Abstract
Purpose of review
This article provides an updated review on mechanistic and molecular studies relating to the effects of n-3 fatty acids (FA) on inhibiting atherogenesis.
Recent findings
The effects of n-3 FA on modulating arterial lipoprotein lipase (LpL) levels link to changes in lipid deposition in the arterial wall. LpL expression in the arterial wall also relates to local macrophage-mediated inflammatory processes. Increasing evidence suggests that n-3 FA ameliorate inflammation, another key component in the development of atherosclerosis, including decreases in pro-inflammatory cytokine production. n-3 FA inhibit atherogenic signaling pathways and modulate the phenotypes of inflammatory leukocytes and their recruitment in the arterial wall.
Summary
New mechanistic insights into the anti-atherogenic action of n-3 FA have emerged. These studies may contribute to future therapeutic advances in preventing mortality and morbidity associated with atherosclerosis.
Keywords: Atherosclerosis, inflammation, lipoprotein lipase, macrophages, n-3 fatty acids
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States [1] and many countries globally. CVD risk factor control remains a challenge for many. Dietary fatty acids (FA) play an important role in the development or prevention of CVD. Diets high in saturated fats increase atherosclerotic CVD morbidity and mortality [2]. On the other hand, omega-3 fatty acids (n-3 FA), especially fish oil-derived eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have emerged as potentially preventive and therapeutic agents to decrease CVD by acting on pathways related to atherosclerosis and myocardial infarction [3]. While controversy continues [4**, 5*], many mechanistic studies point towards beneficial effects of n-3 FA on inhibiting adverse pathways in the pathogenesis of CVD. The aim of this review article is to provide an overview of recent findings on underlying mechanisms of potential anti-atherogenic action of n-3 FA.
Overview of cardio-protective mechanisms of n-3 FA
Dietary n-3 FA are a class of essential polyunsaturated FA (PUFA) with the double bond in the third carbon position (ω-3) from the methyl terminal. n-3 FA can be further converted into signaling molecules, such as eicosanoids and the more recently identified docosanoids which are docosatrienes, protectins, and resolvins [6]. The longer chain n-3 FA, EPA and DHA are generally far more bioactive than their n-3 essential FA precursor, alpha linolenic acid [7]. n-3 FA may be cardio-protective by changing several intermediate determinants of CVD risk [8]. n-3 FA ameliorate hypertriglyceridemia, reduce blood pressure and protect against arrhythmias [6, 9, 10*]. A number of pathways can be mediated directly by incorporation of n-3 FA into cell membrane phospholipids; this results in changes in membrane fluidity by altering the properties of lipid rafts and caveolae, leading to modulation of membrane-associated proteins and receptor activities [11]. Membrane incorporation of n-3 FA decreased the generation of intracellular reactive oxygen species with a subsequent diminished activation of redox-sensitive transcription factors, such as nuclear factor-κB (NF-κB) [12]. n-3 FA signaling through a G-protein coupled receptor, GPR120, is reported to modulate inflammation and insulin-sensitizing effects in monocytes and macrophages [13].
Another action of n-3 FA relating to cardiovascular health is via its interference with cell-membrane ion channels that results in a number of biological effects, including reducing arrhythmias [14, 15]. Although findings on the effects of n-3 FA on reducing recurrent atrial fibrillation in patients are controversial [16, 17], a recent report has demonstrated that n-3 FA were effective in primary prevention of atrial fibrillation [18*].
Other biological effects of n-3 FA are likely mediated through the release of bioactive mediators that suppress pro-inflammatory cytokines and produce anti-inflammatory catabolites, such as protectins and resolvins [8, 19]. n-3 FA have been reported to reduce platelet reactivity, in part, through the modification of platelet fatty acid composition and decreasing production of pro-aggregatory eicosanoids to potentially provide protective action against plaque rupture [20, 21]. Moertl et al reported a role of n-3 FA in controlling thrombosis in patients with chronic heart failure by reducing the levels of mediators that promote thrombosis [22]. However, n-3 FA had little or no effect on markers of platelet and endothelial functions in patients with peripheral arterial disease (PAD) [23]. Higher intake of n-3 FA increased the stability of atherosclerotic plaques in humans [24]. Incorporation of n-3 FA into advanced atherosclerotic plaques increased plaque stability in humans [24, 25] and apoE−/− mice [26] by inducing structural changes of plaques with lower plaque inflammation and by increasing the thickness of fibrous caps [9, 27].
