 (0.70–1.23) | 62/86 (721) | 37/59 (627) | 0.87 (0.69–1.09) |
| Family/unattended/other | 5/11 (455) | 35/55 (636) | 1.40 (0.72–2.74) | 18/27 (667) | 19/38 (500) | 0.75 (0.51–1.10) |
| Location of birth | ||||||
| Home/home with birth attendant | 35/51 (686) | 81/127 (638) | 0.93 (0.71–1.22) | 88/126 (698) | 80/127 (630) | 0.90 (0.74–1.10) |
| Clinic | 6/13 (462) | 18/29 (621) | 1.34 (0.73–2.47) | 20/31 (645) | 11/27 (407) | 0.63 (0.39–1.01) |
| Hospital | 19/32 (594) | 27/39 (692) | 1.17 (0.80–1.71) | 16/35 (457) | 16/27 (593) | 1.30 (0.86–1.95) |
| Birth weight | ||||||
| 500–999 g | 15/16 (938) | 20/25 (800) | 0.85 (0.68–1.08) | 9/11 (818) | 14/17 (824) | 1.01 (0.73–1.38) |
| 1000–1249 g | 26/36 (722) | 44/76 (579) | 0.80 (0.57–1.13) | 39/55 (709) | 59/87 (678) | 0.96 (0.75–1.22) |
| 1250–1499 g | 20/45 (444) | 62/94 (660) | 1.48 (1.01–2.18) | 76/126 (603) | 34/77 (442) | 0.73 (0.56–0.95) |
Data may reflect missing values.
TABLE 3.
Stillbirth Rates According to Characteristics of Infants and Deliveries
|
n/N (Deaths per 1000)
|
RR (95% CI) |
n/N (Deaths per 1000)
|
RR (95% CI) | |||
|---|---|---|---|---|---|---|
| Before ENC Training | After ENC Training | Control | After NRP, Intervention | |||
| Stillbirth | 72/169 (426) | 157/359 (437) | 1.03 (0.80–1.31) | 101/295 (342) | 91/273 (333) | 0.97 (0.57–1.67) |
| Fresh stillbirth | 39/169 (231) | 117/359 (326) | 1.41 (0.97–2.07) | 71/295 (241) | 65/273 (238) | 0.99 (0.54–1.81) |
| Macerated stillbirth | 33/169 (195) | 40/359 (111) | 0.57 (0.38–0.85) | 30/295 (102) | 26/273 (95) | 0.94 (0.39–2.24) |
| Male | 40/89 (449) | 77/174 (443) | 0.98 (0.73–1.33) | 52/155 (336) | 46/136 (338) | 1.01 (0.57–1.79) |
| Female | 31/79 (392) | 80/185 (432) | 1.10 (0.81–1.49) | 49/140 (350) | 45/137 (329) | 0.94 (0.50–1.76) |
| Birth attendant | ||||||
| All birth attendants | 66/151 (437) | 146/291 (502) | 1.15 (0.91–1.45) | 94/261 (360) | 87/231 (377) | 1.05 (0.62–1.77) |
| Physician | 11/33 (333) | 16/46 (348) | 1.04 (0.61–1.78) | 5/29 (172) | 5/13 (385) | 2.23 (0.89–5.58) |
| Nurse/midwife | 25/55 (455) | 42/86 (488) | 1.07 (0.74–1.56) | 31/88 (352) | 46/122 (377) | 1.07 (0.56–2.03) |
| Traditional birth attendants | 30/63 (476) | 88/159 (554) | 1.16 (0.79–1.71) | 58/144 (403) | 36/96 (375) | 0.93 (0.50–1.74) |
| Family/unattended/other | 6/17 (353) | 11/68 (162) | 0.46 (0.14–1.49) | 7/34 (206) | 4/42 (95) | 0.46 (0.12–1.86) |
| Location of birth | ||||||
| Home/home with birth attendant | 38/89 (427) | 99/228 (434) | 1.02 (0.72–1.45) | 64/190 (337) | 41/169 (243) | 0.72 (0.36–1.44) |
| Clinic | 10/23 (435) | 30/59 (509) | 1.17 (0.61–2.24) | 27/58 (466) | 26/53 (491) | 1.05 (0.64–1.73) |
| Hospital | 24/56 (429) | 28/72 (389) | 0.91 (0.63–1.30) | 10/47 (213) | 24/51 (471) | 2.21 (1.10–4.44) |
| Birth weight | ||||||
| 500–999 g | 22/38 (579) | 68/93 (731) | 1.26 (0.91–1.76) | 29/40 (725) | 33/50 (660) | 0.91 (0.65–1.28) |
| 1000–1249 g | 27/63 (429) | 63/139 (453) | 1.06 (0.80–1.40) | 42/97 (433) | 36/124 (290) | 0.67 (0.41–1.09) |
| 1250–1499 g | 23/68 (338) | 26/127 (205) | 0.61 (0.37–0.98) | 30/158 (190) | 22/99 (222) | 1.17 (0.50–2.76) |
Data may reflect missing values.
