Abstract
The pro-inflammatory cytokine IL-1β is known to play a role in several models of aging, neuroinflammation, and neurodegenerative diseases. Here, we document a detailed time- and age-dependent pattern of pro- and anti-inflammatory biomarkers following bilateral intrahippocampal injection of interleukin-1β. During the first 12 hours several pro- and anti-inflammatory cytokines increased in the aged (24 mo old) rats, some of which returned to baseline levels by 24 hours post-injection while others remained elevated for 72 hours post-injection. In contrast, no such increases were observed in the young (3 mo old) rats. Interestingly, young rats up-regulated mRNA of two pro-inflammatory cytokines, interleukin-1β and tumor necrosis factor-α, but did not translate these transcripts into functional proteins, which may be related to expression of suppressor of cytokine signaling type-2. These results contribute to our understanding of how neuroinflammation may contribute to the pathogenesis of age-related neurodegenerative disorders due to an age-related bias toward a hyper-reactive immune response that is not selective for a pro- or anti-inflammatory phenotype following an inflammatory stimulus.
1. Introduction
Neuroinflammation may develop in response to numerous stimuli; the balance of pro- and anti-inflammatory proteins released by local glia and neurons determines whether the outcome is injurious of restorative (Colton 2009). Normal aging is associated with increased neuroinflammation in vulnerable brain regions (Akiyama et al., 2000; Cagnin et al., 2001; Frank-Cannon et al., 2009). The neurodegeneration may be due to the inability of microglia to effectively convert from a pro-inflammatory to an anti-inflammatory and repair-oriented activation state, leading to excessive damage to surrounding neurons (Cerbai et al., 2012). The pro- and anti-inflammatory states within the brain are associated with differential expression of cytokines, growth factors, and oxidative enzymes. The ensemble of cytokines involved in the pro-inflammatory response are tumor necrosis factor α (TNF-α), interleukin (IL)-1α, IL-1β, IL-2, IL-12, IL-6, interferon-γ (IFN-γ), and granulocyte-macrophage colony stimulating factor (GM-CSF) while the cytokines involved in the anti-inflammatory response is associated with release of transforming growth factor-β (TGFβ), IL-4, IL-5, IL-10, and IL-13 (Boche et al., 2013). CX3CR1 on microglia and its ligand, CX3CL1, are involved in maintaining microglia in a resting state (Cardona et al., 2006). We hypothesized that aged animals would have an exaggerated and protracted pro-inflammatory response following pro-inflammatory stimulation of the hippocampus, a region that is selectively vulnerable to age-related neuroinflammation, and that such an altered response would be mediated by blunted anti-inflammatory gene and protein expression. We characterized the time course of hippocampal immune protein and gene expression following intrahippocampal injection of the pro-inflammatory cytokine IL-1β.
2. Materials and Methods
2.1 Animals & Surgery
Male Fisher-344 (NIA) rats 3 months (young) of age and 24 months of age (old) were singly housed with ad libitum food and water and maintained on a 12/12-h light-dark cycle in a temperature-controlled room (22°C). Each rat was anesthetized, placed in a stereotaxic device and holes drilled bilaterally into the skull 3.0 mm posterior and 2.6 mm lateral to bregma. A 25 gauge 2 μl syringe (Hamilton Company, Reno NV) was slowly lowered to −3.5mm (dorsal hippocampus) and injected slowly with 1ul rat recombinant 20 ng/μl IL-1β (R&D Systems, Minneapolis MN) dissolved in sterile saline. This injection procedure was repeated on bilaterally. 3, 6, 12, 24, 48, and 72 hours later both hippocampi were dissected, combined and stored (−80°C) until further processing. Both surgical sham and non-surgical control rats were prepared as well for a total of 8 animals per combination of treatment, group, and timepoint.
2.2 Protein Analysis
A sample of hippocampus was homogenized in Bio-Plex Cell Lysis Buffer (Bio-Rad, Hercules CA) and analyzed using Bio-Plex Pro Rat Th1/Th2 (IL-1α, IL-1 β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, GM-CSF, IFN-γ, and TNF-α) and TGFβ (TGFβ1, TGFβ2, and TGFβ3) multiplex bead immunoassays (Bio-Rad, Hercules CA). Total protein was determined Bradford protein assay (Bio-Rad, Hercules CA).