Effects of dietary n-3 FA on arterial lipid deposition and lipoprotein lipase
Lipid deposition is an initial key step in atherogenesis that begins with the entry of lipoproteins (mainly LDL) into the arterial wall. Lipid deposition initiates a pro-inflammatory cascade that attracts monocytes into the subendothelial space [28]. Infiltrated monocytes differentiate into macrophages that take up the lipoproteins and become foam cells. These foam cells make up the fatty streak that can be the precursor of an atherosclerotic plaque [9].
Incorporation of dietary FA into chylomicron remnants influenced lipid accumulation in arterial macrophages [29]. When compared with remnants rich in n-3 FA, chylomicron remnants rich in saturated FA were taken up more rapidly by cultured macrophages and resulted in greater arterial lipid accumulation [30]. FA can also affect binding and uptake of lipids in the arterial wall by macrophages. We have reported that regulatory effects of dietary FA on arterial lipid deposition were related to expression and distribution of arterial lipoprotein lipase (LpL) [31–33]. In addition to its catalytic activity on triglycerides, LpL can also serve as a bridging or anchoring molecule for LDL and other lipoproteins to cell surfaces [34, 35]. Seo et al reported that a high saturated fat diet increased arterial cholesterol delivery via total LDL and selective LDL-cholesterol uptake in mice, and that this was associated with increased arterial wall LpL levels [31]. However, little is known about how n-3 FA might affect pathways in the early stage of atherosclerosis, i.e., cholesterol delivery and LpL at the levels of arterial wall.
We investigated pathways underlying regulation of arterial LpL and the role of LpL in mediating arterial lipid deposition in the development of atherosclerosis in several mouse models. We demonstrated that specific conditions, such as high intakes of saturated FA and insulin resistance, altered recruitment of different cell populations to the arterial wall, particularly accumulation of macrophages that secrete LpL, and thus favor the development of atherosclerosis [32, 33]. n-3 FA decreased the presence of inflammatory cells and hence, macrophage-secreted arterial LpL, which was associated with decreases in arterial cholesterol delivery, inflammation and atherosclerosis. The presence of arterial LpL itself also appears important for the presence of macrophages in the arterial wall [33]. We recently used LDLR−/− mice to evaluate impact of dietary saturated FA being replaced with n-3 FA on the progression of atherosclerosis and arterial LpL levels and localization. Our preliminary data demonstrate that increasing replacement of saturated FA by n-3 FA intake abrogated the adverse effects mediated by saturated FA by improving mouse plasma lipid profiles and lowering total and LDL cholesterol levels. Incremental inclusion of n-3 FA also decreased aortic macrophage-associated LpL, as well as aortic macrophage markers and pro-inflammatory markers [36].
Emerging mechanisms relating to anti-inflammatory actions of n-3 FA in atherosclerosis
Higher intakes of dietary n-3 FA decreased serum levels of pro-inflammatory biomarkers, including interleukin-6 ( IL-6), soluble E-selectin, ICAM-1, VCAM-1 and C-reactive protein (CRP) [37]. As well, a number of studies highlight the importance of leukocyte recruitment in atherosclerosis. Leukocytes recruited during the inflammatory processes include neutrophils, monocytes and T cells, and to a less extent, B cells, dendritic cells (DC) and mast cells [38]. Specific leukocyte subsets are recruited by a unique combination of chemokines and their corresponding receptors. Studies on arterial monocytes and macrophages, and their contributions to innate versus adaptive immunity have been previously reviewed [39]. We will now focus on recent aspects of n-3 FA-mediated effects on monocytes and macrophage recruitment and phenotypes and on previously less appreciated cellular players, such as neutrophils and DC. These highlight the emergence of additional pathways related to murine atherosclerosis that may guide future studies relating to effects of n-3 FA.
Effects of n-3 FA on monocytes and macrophages in atherosclerosis
Monocytes/macrophages are heterogeneous populations of cells that play a key role in innate or adaptive immune response in atherosclerosis. Subpopulations of monocytes and macrophages function differently and are identified by differential expression of selected surface markers in the inflammatory processes [40]. In humans, the presence of CD16 classifies a pro-inflammatory monocyte subset. Mouse monocyte profiling is characterized mainly by the expression of Ly6C; CCR2+ (MCP-1 receptor) Ly6Chi monocytes are recruited to inflamed tissues, whereas the CCR2−Ly6Clo subset is recruited to non-inflamed tissues. Monocyte subsets with expression of Ly6C and CCR2 were readily recruited to atherosclerotic lesions and developed into pro-inflammatory macrophages in mice [41, 42]. n-3-rich diets reduced Ly6Chi monocyte subset populations and monocyte recruitment to aortic lesions, resulting in reduced atherosclerosis in LDLR−/− and apoE−/− mice, independent of plasma cholesterol levels [43*].