TABLE 4.
Perinatal Mortality Rates According to Characteristics of Infants and Deliveries
|
n/N (Deaths per 1000)
|
RR (95% CI) |
n/N (Deaths per 1000)
|
RR (95% CI) | |||
|---|---|---|---|---|---|---|
| Before ENC Training | After ENC Training | Control | After NRP, Intervention | |||
| Perinatal death | 133/169 (787) | 283/352 (804) | 1.02 (0.91–1.14) | 225/293 (768) | 198/272 (728) | 0.95 (0.84–1.07) |
| Male | 76/89 (854) | 137/169 (811) | 0.95 (0.84–1.07) | 118/154 (766) | 105/136 (772) | 1.01 (0.87–1.16) |
| Female | 56/79 (709) | 146/183 (798) | 1.13 (0.94–1.35) | 107/139 (770) | 93/136 (684) | 0.89 (0.75–1.05) |
| Birth attendant | ||||||
| All birth attendants | 121/151 (801) | 237/286 (829) | 1.03 (0.93–1.15) | 200/259 (772) | 175/230 (761) | 0.99 (0.88–1.10) |
| Physician | 23/33 (697) | 32/42 (762) | 1.09 (0.80–1.50) | 13/27 (482) | 12/13 (923) | 1.92 (1.39–2.65) |
| Nurse/midwife | 45/55 (818) | 71/85 (835) | 1.02 (0.87–1.20) | 67/88 (761) | 90/122 (738) | 0.97 (0.84–1.12) |
| Traditional birth attendants | 53/63 (841) | 134/159 (843) | 1.00 (0.87–1.15) | 120/144 (833) | 73/95 (768) | 0.92 (0.80–1.06) |
| Family/unattended/other | 11/17 (647) | 46/66 (697) | 1.08 (0.65–1.79) | 25/34 (735) | 23/42 (548) | 0.74 (0.53–1.05) |
| Location of birth | ||||||
| Home/home with birth attendant | 73/89 (820) | 180/226 (797) | 0.97 (0.83–1.13) | 152/190 (800) | 121/168 (720) | 0.90 (0.77–1.06) |
| Clinic | 16/23 (696) | 48/59 (814) | 1.17 (0.83–1.64) | 47/58 (810) | 37/53 (698) | 0.86 (0.71–1.04) |
| Hospital | 43/56 (768) | 55/67 (821) | 1.07 (0.88–1.30) | 26/45 (578) | 40/51 (784) | 1.36 (1.04–1.77) |
| Birth weight | ||||||
| 500–999 g | 37/38 (974) | 88/93 (946) | 0.97 (0.91–1.04) | 38/40 (950) | 47/50 (940) | 0.99 (0.90–1.09) |
| 1000–1249 g | 53/63 (841) | 107/139 (770) | 0.92 (0.77–1.09) | 81/97 (835) | 95/123 (772) | 0.92 (0.80–1.07) |
| 1250–1499 g | 43/68 (632) | 88/120 (733) | 1.16 (0.93–1.45) | 106/156 (680) | 56/99 (566) | 0.83 (0.68–1.02) |
Data may reflect missing values.
