Abstract
Background and Objectives
Policies for genetic testing in children (GTIC) focus on medical or psychosocial benefit to the child, discouraging or prohibiting carrier testing, and advising caution regarding pre-symptomatic diagnosis if no treatment exists. This study sought to understand parents’ perspectives on these issues and determine their experiences and knowledge related to genetic testing for Batten disease – a set of inherited neurodegenerative diseases of childhood onset for which no disease modifying therapies yet exist.
Methods
Parents of children with Batten disease completed a survey of their knowledge of genetics, experience with genetic testing, and opinions regarding GTIC.
Results
54% had sought genetic testing for non-affected family members, including predictive diagnosis of healthy, at-risk children. Participation in any genetic counseling was associated with greater knowledge on questions about genetics. The majority of parents felt it was better to know ahead of time that a child would develop Batten disease, believed that this knowledge would not alter how they related to their child, and that parents should have the final say in deciding whether to obtain GTIC.
Conclusions
Parents of children with an inherited disease are knowledgeable about genetics and wish to establish predictive or carrier status of at-risk children.
Keywords: Child, Genetic testing, Neurologic disease/disorder, Parental attitudes
1. Introduction
The neuronal ceroid lipofuscinoses (NCLs; Batten disease) are rare lysosomal storage diseases with distinct genetic etiologies but overlapping clinical features of vision loss, motor decline, seizures, dementia, and premature death. Collectively, Batten disease variants have an estimated birth incidence ranging from 1 to 7: 100,000 [1]. Excluding the adult-onset, autosomal-dominant Parry disease (CLN4), these are autosomal-recessive, childhood-onset conditions. The NCL Database contains a list of known disease-causing mutations: http://www.ucl.ac.uk/ncl/mutation.shtml [2].
For autosomal recessive forms of Batten disease, carrier genetic testing establishes whether an individual carries one copy of a Batten-causing mutation. Carrier status poses no known health risks but may inform reproductive health choices. Predictive genetic testing establishes whether a currently healthy child will phenoconvert to symptomatic Batten disease. With no disease-modifying treatments yet available for any Batten diagnosis, predictive diagnosis confers no therapeutic or preventive benefits. For Batten disease variants, non-genetic tests such as enzyme analysis and electron microscopy can aid in diagnosis of symptomatic children, though genetic testing is generally considered definitive. The utility of various genetic and non-genetic diagnostic methods and a set of diagnostic algorithms, are described on the NCL Database website [2].
Although parents of Batten-affected individuals may wish to know if their other children are carriers or will phenoconvert, this desire may contrast with established policies. For example, the current joint policy statement of the American Academy of Pediatrics and American College of Medical Genetics (AAP/ACMG) advises against carrier testing “when carrier status has no medical relevance” for children (p. 236) [3, 4]. Similarly, the European Society of Human Genetics (ESHG) discourages carrier testing until children are sufficiently mature to consent to the genetic test, and suggests parents understand the risk of an incidental carrier test finding in a child [5]. Regarding predictive diagnosis, the ESHG notes this testing carries “both benefits and risks”, with “psychological or social benefit” (p. 721) possible reasons to proceed with pre-symptomatic diagnostic testing. [5, 6]. The joint AAP/ACMG statement does not explicitly address childhood-onset conditions that lack disease-modifying treatments, but broadly states, “parents may authorize predictive genetic testing for asymptomatic children at risk of childhood-onset conditions” (p. 621) [4]. The AAP/ACMG also recommend proceeding with caution because of potential medical or psychosocial risk to the patient and family, and suggests obtaining child assent, if possible. Of note, all childhood forms of Batten disease have onset at an age (from infancy to about 7 years old) when children may still lack maturity to provide assent. By contrast, Batten carrier testing can be safely deferred until sufficient maturity is attained for an informed consent process. Finally, the Institute of Medicine (IOM) only recommends predictive diagnostic testing when “an effective curative or preventive treatment [exists] that must be instituted early in life to achieve maximum benefit” (p. 10) [7].