2.3 mRNA Analysis
RNA was extracted from the remaining hippocampal tissue using PureZOL RNA Isolation Reagent (Bio-Rad, Hercules CA) and NucleoSpin RNA II (Machery-Nagel, Allentown PA). cDNA templates were generated using iScript Reverse Transcription kit (Bio-Rad, Hercules CA). The target cDNA (IL-1β, CX3CR1, CX3CL1, BDNF, TNF-α, TGFβ1, p38 mitogen-activated kinase (p38-MAPK) and suppressor of cytokine signaling 2 (SOCS2) and the reference target glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were amplified simultaneously with SsoAdvanced SYBR Green Supermix in a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules CA). Data were analyzed using the comparative Ct (cycle threshold) method comparing samples to age-matched non-surgical controls.
2.4 Statistics
All results, expressed as fold-change over age-matched nonsurgical controls were analyzed using a 3-way analysis of variance (ANOVA), followed by 1-way ANOVA with Holm-Sidak post-hoc test, with significance as p<0.05. § indicates significant difference between IL1-treated and shams within the same age and time group. # indicates significant difference between young and old within the same time and treatment group. † indicates significant difference between time groups within age and treatment group. Data expressed as mean + standard error of the means (SEM).
3. Results
3.1 IL-1β induced a differential expression of a variety of pro-inflammatory cytokines in the young and old rat brain
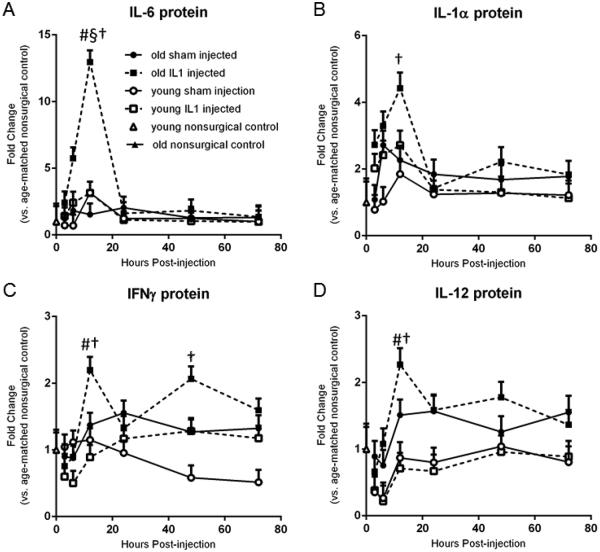
A 3-Way ANOVA revealed main effects of age and time (p<0.05) on the protein expression of all the pro-inflammatory cytokines measured, including TNF-α, IL-1α, IL-1β, IL-2 IL-5, IL-12, IL-6, IFN-γ and GM-CSF. There was a main effect of IL-1β treatment on protein expression of IL-1α and IL-6 as well as a significant interaction between treatment and time. Furthermore, there were significant interactions between and age, treatment, and time on IL-6 protein levels, with old animals expressing significantly more IL-6 protein at 12 hr compared to young rats at 12 hr. Subsequent post-hoc tests revealed significant (p<0.05) upregulation in protein expression of IL-6 and IL-1α at 12 hr post-treatment in IL-1β treated old rats (Figures 1a and 1b) compared to the other time points. Additionally, the pro-inflammatory cytokines IFNγ and IL-12 (Figures 1c and 1d) showed prolonged elevation in aged animals. These two cytokines were significantly elevated in aged rats at 12 hr post-injection compared to 3 hr post-injection (p<0.001 and p=0.002, respectively), as well as compared to young IL-1-treated rats at the same time (p<0.001 and p=0.003, respectively). Later time points were not significantly different from either baseline or the 12 hr elevation, suggesting only a partial restoration of baseline levels for these two cytokines. Additionally, IFNγ protein levels remained significantly elevated in IL-1β treated aged rats 48 hours post-injection (p=0.003), further supporting the concept that aged rats demonstrate a protracted elevation of some pro-inflammatory cytokines. IL-2 and IL-5 levels were unchanged according to post-hoc analyses (data not shown).