NRP Randomized Trial
There was no difference in the demographic characteristics between the randomly assigned groups. All-cause, 7-day neonatal mortality, stillbirth, and perinatal mortality rates did not decrease for the infants born in clusters assigned to receive training in the NRP, compared with control clusters (Tables 2, 3, and 4). Of the stillbirths, the proportions of fresh stillbirths averaged 71% and did not differ between intervention and control clusters.
Resuscitation
Overall, 15.3% of VLBW infants (168 of 1096 VLBW infants) received bag-mask ventilation; the majority of infants who did not receive bag-mask ventilation were stillborn. Of the infants who received bag-mask ventilation, 10.7% were stillborn. Of the live-born infants, 22% received bag-mask ventilation. Of the live-born infants, 25% survived to day 7. These results did not differ significantly according to study phase. Of the live-born infants who did not receive bag-mask ventilation, 40% survived to day 7.
Causes of Death
Overall, the primary causes of deaths were VLBW (76%), birth asphyxia (16%), and infections (6%). After ENC training, there was a decrease in the rate of deaths attributable to birth asphyxia (from 16.5 deaths per 1000 live births to 2.6 deaths per 1000 live births; P < .001) among VLBW infants.
Neurologic Examination Results
Neurologic examination results were available for only 59 of the 7-day VLBW survivors. Twenty (34%) of the 59 infants who were seen at 7 days had examination results consistent with moderate/severe encephalopathy. These results did not change significantly according to study phase.
DISCUSSION
This multicenter study conducted in rural communities in developing countries demonstrated that neither ENC training nor NRP training reduced mortality rates for VLBW infants. This result is in contrast to the reductions in stillbirth and perinatal mortality rates for infants with birth weights of >1500 g that were observed after training of the same birth attendants with the ENC course13 and the reductions in neonatal mortality rates in a facility-based study of the same intervention.11 The majority of the deliveries occurred at home and were attended by traditional birth attendants or family members. Maternal and neonatal transportation and advanced neonatal care generally were unavailable or were limited in the study communities, which may explain in part the lack of impact on mortality rates in this high-risk population.
The study strengths are the population-based design, the rigorous training using master instructors, the exclusive use of local trainers to train birth attendants, the use of pregnancy/birth registries to capture all births, the inclusion of all birth attendants, the relatively large sample size, and the high consent and 7-day follow-up rates. Importantly, whereas most low-income countries register live births but not stillbirths,4 birth attendants were trained to report outcomes of all pregnancies, which allowed ascertainment of the contributions of stillbirths and very early neonatal deaths to perinatal mortality rates. In addition, many studies of perinatal interventions at the community level did not train birth attendants in the differentiation between stillbirths and neonatal deaths, as was performed in the current study. To our knowledge, this is the largest study of VLBW infants born in rural communities in low- and middle-income countries.
One weakness of this study was the before/after design to evaluate the impact of the ENC course. To minimize this weakness, all training, except for ENC training, was conducted before the initiation of data collection. This active baseline design minimizes biases and decreases the likelihood that other concurrent changes in practice influenced the outcomes.14 With high-quality design and data collection, before/after analyses can provide meaningful results and supplement studies using random allocation.18 However, the possibility that differences between the pre–ENC training and post–ENC training periods might confound and mask potential differences between the periods cannot be excluded. Another weakness is that some infants considered VLBW were not weighed at birth and therefore, were not included in the study. However, analyses including infants with estimated birth weights did not change the study conclusions. Because infants thought to be most immature might have been excluded, smaller VLBW infants are likely to be underrepresented in the reported cohort, as suggested by the birth weight distributions. Another important limitation is that many VLBW infants did not receive bag-mask ventilation and might not have received other care because of anticipated low chances for survival. However, the use of bag-mask ventilation increased almost fourfold during the study periods. Despite the increased use of bag-mask ventilation, mortality rates were not decreased.