Effects of n-3 FA on the chemotaxis of monocytes have been reported. Grenon et al demonstrated that n-3 EPA, but not n-6 arachidonic acid (AA), decreased monocyte adhesion to endothelial cells, independent of the stimulation of TNF-α, and that this was associated with reduced mRNA expression of adhesion molecules, such as ICAM-1, VCAM-1, and E-selectin, and pro-inflammatory mediators, such as IL-6 and TNF-α [44*]. Still, Luu et al has reported little effect on migration and adhesion of monocytes isolated from patients with peripheral arterial disease (PAD) receiving fish oil supplementation [45].
Macrophages that reside in the arterial wall mediate inflammatory processes. Jung et al has demonstrated that n-3 EPA attenuated pro-inflammatory markers in cultured murine macrophages by decreasing mRNA expression levels of pro-inflammatory cytokines, such as IL-6; pathways inhibited in part, by decreasing PPARγ levels [46*]. The specific mechanisms on how and which dietary FA influence macrophage-mediated inflammatory responses are still not well-understood. Earlier studies reported that saturated FA triggered the accumulation of macrophages in arterial wall which contributeed to atherogenesis by local secretion of pro-inflammatory mediators.[47, 48]. Saturated FA can activate inflammation through the activation of toll-like receptor (TLR)/ NF-κB-mediated signaling in macrophages [49, 50]. On the other hand, EPA and DHA blocked LPS-induced NF-κB activation in macrophages [50]. DHA inhibited TLR4/NF-κB activation by inhibiting TLR2 dimerization with TLR6 or TLR1 [51].The incorporation of DHA into membranes can alter lipid composition in lipid rafts, resulting in disruption of TLR4 recruitment into lipid rafts and reduced downstream signaling [52]. Interestingly, other pro-inflammatory mediators, such as ceramide [53] and NADPH oxidase (NOX), [54], have been linked to saturated FA exacerbating vascular injury. The specific interaction between n-3 FA and ceramide and/or NOX has yet to be established.
Changes of macrophage phenotypes have been observed during the development of atherosclerosis [55]. As atherosclerotic lesions progress, macrophages exhibit a pro-inflammatory phenotype (M1 macrophages) and produce inflammatory cytokines that are associated with “classical activation”. Alternatively, macrophages in the presence of cytokines such as IL-4 or IL-13 are activated to promote angiogenesis and matrix remodeling while suppressing adverse inflammatory responses (M2 macrophages) [56]. Recently, subsets of macrophages, including Mres, have been identified during the resolution phase of inflammation, that are associated with elevated cAMP levels and feature the properties of both M1 and M2 [57]. Another subset, Mox, develops in response to oxidized phospholipids and is related to a redox-sensitive transcription factor- NF-E2-related factor 2 (Nrf2) [58].
It is possible that macrophage polarization is primed by circulating monocytes. Macrophages produce a class of DHA-derived anti-inflammatory and pro-resolving products, maresins, in resolving inflammation. Lipoxin A4, resolvin E1/ D1/E2, and maresin1 enhance phagocytosis of apoptoic neutrophils by macrophages without inducing pro-inflammatory gene expression [59, 60]. Resolvins produced in human vasculature reduced or blocked polymorphonuclear leukocyte transmigration in vivo [61]. Details on the effects of n-3 FA on macrophage phenotypes in atherosclerosis are still limited. Still, the potential action of n-3 FA on M2 skewing and M1 inhibition of macrophages has been demonstrated in adipose tissue and this linked to macrophage cell-surface receptor, GPR120 [13]. Carotid endarterectomy patients with EPA supplementation had decreased plaque inflammation with fewer T cells and foam cells [25].