Figure 1.
IL-1β intrahippocampal injection triggers increases in pro-inflammatory cytokine expression in old rats. A. IL-6 was significantly upregulated at 12 hr post treatment in IL-1β treated old rats compared to all other timepoints as well as compared to old sham injected rats and young IL-1β treated rats. B. IL-1α was significantly upregulated at 12 hr post treatment in IL-1β treated old rats compared to all other timepoints. C. IFNγ was significantly elevated in aged rats at 12 hr post-injection compared to 3 hr post-injection as well as compared to young IL-1β-treated rats at the same timepoint. IFNγ was also significantly elevated at 48 hr post injection in IL-1β treated aged rats. D. IL-12 was significantly elevated in aged rats at 12 hr post-injection compared to 3 hr post-injection and later time points were not significantly different from either baseline or the 12 hr elevation. A-D. n=8/group Data expressed as mean + SEM. § indicates significant difference between IL1-treated and shams within the same age and time group. # indicates significant difference between young and old within the same time and treatment group. † indicates significant difference between time groups within age and treatment group.
3.2 IL-1β induced a differential expression of a variety of anti-inflammatory cytokines in the young and old rat brain
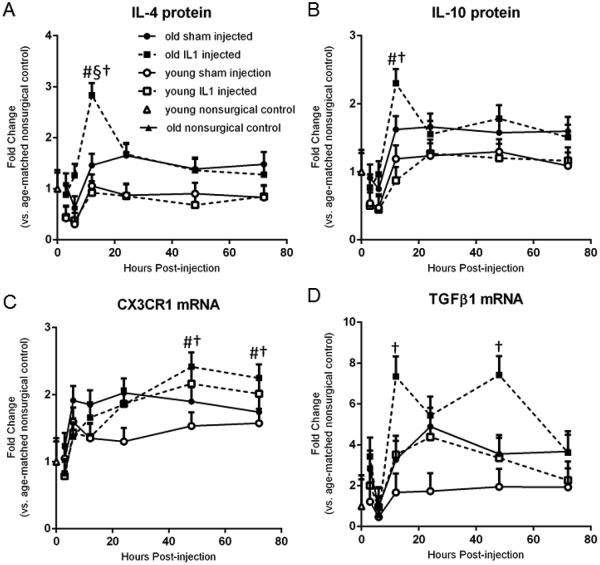
A 3-Way ANOVA revealed a main effect of age (p<0.05) on the expression of most of the anti-inflammatory cytokines measured, except TGFβ2 protein and CX3CL1 mRNA (data not shown). There was a main effect of time post-treatment (p<0.05) on the expression of all the anti-inflammatory cytokines measured except TGFβ1 protein (data not shown). There was a significant age x time interaction for CX3CR1 gene expression (Figure 2c). Post hoc analyses of IL-4 and IL-10 protein expression (Figures 2a and 2b) showed significant (p<0.05) upregulation in IL-1β-treated old rats at 12 hr post-treatment compared to IL-1β-treated young rats in the at the same time post-injection, as well as compared to IL-1-treated old rats in the 3 and 6 hr time points. Additionally, IL-4 levels peaked in old IL-1 treated rats at 12 hours compared to other timepoints as well as compared to old shams. Old rats treated with IL-1β had significant (p<0.05) upregulation of TGFβ1 gene expression 12 and 48 hr post-injection (Figure 2d). CX3CR1 gene expression also increased in both young and old IL-1-treated rats at 48 and 72 hours post-injection (Figure 2c). BDNF gene expression showed only a trend (p=0.068) towards upregulation in young IL-1β treated animals 3 hours post-treatment (data not shown), but showed no change in old animals. IL-13 and TGFβ3 levels were unchanged according to post-hoc analyses (data not shown).
Figure 2.