This study focused on VLBW infants, who, because of their high risk of death, were not included in the First Breath Trial results. Birth weight is a predictor of neonatal death,19,20 causes of neonatal death,21 and birth asphyxia20,22 in less-developed countries. Gestational age also is a strong predictor of neonatal death.19 A combination of birth weight and gestational age was almost as effective in predicting neonatal death as a more-complex model that was dependent on variables that rarely are available at first-level facilities or through retrospective data collection in the developing world.19 Therefore, focusing on the effects of interventions that affect birth weight and gestational age is important.
Improved survival rates for VLBW infants are likely to require advanced care. Survival rates for VLBW infants increase as care practices, including use of prenatal steroid treatment, cesarean section, exogenous surfactant administration, ventilation, and continuous positive airway pressure therapy, are introduced in NICUs.23 However, these advanced care practices frequently are not available for pregnant women at high risk or their infants at first-level facilities or in home deliveries in developing countries. Perinatal and neonatal care in these circumstances would require maternal referral to more-advanced facilities or stabilization and prompt transportation soon after birth. There is some evidence that neonatal care intervention packages6,7,11–13,18 and newborn resuscitation5,24–27 can be effective in reducing mortality rates in a general population of neonates. However, those studies did not include VLBW infants or reported on only a small population of VLBW infants, without specific analyses of the effects of the interventions on those infants. The current study is particularly important because a training package of neonatal interventions and resuscitation was tested among VLBW infants born at the community level. However, the current data cannot be used to determine whether these interventions would be effective if maternal and/or neonatal referral and advanced care practices were available. In a large, population-based study in a developed country, being born at a higher-level facility was associated with improved survival rates for VLBW infants.28
CONCLUSIONS
Training of birth attendants in ENC and in NRP did not decrease early neonatal mortality, stillbirth, or perinatal mortality rates for VLBW infants born at a first-level facility or at home. In view of the advanced practices found to be effective for these high-risk infants, transportation of pregnant women to facilities with high levels of care should be considered when delivery of a VLBW infant or an infant at <30 weeks of gestation is expected. However, VLBW infants constitute only ~1% of births, and ENC training should continue to be advocated for all births, because it can reduce markedly the rates of stillbirths,13 neonatal deaths,11 and perinatal deaths.13
WHAT’S KNOWN ON THIS SUBJECT
Perinatal and/or neonatal mortality rates for infants born in community settings in developing countries can be decreased with implementation of a package of neonatal health care interventions. VLBW infants born in these settings have high mortality risk.
WHAT THIS STUDY ADDS
A neonatal health care intervention package that was effective in reducing perinatal and/or early neonatal mortality rates did not improve outcomes for VLBW infants born in community settings in developing countries.
Acknowledgments
This work was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Global Network for Women’s and Children’s Health Research (grants U01 HD040477, U01 HD043475, U01 HD043464, U01 HD040657, U01 HD042372, U01 HD040607, U01 HD040636, and U01 HD040574) and the Bill and Melinda Gates Foundation. Eunice Kennedy Shriver National Institute of Child Health and Human Development staff members participated in the study design, study conduct, data interpretation, and manuscript editing. Neither funding agency limited the ability to complete the research as planned. The authors had full control of the primary data.