Transcriptional control of macrophage function has been reported. Szanto et al showed that activation of M2 macrophages with IL-4 stimulated the activity of PPARγ. This effect was likely associated with a signal transducer and activator of transcription 6 (SATA6), which acted as a facilitating factor for PPARγ by promoting DNA binding and consequently increased the levels of regulated genes and responses [62]. Global gene expression analysis validated the cross-link of IL-4 and PPARγ in mouse and human macrophages as well as in DC [63]. In response to IL-4, STAT6 and PPARγ-coactivator-1β (PGC-1β) induced macrophage FA oxidation and attenuated macrophage inflammation [64]. Potential regulatory effects of n-3 FA on modulating PPARγ levels that affect inflammatory responses in macrophages have been reported [46*]. Additional studies on the relation of n-3 FA and PPARγ should provide further insights into the regulation of macrophage phenotypes in atherosclerosis.
Effects of n-3 FA on emerging contributors to atherogenesis
Transient neutrophilic adhesion to the vasculature endothelium is termed rolling and is a first step in leukocyte migration across the endothelial wall. Hyperlipidemia-induced neutrophilia correlated with increased lesion formation in apoE−/− mice [65]. Neutropenic mice displayed a rescued phenotype with decreased lesion formation at early but not at the late stages of atherosclerotic lesion development [66]. Doring et al demonstrated that neutrophil secondary granule protein cathelicidin directly promoted atherosclerosis by enhancing the recruitment of inflammatory monocytes [67*]. The relationships of n-3 FA with arterial neutrophils are still not clear in atherogenesis. Neutrophils typically have high contents of AA, but oral administration of EPA and DHA resulted in proportional increases of n-3 FA levels in humans [68]. Tull et al reported that n-3 FA reduced the transient migration of neutrophils across endothelial cell monolayers through the modulation of cellular phospholipid composition and production of eicosanoids, such as cyclooxgenase-2 (COX-2) and prostaglandin D2 (PGD2) or PGD3 [69].
Dendritic cells (DC) are antigen-presenting cells that represent an important cellular link between innate and adaptive immunity. They also have a role in tolerance for self-antigens [38]. The impact of n-3 FA on DC maturation and DC-derived pro-inflammatory cytokines has been investigated. EPA and DHA attenuated LPS-induced DC maturation by suppressing IL-12 production and expression of CD40, CD80, CD86 and MHCII, while increasing IL-10 production and expression of IL-10 [70]. These effects were likely modulated through suppressing NF-κB-mediated signaling pathway. Similar findings on reduced DC maturation mediated by n-3 FA in spleen and central nerve system have been reported in mice [71, 72]. Likely, n-3 FA impaired p38 mitogen-activated protein kinase (MAPK) activity that blocked LPS-induced DC maturation [73]. Nakajima et el has further demonstrated that a 5% EPA diet was able to induce atherosclerotic lesion regression by increasing immature arterial DC in LDLR−/− mice [74]. In addition, it has been shown that when bone marrow-derived DC were exposed to n-3-derived resolvin E1 and pathogens, these DC remained at the inflammatory sites, instead of migrating to lymph nodes, and induced apoptosis of activated arterial CD4+ T cells [75].