IL-1β intrahippocampal injection triggers increases in anti-inflammatory cytokine expression in old rats. A. IL-4 levels peaked in old IL-1 treated rats at 12 hours compared to 3 and 6 hr timepoints as well as compared to old shams and young IL-1 treated rats. B. IL-10 levels peaked in old IL-1 treated rats at 12 hours compared to IL-1β-treated young rats in the at the same timepoint, as well as compared to IL-1-treated old rats in the 3 and 6 hr time points but were no different than old sham rats. C. CX3CR1 gene expression was increased in both young and old IL-1-treated rats at 48 and 72 hours post-injection. D. Old rats treated with IL-1β had significant upregulation of TGFβ1 gene expression 12 and 48 hr post-injection. A-D. n=8/group Data expressed as mean + SEM. § indicates significant difference between IL1-treated and shams within the same age and time group. # indicates significant difference between young and old within the same time and treatment group. † indicates significant difference between time groups within age and treatment group.
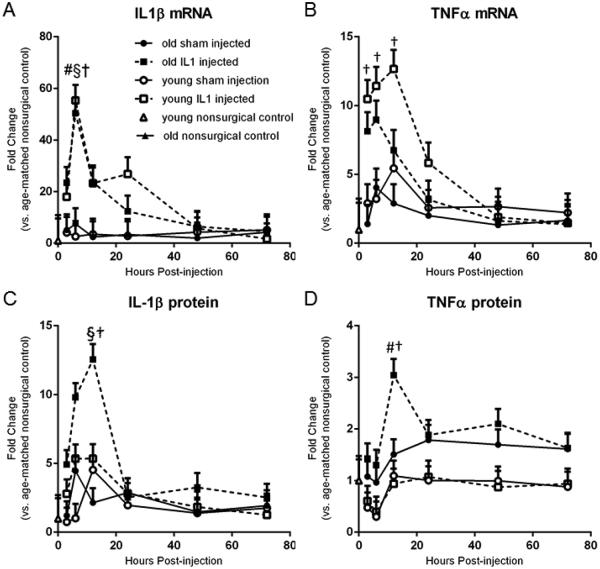
3.3 Old and young rats increased IL-1β and TNFα mRNA but only old rats increased protein levels
IL-1β and TNFα mRNA increased in old and young rats in the first 12 hours following IL-1 injection, but only old IL-1 treated rats showed a subsequent increase in protein levels of these two markers. A 3-way ANOVA on gene expression of IL-1β (Figure 3a) revealed significant main effects of treatment with IL-1β and time post-treatment as well as a significant treatment x time interaction. Old and young rats showed a significant (p<0.05) upregulation of IL-1β gene expression at 6 hours post-treatment compared to age-matched sham-operated rats, non-surgical controls, as well as compared to IL-1 treated rats of the same age at later time points. Regarding subsequent protein expression of IL-1β (Figure 3c), an ANOVA of the protein expression of IL-1β (Figure 3c) revealed significant main effects for age, IL-1β treatment, and time post-treatment as well as a significant age x treatment interaction, a significant treatment x time interaction, and a 3-way interaction between age x treatment x time. Post-hoc analyses of IL-1β protein expression revealed significant (p<0.05) upregulation in only the old IL-1β treated animals at 6 and 12 hr post-treatment compared to non-surgical controls as well as later time points (24, 48, and 72 hr).
Figure 3.
IL-1β and TNFα mRNA increased in old and young rats in the first 12 hours following IL-1 injection, but only old IL-1 treated rats showed a subsequent increase in protein levels of these two markers. A. Old and young rats showed a significant upregulation of IL-1β gene expression at 6 hours post-treatment compared to age-matched sham-injected rats, non-surgical controls, and IL-1 treated rats of the same age at later time points. B. In young IL-1β treated rats, TNFα mRNA is significantly upregulated at 3, 6 and 12 hr compared to non-surgical controls, and the 6 and 12 hr points compared to the 48 and 72 hr points. C. Old IL-1β treated animals upregulated IL-1β protein at 6 and 12 hr post-treatment compared to non-surgical controls as well as later time points. D. Old rats treated with IL-1β had significant elevations in TNFα protein at 6 hr compared to young IL-1β treated rats at the same time point or compared to old IL-1β treated rats at the 6 hr timepoint. A-D. n=8/group Data expressed as mean + SEM. § indicates significant difference between IL1-treated and shams within the same age and time group. # indicates significant difference between young and old within the same time and treatment group. † indicates significant difference between time groups within age and treatment group.