The First Breath Study Group of the Global Network for Women’s and Children’s Health Research included the following: Argentina: Fernando Althabe, Jose M. Belizan, and Edgardo Szyld (Institute for Clinical Effectiveness and Health Policy); Democratic Republic of Congo: John Ditekemena and Antoinette Tshefu (Kinshasa School of Public Health); Guatemala: Ana Garces (San Carlos University); India: Arjit Mohapatra and Sailajanandan Parida (Sriramchandra Bhanja Medical College) and Shivaprasad S. Goudar and Bhalchandra S. Kodkany (Jawaharlal Nehru Medical College); Pakistan: Imtiaz Jehan, Syed Rafat Jafri, and Omrana Pasha (Aga Khan University); Zambia: Margaret Mbelenga (Centre for Infectious Disease Research) and Elwyn Chomba (University of Zambia); United States: Waldemar A. Carlo (University of Alabama at Birmingham), Nancy Krebs and Michael Hambidge (University of Colorado), Robert L. Goldenberg (Drexel University), Pinaki Panigrahi (University of Maryland, Baltimore), Richard J. Derman (University of Missouri, Kansas City), Carl Bose (University of North Carolina at Chapel Hill), Pierre Buekens (Tulane School of Public Health and Tropical Medicine), Linda L. Wright, Macaya Douoguih, and Anne Willoughby (Eunice Kennedy Shriver National Institute of Child Health and Human Development), and Hillary Harris, Elizabeth M. McClure, Hrishikesh Chakraborty, Janet Moore, and Ty Hartwell (RTI International).
Funded by the National Institutes of Health (NIH).
ABBREVIATIONS
- ENC
Essential Newborn Care
- NRP
Neonatal Resuscitation Program
- VLBW
very low birth weight
- RR
relative risk
- CI
confidence interval
Footnotes
This trial has been registered at www.clinicaltrials.gov (identifier NCT00136708).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.World Health Organization. Neonatal and Perinatal Mortality: Country, Regional and Global Estimates. Geneva, Switzerland: World Health Organization; 2006. [Accessed February 11, 2010]. Available at: www.who.int/making_pregnancy_safer/publications/en/index.html. [Google Scholar]
- 2.Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? where? why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Laakso T, Shibuya K, Hill K, Lopez AD. Can we achieve Millennium Development Goal 4? New analysis of country trends and forecasts of under-5 mortality to 2015. Lancet. 2007;370(9592):1040–1054. doi: 10.1016/S0140-6736(07)61478-0. [DOI] [PubMed] [Google Scholar]
- 4.Lawn J, Shibuya K, Stein C. No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull World Health Organ. 2005;83(6):409–417. [PMC free article] [PubMed] [Google Scholar]
- 5.Deorari AK, Paul VK, Singh M, Vidyasagar D. Medical Colleges Network. Impact of education and training on neonatal resuscitation practices in 14 teaching hospitals in India. Ann Trop Paediatr. 2001;21(1):29–33. [PubMed] [Google Scholar]
- 6.Darmstadt GL, Bhutta ZA, Cousens S, et al. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365(9463):977–988. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 7.Haws RA, Thomas AL, Bhutta ZA, Darmstadt GL. Impact of packaged interventions on neonatal health: a review of the evidence. Health Policy Plan. 2007;22(4):193–215. doi: 10.1093/heapol/czm009. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Pregnancy, Childbirth, Postpartum and Newborn Care: A Guide for Essential Practice. 2. Geneva, Switzerland: World Health Organization; 2006. [PubMed] [Google Scholar]
- 9.Lang S. Essential Newborn Care Course. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 10.McClure EM, Carlo WA, Wright LL, et al. Evaluation of the educational impact of the WHO Essential Newborn Care course in Zambia. Acta Paediatr. 2007;96(8):1135–1138. doi: 10.1111/j.1651-2227.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 11.Wright LL, Garces A, Carlo WA, McClure EM First Breath Study Group. Effect of Essential Newborn Care (ENC) training by birth attendant type: a global network study. Presented at the annual meeting of the Pediatric Academic Societies; May 2–5, 2009; Baltimore, MD. [Google Scholar]