Conclusion
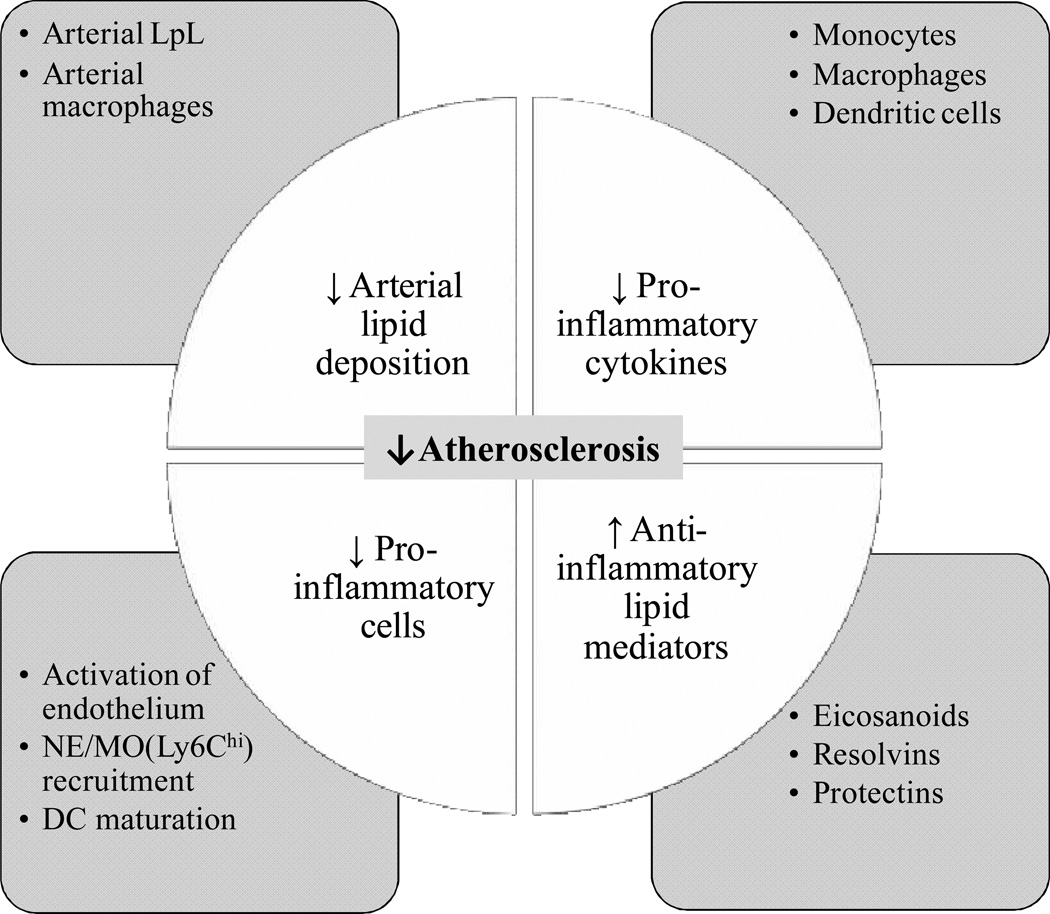
Findings outlined in this review suggest that anti-atherogenic effects of n-3 FA can affect multiple steps in atherosclerotic lesion development. Pathways reviewed herein also contribute to mechanisms important for arterial plaque stability and rupture [76]. As summarized in Figure 1, these effects include modulation of arterial initial uptake and binding of LDL by modulating arterial LpL and macrophage levels. n-3 FA inhibit pro-inflammatory processes by reducing the production of inflammatory mediators and the recruitment of inflammatory leukocytes. Systems that can be used to study the underlying mechanisms include the use of various agonists, antagonists or genetic modifications to elucidate further the role of n-3 FA in different steps in the development of atherosclerosis. These findings of “beneficial effects” of dietary n-3 FA on processes related to atherosclerosis are relevant to future strategies for prevention and treatment of CVD.
Figure 1. Summary of potential “beneficial effects” of n-3 FA in atherosclerosis.
n-3 FA modulate atherosclerosis by affecting uptake and binding of LDL to the arterial wall. This is associated with reduced arterial LpL and macrophage levels. n-3 FA are protective against inflammation in the arterial wall by reducing the production of pro-inflammatory cytokines in monocytes (MO), macrophages or dendritic cells (DC) and decreasing the recruitment of inflammatory leukocytes to the arterial wall, including neutrophils (NE) and MO. Lastly, n-3 FA and n-3-derived eicosanoids, resolvins and protectins are potential anti-inflammatory lipid mediators in atherosclerosis. Specific examples of pathways related to each area are shown in the square boxes.
Key points.
-
.
Molecular and cellular anti-atherogenic targets of n-3 FA have been identified.
-
.
n-3 FA modulate arterial lipid deposition and inflammatory responses through regulating arterial LpL levels early in development of atherosclerosis.
-
.
Growing understanding of the inflammatory pathways and mediators has unveiled new mechanisms that can be considered as part of strategic approaches to decrease risks for CVD using n-3 FA and related molecules in humans.
Acknowledgements
Source of funding
This work was supported by National Institutes of Health grant HL40404 (R.J.D.), T32-DK007647 and T32- HL007343 (C.L.C.)
Footnotes
Conflict of interest
None.
REFERENCES
- 1.Hoyert DL. 75 years of mortality in the United States, 1935–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. 2010;45:893–905. doi: 10.1007/s11745-010-3393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Harris WS, Chung M, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 4. Harris WS. Are n-3 fatty acids still cardioprotective? Curr Opin Clin Nutr Metab Care. 2013;16:141–149. doi: 10.1097/MCO.0b013e32835bf380. In this review, clinical trials and meta-analyses of n-3 FA in CVD were summarized and discussed. The author concludes that evidence for n-3 FA reducing risk for CVD remains favorable.