For TNFα gene expression (Figure 3b), an ANOVA revealed a main effect of age, IL-1β treatment, and a significant interaction between age x time. Post-hoc analyses revealed that young rats treated with IL-1β had significant (p<0.05) gene upregulation of TNFα at 3, 6 and 12 hr compared to non-surgical controls, and the 6 and 12 hr points compared to the 48 and 72 hr points. Analysis of TNFα protein expression (Figure 1d) revealed main effects of age, treatment, and time. Post-hoc analyses revealed that only aged rats treated with IL-1β had significant elevations in TNFα protein at 6 hr compared to young IL-1 treated rats at the same time point (p<0.001) or compared to old treated rats at the earlier 6 hr time point (p=0.022).
3.4 The effect of post-transcriptional control on the inflammatory response in the aged brain
To address the contradictory results between gene and protein expression of IL-1β and TNFα, we investigated the mRNA transcripts for two markers related to post-transcriptional or post-translational control of cytokine expression and action: p38-MAPK and SOCS2. p38-MAPK activation is important for stabilization of cytokine mRNA, allowing proteins to be translated (Brook et al., 2000). A 3-way ANOVA revealed significant effects of age, treatment, and time post-treatment on p38-MAPK gene expression, as well as a significant interaction of treatment x time (Figure 4a). However, subsequent post-hoc analyses demonstrated no specific differences between groups at any of the time points evaluated. SOCS2 expression (Figure 4b), in contrast to p38-MAPK, is a feedback mechanism that decreases cytokine expression (for review, see Wang & Campbell, 2002). SOCS2 expression was significantly affected by treatment and time post-treatment, as well as an interaction between the two. However, post-hoc analyses only revealed significant changes in young IL-1 treated animals at 6 hr compared to young shams at 3 hr (p=0.015). This slight increase in SOCS2 gene expression in young animals may be indicative of a subtle difference in a feedback mechanism on cytokine expression between young and old animals, but additional analysis is required.
Figure 4.
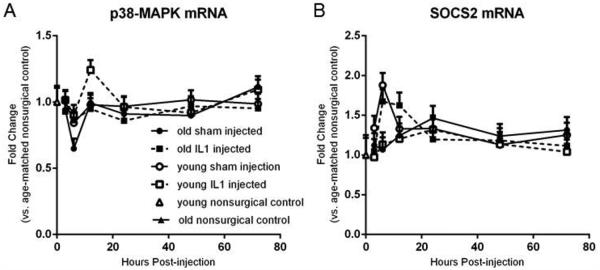
Post-transcriptional and post-translational control of cytokine expression. A. 3-way ANOVA showed significant effects of age, treatment, and time post-treatment on p38-MAPK gene expression, as well as a significant interaction of treatment x time. Post-hoc analyses demonstrated no specific differences between groups at any of the time points evaluated. B. 3-way ANOVA on SOCS2 expression was significantly affected by treatment and time post-treatment, as well as an interaction between the two. Post-hoc analyses revealed significant changes in young IL-1 treated animals at 6 hr compared to young shams at 3 hr. A and B. n=8/group Data expressed as mean + SEM.
4. Discussion
We characterized the influence of aging upon the time-dependent response of the hippocampus to a pro-inflammatory challenge by the cytokine IL-1β. Our results revealed a series of time-dependent and age-dependent changes in pro- and anti-inflammatory proteins and an age-dependent alteration in post-transcriptional regulation of pro-inflammatory cytokines.