- 12.Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115(2 suppl):519–617. doi: 10.1542/peds.2004-1441. [DOI] [PubMed] [Google Scholar]
- 13.Carlo WA, Goudar SS, Jehan I, et al. Newborn-care training and perinatal mortality in developing countries. N Engl J Med. 2010;362(7):614–623. doi: 10.1056/NEJMsa0806033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spilker B. Guide to Clinical Trials. 1. Philadelphia, PA: Lippincott Williams & Wilkins; 1991. p. 75. [Google Scholar]
- 15.Ellis M, Manandhar DS, Manandhar N, Wyatt J, Bolam AJ, Costello AM. Stillbirths and neonatal encephalopathy in Kathmandu, Nepal: an estimate of the contribution of birth asphyxia to perinatal mortality in low-income urban population. Paediatr Perinat Epidemiol. 2000;14(1):39–52. doi: 10.1046/j.1365-3016.2000.00233.x. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Basic Newborn Resuscitation: A Practical Guide. Geneva, Switzerland: World Health Organization; 1997. p. 32. [Google Scholar]
- 17.Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System. 2. Cary, NC: SAS Institute; 2009. [Google Scholar]
- 18.Bhutta ZA, Memon ZA, Soofi S, Salat MS, Cousens S, Martines J. Implementing community-based perinatal care: results from a pilot study in rural Pakistan. Bull World Health Organ. 2008;86(6):452–459. doi: 10.2471/BLT.07.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg RE, Ahmed S, Saha SK, et al. Simplified age-weight mortality risk classification for very low birth weight infants in low-resource settings. J Pediatr. 2008;153(4):519–524. doi: 10.1016/j.jpeds.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 20.Adhikari M, Gouws E, Desai PK. Periventricular hemorrhage in a developing world: is drug intervention appropriate? Brain Dev. 1995;17(3):164–168. doi: 10.1016/0387-7604(95)00016-5. [DOI] [PubMed] [Google Scholar]
- 21.Hotrakitya S, Tejavej A, Siripoonya P. Early neonatal mortality and causes of death in Ramathibodi Hospital: 1981–1990. J Med Assoc Thai. 1993;76(suppl 2):119–129. [PubMed] [Google Scholar]
- 22.Kolatat T, Aunganon K, Yosthiem P. Airway complications in neonates who received mechanical ventilation. J Med Assoc Thai. 2002;85(suppl 2):S455–S462. [PubMed] [Google Scholar]
- 23.Ho JJ, Chang AS. Changes in the process of care and outcome over a 10-year period in a neonatal nursery in a developing country. J Trop Pediatr. 2007;53(4):232–237. doi: 10.1093/tropej/fmm050. [DOI] [PubMed] [Google Scholar]
- 24.Cowles W. Abstracts from India’s Second International Emergency Medicine and Disaster Preparedness Conference. Acad Emerg Med. 2007;14(5):e109–e113. [Google Scholar]
- 25.Zhu XY, Fang HQ, Zeng SP, Li YM, Lin HL, Shi SZ. The impact of the neonatal resuscitation program guidelines (NRPG) on the neonatal mortality in a hospital in Zhuhai, China. Singapore Med J. 1997;38(11):485–487. [PubMed] [Google Scholar]
- 26.O’Hare BA, Nakakeeto M, Southall DP. A pilot study to determine if nurses trained in basic neonatal resuscitation would impact the outcome of neonates delivered in Kampala, Uganda. J Trop Pediatr. 2006;52(5):376–379. doi: 10.1093/tropej/fml027. [DOI] [PubMed] [Google Scholar]
- 27.Opiyo N, Were F, Govedi F, Fegan G, Wasunna A, English M. Effect of newborn resuscitation training on health worker practices in Pumwani Hospital, Kenya. PLoS One. 2008;3(2):e1599. doi: 10.1371/journal.pone.0001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cifuentes J, Bronstein J, Phibbs CS, Phibbs RH, Schmitt SK, Carlo WA. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics. 2002;109(5):745–751. doi: 10.1542/peds.109.5.745. [DOI] [PubMed] [Google Scholar]