- 5. Rizos EC, Ntzani EE, Bika E, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. This is a meta-analysis reporting negative effects of n-3 FA on reducing risk of CVD.
- 6.Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21:781–792. doi: 10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Jung UJ, Torrejon C, Tighe AP, Deckelbaum RJ. n-3 Fatty acids and cardiovascular disease: mechanisms underlying beneficial effects. Am J Clin Nutr. 2008;87:2003S–2009S. doi: 10.1093/ajcn/87.6.2003S. [DOI] [PubMed] [Google Scholar]
- 8.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 9.Sudheendran S, Chang CC, Deckelbaum RJ. N-3 vs. saturated fatty acids: effects on the arterial wall. Prostaglandins Leukot Essent Fatty Acids. 2010;82:205–209. doi: 10.1016/j.plefa.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259. This review summarizes recent findings on molecular and cellular mechanisms relating to n-3 FA and CVD.
- 11.Chen W, Jump DB, Esselman WJ, Busik JV. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2007;48:18–26. doi: 10.1167/iovs.06-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massaro M, Basta G, Lazzerini G, et al. Quenching of intracellular ROS generation as a mechanism for oleate-induced reduction of endothelial activation and early atherogenesis. Thromb Haemost. 2002;88:335–344. [PubMed] [Google Scholar]
- 13.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair SS, Leitch JW, Falconer J, Garg ML. Prevention of cardiac arrhythmia by dietary (n-3) polyunsaturated fatty acids and their mechanism of action. J Nutr. 1997;127:383–393. doi: 10.1093/jn/127.3.383. [DOI] [PubMed] [Google Scholar]
- 15.von Schacky C. Omega-3 fatty acids and cardiovascular disease. Curr Opin Clin Nutr Metab Care. 2004;7:131–136. doi: 10.1097/00075197-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kowey PR, Reiffel JA, Ellenbogen KA, et al. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304:2363–2372. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Sutherland F, Morton JB, et al. Long-term omega-3 polyunsaturated fatty acid supplementation reduces the recurrence of persistent atrial fibrillation after electrical cardioversion. Heart Rhythm. 2012;9:483–491. doi: 10.1016/j.hrthm.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 18. Wu JH, Lemaitre RN, King IB, et al. Association of plasma phospholipid long-chain omega-3 fatty acids with incident atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2012;125:1084–1093. doi: 10.1161/CIRCULATIONAHA.111.062653. This study reports an inverse association between plasma n-3 FA levels and risk of atrial fibrillation in older adults with no history of heart failure or atrial fibrillation, suggesting n-3 FA might have a role in primary prevention of atrial fibrillation.
- 19.Buckley CD, Gilroy DW, Serhan CN, et al. The resolution of inflammation. Nat Rev Immunol. 2012;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- 20.Gajos G, Zalewski J, Rostoff P, et al. Reduced thrombin formation and altered fibrin clot properties induced by polyunsaturated omega-3 fatty acids on top of dual antiplatelet therapy in patients undergoing percutaneous coronary intervention (OMEGA-PCI clot) Arterioscler Thromb Vasc Biol. 2011;31:1696–1702. doi: 10.1161/ATVBAHA.111.228593. [DOI] [PubMed] [Google Scholar]
- 21.Calder PC. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res. 2012;56:1073–1080. doi: 10.1002/mnfr.201100710. [DOI] [PubMed] [Google Scholar]
- 22.Moertl D, Berger R, Hammer A, et al. Dose-dependent decrease of platelet activation and tissue factor by omega-3 polyunsaturated fatty acids in patients with advanced chronic heart failure. Thromb Haemost. 2011;106:457–465. doi: 10.1160/TH11-03-0169. [DOI] [PubMed] [Google Scholar]