Within 6 hr after the pro-inflammatory challenge, IL-1β gene expression was significantly elevated in both young and old rats, but only the aged rats displayed a significant increase in IL-1β protein levels. The changes in IL-1β gene and protein expression in the aged rats correlated significantly with each other (r=0.437, p<0.001), implicating that we are measuring de novo protein synthesis as opposed to measuring the IL-1β we injected. Furthermore, previous studies have shown that the in vivo half-life of IL-1β protein is 3-4 hours (Klapproth et al., 1989). TNFα gene expression increased rapidly in young rats, but not old rats; in contrast, TNFα protein expression increased only in the old rats. These discrepancies between cytokine mRNA and protein levels may be related to age-related differences in mechanisms of post-transcriptional control. We initially examined p38-MAPK to explore this possibility. The transcripts of many pro-inflammatory cytokines contain an adenine/uridine-rich element that target them for degradation, and this process is regulated by the activation state of the immune cell producing the transcript (Stoecklin & Anderson, 2006). Stimulation of macrophages with the bacterial endotoxin lipopolysaccharide stabilizes production of TNFα via p38-MAPK dependent mechanisms (Brook et al., 2000). IL-1β can activate the p38-MAPK pathway via phosphorylation and increase expression of p38-MAPK mRNA (Sheng et al., 2001). Drugs that inhibit the p38-MAPK pathway reduce the protein expression of several pro-inflammatory cytokines in microglia, including TNFα and IL-1β (Bachstetter et al., 2011). p38-MAPK phosphorylation is elevated in the brains of aged animals (Li et al., 2011). However, we found no changes in the expression of p38-MAPK mRNA in old or young animals that would account for the discrepancy in cytokine mRNA and protein expression we observed.
We also examined SOCS2 gene expression as another method for evaluating post-transcriptional control of cytokine expression. SOCS2 is highly expressed in the brain (Wang & Campbell, 2002). While SOCS2 is a well-established negative regulator of growth hormone signaling (Greenhalgh et al., 2002), SOCS2 deficient mice also have exaggerated pro-inflammatory cytokine production and mortality following peripheral infection (Machado et al., 2006). Insufficient SOCS2 expression in aging may lead to poorly regulated production of pro-inflammatory cytokines, similar to our observation with TNFα and IL-1β protein. We saw a small but significant increase in SOCS2 production in young IL-1 treated animals, but no change in SOCS2 production in aged animals, indicating that there may be a deficit in SOCS2 production that underlies our observation of increased cytokine expression following acute IL-1 treatment.
Within 12 hr after the pro-inflammatory challenge, IL-6 and IL-1α protein levels were significantly upregulated in old rats. IL-6 can induce a pro-inflammatory state in microglia (Basu et al., 2002). Elevated levels of IL-6 are associated with Alzheimer’s disease pathology (Smith et al., 2012) and are correlated with decreased function in Parkinson’s disease (Hofmann et al., 2009). IL-12 and IFN-γ were also significantly increased in old rats 12 hr after the pro-inflammatory challenge. The sustained upregulation of these markers is indicative of a prolonged pro-inflammatory immune response in the aged brain; young rats did not show increases in any of these markers. While IFN-γ can also induce a pro-inflammatory activation state in microglia and macrophages, microglia do not respond as robustly to this cytokine as macrophages (Melief et al., 2012). IL-12 is secreted by microglia in response to IFN-γ (Aloisi et al., 1997), so it was not surprising that these two markers were significantly correlated (r=0.905, p<0.001) in the current study.
Within 12 hr after the pro-inflammatory challenge, aged rats also upregulated the anti-inflammatory cytokines IL-4 and IL-10. IL-4 returned to baseline levels by 48 hr, while IL-10 did not fully return to baseline by 72 hr. In contrast, young rats did not upregulate these anti-inflammatory cytokines above baseline levels. IL-4 and IL-10 are sufficient to initiate an anti-inflammatory microglia response (Wei & Jonakait, 1999); the expression of these cytokines may represent an attempt by the aged brain to quell the hyperactive pro-inflammatory response. Fractalkine receptor (CX3CR1) gene expression, which is responsible for maintaining microglia in a resting state and is considered overall anti-inflammatory, was increased at later time points in both young and old IL-1β-exposed rats. TGFβ is associated with conversion of microglia to a repair-oriented activation state and regulation of fractalkine activity, however only TGFβ1 gene expression was increased in the aged rats with no increase in protein levels. The appearance of an anti-inflammatory response in the old rats but not the young rats may be indicative of a greater amount of tissue injury in the old rats, possibly due to their exaggerated response. This idea is supported by data from spinal cord injury patients showing significant correlations between anti-inflammatory markers and severity of tissue injury (Ochoa et al., 2001).