- 23.Mackay I, Ford I, Thies F, et al. Effect of omega-3 fatty acid supplementation on markers of platelet and endothelial function in patients with peripheral arterial disease. Atherosclerosis. 2012;221:514–520. doi: 10.1016/j.atherosclerosis.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 24.Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 25.Cawood AL, Ding R, Napper FL, et al. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;212:252–259. doi: 10.1016/j.atherosclerosis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto M, Sata M, Fukuda D, et al. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis. 2008;197:524–533. doi: 10.1016/j.atherosclerosis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Loree HM, Kamm RD, Stringfellow RG, Lee RT. Effects of fibrous cap thickness on peak circumferential stress in model atherosclerotic vessels. Circulation research. 1992;71:850–858. doi: 10.1161/01.res.71.4.850. [DOI] [PubMed] [Google Scholar]
- 28.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 29.Botham KM, Bravo E, Elliott J, Wheeler-Jones CP. Direct interaction of dietary lipids carried in chylomicron remnants with cells of the artery wall: implications for atherosclerosis development. Curr Pharm Des. 2005;11:3681–3695. doi: 10.2174/138161205774580732. [DOI] [PubMed] [Google Scholar]
- 30.De Pascale C, Avella M, Perona JS, et al. Fatty acid composition of chylomicron remnant-like particles influences their uptake and induction of lipid accumulation in macrophages. FEBS J. 2006;273:5632–5640. doi: 10.1111/j.1742-4658.2006.05552.x. [DOI] [PubMed] [Google Scholar]
- 31.Seo T, Qi K, Chang C, et al. Saturated fat-rich diet enhances selective uptake of LDL cholesteryl esters in the arterial wall. J Clin Invest. 2005;115:2214–2222. doi: 10.1172/JCI24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang CL, Seo T, Matsuzaki M, et al. n-3 fatty acids reduce arterial LDL-cholesterol delivery and arterial lipoprotein lipase levels and lipase distribution. Arterioscler Thromb Vasc Biol. 2009;29:555–561. doi: 10.1161/ATVBAHA.108.182287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CL, Seo T, Du CB, et al. n-3 Fatty acids decrease arterial low-density lipoprotein cholesterol delivery and lipoprotein lipase levels in insulin-resistant mice. Arterioscler Thromb Vasc Biol. 2010;30:2510–2517. doi: 10.1161/ATVBAHA.110.215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yagyu H, Chen G, Yokoyama M, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merkel M, Heeren J, Dudeck W, et al. Inactive lipoprotein lipase (LPL) alone increases selective cholesterol ester uptake in vivo, whereas in the presence of active LPL it also increases triglyceride hydrolysis and whole particle lipoprotein uptake. J Biol Chem. 2002;277:7405–7411. doi: 10.1074/jbc.M107914200. [DOI] [PubMed] [Google Scholar]
- 36.Torrejon C, Jung U, Deckelbaum R, Uauy R. n-3 fatty acids as part of a saturated fatty acid rich diet reduces plasma lipids levels, and arterial inflammatory markers in LDLR KO mice. FASEB J. 2011;25:339.8. (Abstract) [Google Scholar]
- 37.Miles EA, Banerjee T, Calder PC. The influence of different combinations of gamma-linolenic, stearidonic and eicosapentaenoic acids on the fatty acid composition of blood lipids and mononuclear cells in human volunteers. Prostaglandins Leukot Essent Fatty Acids. 2004;70:529–538. doi: 10.1016/j.plefa.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 39.Rader DJ, Pure E. Lipoproteins, macrophage function, and atherosclerosis: beyond the foam cell? Cell Metab. 2005;1:223–230. doi: 10.1016/j.cmet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 41.Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brown AL, Zhu X, Rong S, et al. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler Thromb Vasc Biol. 2012;32:2122–2130. doi: 10.1161/ATVBAHA.112.253435. This study reports that n-3 FA-rich diets decreased Ly6Chi pro-inflammatory monocytes, and that this was associated with decreased atherosclerosis in murine atherosclerosis-susceptible models.
- 44. Grenon SM, Aguado-Zuniga J, Hatton JP, et al. Effects of fatty acids on endothelial cells: inflammation and monocyte adhesion. J Surg Res. 2012;177:e35–e43. doi: 10.1016/j.jss.2012.04.010. Co-culturing endothelial cells and monocytes with n-6 AA, but not n-3 EPA, increased monocyte adhesion to endothelial cells with or without the stimulation of TNF-α. Possible mechanisms include the modulation of mRNA expression of adhesion molecules, such as ICAM-1, VCAM-1, and E-selectin, and pro-inflammatory mediators, such as IL-6 and TNF-α.
- 45.Luu NT, Madden J, Calder PC, et al. Dietary supplementation with fish oil modifies the ability of human monocytes to induce an inflammatory response. J Nutr. 2007;137:2769–2774. doi: 10.1093/jn/137.12.2769. [DOI] [PubMed] [Google Scholar]
- 46. Jung UJ, Torrejon C, Chang CL, et al. Fatty acids regulate endothelial lipase and inflammatory markers in macrophages and in mouse aorta: a role for PPARgamma. Arterioscler Thromb Vasc Biol. 2012;32:2929–2937. doi: 10.1161/ATVBAHA.112.300188. n-3 EPA reduced expression of endothelial lipase and pro-inflammatory mediators, including IL-6, in cultured murine macrophages and in aorta of LDLR−/− mice. This study provides evidence that n-3 FA-mediated effects on reducing inflammatory makers in aorta were related to PPARγ expression.
- 47.Li L, Beauchamp MC, Renier G. Peroxisome proliferator-activated receptor alpha and gamma agonists upregulate human macrophage lipoprotein lipase expression. Atherosclerosis. 2002;165:101–110. doi: 10.1016/s0021-9150(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 48.Hayek T, Hussein K, Aviram M, et al. Macrophage foam-cell formation in streptozotocin-induced diabetic mice: stimulatory effect of glucose. Atherosclerosis. 2005;183:25–33. doi: 10.1016/j.atherosclerosis.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 50.Lee JY, Zhao L, Youn HS, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 51.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Wong SW, Kwon MJ, Choi AM, et al. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maloney E, Sweet IR, Hockenbery DM, et al. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol. 2009;29:1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson JL, Newby AC. Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol. 2009;20:370–378. doi: 10.1097/MOL.0b013e3283309848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 57.Shapiro H, Lutaty A, Ariel A. Macrophages, meta-inflammation, and immuno-metabolism. Scientific World Journal. 2011;11:2509–2529. doi: 10.1100/2011/397971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serhan CN, Yang R, Martinod K, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merched AJ, Ko K, Gotlinger KH, et al. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serhan CN, Clish CB, Brannon J, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szanto A, Balint BL, Nagy ZS, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Villanueva CJ, Waki H, Godio C, et al. TLE3 is a dual-function transcriptional coregulator of adipogenesis. Cell Metab. 2011;13:413–427. doi: 10.1016/j.cmet.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rotzius P, Thams S, Soehnlein O, et al. Distinct infiltration of neutrophils in lesion shoulders in ApoE-/- mice. Am J Pathol. 2010;177:493–500. doi: 10.2353/ajpath.2010.090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drechsler M, Megens RT, van Zandvoort M, et al. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 67. Doring Y, Drechsler M, Wantha S, et al. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res. 2012;110:1052–1056. doi: 10.1161/CIRCRESAHA.112.265868. This study provides evidence that neutrophils may play a role in influencing atherosclerosis.
- 68.Healy DA, Wallace FA, Miles EA, et al. Effect of low-to-moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids. 2000;35:763–768. doi: 10.1007/s11745-000-0583-1. [DOI] [PubMed] [Google Scholar]
- 69.Tull SP, Yates CM, Maskrey BH, et al. Omega-3 Fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol. 2009;7:e1000177. doi: 10.1371/journal.pbio.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Draper E, Reynolds CM, Canavan M, et al. Omega-3 fatty acids attenuate dendritic cell function via NF-kappaB independent of PPARgamma. J Nutr Biochem. 2011;22:784–790. doi: 10.1016/j.jnutbio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 71.Kong W, Yen JH, Vassiliou E, et al. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis. 2010;9:12. doi: 10.1186/1476-511X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011;25:872–882. doi: 10.1016/j.bbi.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, Hao Q, Li QR, et al. Omega-3 polyunsaturated fatty acids affect lipopolysaccharide-induced maturation of dendritic cells through mitogen-activated protein kinases p38. Nutrition. 2007;23:474–482. doi: 10.1016/j.nut.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Nakajima K, Yamashita T, Kita T, et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1963–1972. doi: 10.1161/ATVBAHA.111.229443. [DOI] [PubMed] [Google Scholar]
- 75.Vassiliou EK, Kesler OM, Tadros JH, Ganea D. Bone marrow-derived dendritic cells generated in the presence of resolvin E1 induce apoptosis of activated CD4+ T cells. J Immunol. 2008;181:4534–4544. doi: 10.4049/jimmunol.181.7.4534. [DOI] [PubMed] [Google Scholar]
- 76.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]