Microglia in the aged brain may be less capable of effectively responding to elevations of anti-inflammatory cytokines and switching their activation phenotype. Previous studies have shown that microglia from aged mice do not shift to an anti-inflammatory profile when exposed to IL-4 ex vivo, and do not upregulate the IL-4 receptor (Fenn et al., 2012). The slight but extended elevations of the pro-inflammatory cytokines IL12 and IFN-γ that we observed in the current study, despite increases in IL-4 and IL-10, support this concept. The simultaneous expression of pro- and anti-inflammatory markers has been previously described in aged mice following peripheral inflammation (Henry et al. 2009), in aged transgenic Alzheimer’s disease mice (Colton et al., 2006) and during the early stages of Alzheimer’s disease (Sudduth et al., 2013). Taken together with the work herein, these studies suggest that the inflammatory response the aged brain, and their contributions to pathology, may not be as straightforward as pro-inflammatory “priming.” Taking into account these mixed phenotypes may offer a valid approach for characterizing patients with age-related neurodegenerative disorders and their potential for responding to immunomodulatory therapies (Leoutsakos et al., 2011).
In summary, both young and old rats respond to the presence of IL-1β by upregulation of mRNA levels of endogenous IL-1β as well as TNFα. Old animals additionally respond by upregulating the protein levels of several pro- and anti-inflammatory cytokines, while young rats did not upregulate the protein levels of any cytokines. Overall, young rats appear to have a tightly controlled immune response while aged animals have an exaggerated and mixed response. This may be due to hyper-reactive microglia in the aged brain that are not selective for either a pro- or anti-inflammatory phenotype, or due to a failure of old animals increase expression of SOCS2 for regulation of the cytokine response. These data add to a growing body of literature related to the heterogeneity of the immune response in the aging brain and neurodegenerative disorders.
Highlights.
Normal aging alters the time-dependent response of the hippocampus to a pro-inflammatory challenge.
Age and young rats demonstrate a discrepancy in whether their brains translate cytokine mRNA into proteins.
Age rats demonstrate an exaggerated response to a pro-inflammatory challenge.
Overall, young rats appear to have a tightly controlled immune response while aged animals have an exaggerated and mixed response.
Acknowledgements
Supported by U.S. Public Health Service, RO1 AG030331, RO1 AG037320 and The Ohio State University Women and Philanthropy Program to GLW. SCH is supported by a Howard Hughes Medical Institute (HHMI) Med into Grad fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer disease and associated disorders. 2000;14(Suppl 1):S47–53. doi: 10.1097/00002093-200000001-00008. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Penna G, Cerase J, Menéndez Iglesias B, Adorini L. IL-12 production by central nervous system microglia is inhibited by astrocytes. Journal of immunology. 1997;159(4):1604–12. [PubMed] [Google Scholar]
- Bachstetter AD, Xing B, De Almeida L, Dimayuga ER, Watterson DM, Van Eldik LJ. Microglial p38α MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Aβ) Journal of neuroinflammation. 2011;8:79. doi: 10.1186/1742-2094-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain, behavior, and immunity. 2009;23(1):46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Krady JK, Enterline JR, Levison SW. Transforming growth factor beta1 prevents IL-1beta-induced microglial activation, whereas TNFalpha- and IL-6-stimulated activation are not antagonized. Glia. 2002;40(1):109–20. doi: 10.1002/glia.10118. [DOI] [PubMed] [Google Scholar]
- Boche D, Perry VH, Nicoll JAR. Review: Activation patterns of microglia and their identification in the human brain. Neuropathology and applied neurobiology. 2013;39(1):3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- Boche D, Perry VH, Nicoll JAR. Review: Activation patterns of microglia and their identification in the human brain. Neuropathology and applied neurobiology. 2013;39(1):3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- Brook M, Sully G, Clark AR, Saklatvala J. Regulation of tumour necrosis factor alpha mRNA stability by the mitogen-activated protein kinase p38 signalling cascade. FEBS letters. 2000;483(1):57–61. doi: 10.1016/s0014-5793(00)02084-6. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, et al. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358(9280):461–7. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer’s disease. Neurobiology of disease. 2010;37(3):503–9. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbai F, Lana D, Nosi D, Petkova-Kirova P, Zecchi S, Brothers HM, Wenk GL, et al. The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PloS one. 2012;7(9):e45250. doi: 10.1371/journal.pone.0045250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of Microglial Activation in the Innate Immune Response in the Brain. J. Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. Journal of neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain, behavior, and immunity. 2012;26(5):766–77. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Molecular neurodegeneration. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh CJ, Bertolino P, Asa SL, Metcalf D, Corbin JE, Adams TE, Davey HW, et al. Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b) Molecular endocrinology (Baltimore, Md.) 2002;16(6):1394–406. doi: 10.1210/mend.16.6.0845. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-lbeta and anti-inflammatory IL-10 cytokines. Brain, behavior, and immunity. 2009;23(3):309–17. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann KW, Schuh AFS, Saute J, Townsend R, Fricke D, Leke R, Souza DO, et al. Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochemical research. 2009;34(8):1401–4. doi: 10.1007/s11064-009-9921-z. [DOI] [PubMed] [Google Scholar]
- Klapproth J, Castell J, Geiger T, Andus T, Heinrich PC. Fate and biological action of human recombinant interleukin 1 beta in the rat in vivo. Eur J Immunol. 1989 Aug;19(8):1485–90. doi: 10.1002/eji.1830190821. [DOI] [PubMed] [Google Scholar]
- Koenig HG, Meador KG, Cohen HJ, Blazer DG. Depression in elderly hospitalized patients with medical illness. Archives of internal medicine. 1988;148(9):1929–36. [PubMed] [Google Scholar]
- Leoutsakos J-MS, Muthen BO, Breitner JCS, Lyketsos CG. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: findings from the randomized controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial. International journal of geriatric psychiatry. 2012;27(4):364–74. doi: 10.1002/gps.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li J, Bu X, Liu X, Tankersley CG, Wang C, Huang K. Age-induced augmentation of p38 MAPK phosphorylation in mouse lung. Experimental gerontology. 2011;46(8):694–702. doi: 10.1016/j.exger.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Lynch MA. The age-related increase in IL-1 type I receptor in rat hippocampus is coupled with an increase in caspase-3 activation. The European journal of neuroscience. 2002;15(11):1779–88. doi: 10.1046/j.1460-9568.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nature medicine. 2006;12(3):330–4. doi: 10.1038/nm1355. doi:10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25(12):677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Melief J, Koning N, Schuurman KG, Van De Garde MDB, Smolders J, Hoek RM, Van Eijk M, et al. Phenotyping primary human microglia: tight regulation of LPS responsiveness. Glia. 2012;60(10):1506–17. doi: 10.1002/glia.22370. [DOI] [PubMed] [Google Scholar]
- Ochoa JB, Bernard AC, O’Brien WE, Griffen MM, Maley ME, Rockich AK, Tsuei BJ, et al. Arginase I expression and activity in human mononuclear cells after injury. Annals of surgery. 2001;233(3):393–9. doi: 10.1097/00000658-200103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Jones RA, Zhou XQ, McGinness JM, Van Eldik LJ, Mrak RE, Griffin WS. Interleukin-1 promotion of MAPK-p38 overexpression in experimental animals and in Alzheimer’s disease: potential significance for tau protein phosphorylation. Neurochemistry international. 2001;39(5-6):341–8. doi: 10.1016/s0197-0186(01)00041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklin G, Anderson P. Posttranscriptional mechanisms regulating the inflammatory response. Advances in immunology. 2006;89:1–37. doi: 10.1016/S0065-2776(05)89001-7. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Progress in neurobiology. 1999;57(6):563–81. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Sudduth TL, Schmitt FA, Nelson PT, Wilcock DM. Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiology of aging. 2013;34(4):1051–9. doi: 10.1016/j.neurobiolaging.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain, behavior, and immunity. 2009;23(1):46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R, Jonakait GM. Neurotrophins and the anti-inflammatory agents interleukin-4 (IL-4), IL-10, IL-11 and transforming growth factor-beta1 (TGF-beta1) down-regulate T cell costimulatory molecules B7 and CD40 on cultured rat microglia. Journal of neuroimmunology. 1999;95(1-2):8–18. doi: 10.1016/s0165-5728(98)00248-3. [DOI] [PubMed] [Google Scholar]