Except for the IOM policy, there is a trend towards supporting predictive diagnostic GTIC for either medical or psychosocial benefit. Even so, there are disparities among published guidelines regarding what constitutes a child’s best interests, including whether these extend to the parents’ own state of mind [8]. Ross [9] suggests that each family, referencing its personal values, will assess whether psychosocial benefits outweigh psychosocial risks of such testing. She also emphasizes the importance of genetic counseling so parents understand risks and benefits of testing. The joint AAP/ACMG statement similarly notes, “genetic testing is best offered in the context of genetic counseling” [4] (p. 621) and the ESHG requires genetic counseling for GTIC in asymptomatic children. [5].
Actual lab practices regarding predictive and carrier GTIC vary considerably. Wertz & Reilly [10] surveyed 105 clinical labs and found only 25 with clear policies against predictive testing for untreatable conditions. Others lacked policies altogether or were more flexible, e.g., case-by-case determinations, refusing predictive testing selected diagnoses (e.g., HD), only providing carrier testing, or requiring genetic counseling. A more recent international survey of clinical geneticists focused on predictive GTIC for untreatable, adult-onset conditions [11]. Only 12% of participants had participated in such testing, citing reasons such as: psychosocial benefit to the family, and enabling families to plan ahead. Over half (53%) had refused to do so, citing reasons such as: no medical benefit, potential harm, policy compliance, and child autonomy.
AAP/ACMG and ESHG guidelines acknowledge the relevance of parental input for GTIC decisions, and several investigations share the voice of parents and families who might seek GTIC. In Tarini et al. [12], approximately one-third of parents indicated they would definitely or probably seek GTIC, in response to hypothetical scenarios describing non-specific inherited, life-limiting, and potentially untreatable conditions. Also, about 30% of parents supported GTIC absent any treatment; another approximately 30% of parents supported GTIC only if treatment were available. The authors acknowledged that hypothetical scenarios limited their conclusions [12].
Several other investigations have evaluated psychosocial outcomes following genetic diagnosis disclosure to children at-risk for the treatable, inherited condition of familial adenomatous polyposis (FAP) [13–15]. Most children displayed normal emotional and behavioral functioning before and after receiving a positive test result. Compared to gene-negative children, those with a positive test had greater worries about developing FAP, but did not experience generalized or clinically impairing anxiety or depression.
The present investigation attempts to address the limitations of these prior studies by focusing on a specific and life-limiting condition – Batten disease. Although there are no treatments yet available to prevent or modify Batten disease, there are ongoing and emerging clinical trials in the infantile, late infantile and juvenile Batten variants [16–18].Therefore, the definition of reasonable medical or psychosocial benefit may evolve as clinical trial outcomes could have implications for establishing a pre-symptomatic genetic diagnosis of Batten disease. Our primary intent was to survey parents’ current opinions and practices related to GTIC. Our secondary aims were to collect general information about genetic testing experiences, and about genetics knowledge of Batten disease, which had also not been previously studied.
2. Participants and Methods
2.1 Genetic testing survey
The University of Rochester Batten Center (URBC) developed a survey to assess experiences, knowledge, and opinions about genetic testing topics in Batten disease, including opinions about GTIC. The survey was modeled after a questionnaire developed previously, to study attitudes and knowledge regarding another neurodegenerative condition - Parkinson’s disease (M. Nance, personal communication). The present study was the first use of the Batten survey, thus has not yet undergone formal psychometric testing. Face validity was established by Dr. Nance, who provided input on survey development, drawing on her experience with the Parkinson’s disease survey and her expertise with Juvenile Huntington disease (HD). HD is a neurodegenerative condition with no currently available disease-modifying therapy. Due to genetic anticipation, HD sometimes has childhood-onset; predictive diagnostic testing is strongly discouraged. For the Batten survey, we obtained demographic information (respondent age, educational background, child’s Batten diagnosis). Parents answered nine ‘opinion’ statements related to GTIC, such as predictive diagnosis, childhood carrier testing, and parental autonomy in seeking GTIC; items were rated on a 5-point scale: 1 = ‘strongly disagree’, 5 = ‘strongly agree’. Parents also reported their family history of genetic testing, including if other family members had completed carrier or predictive diagnostic genetic testing for Batten disease. We defined carrier testing as, “to establish if someone – parent or sibling – is a carrier” of Batten disease, and predictive testing as, “to establish if someone – another child in the family – will develop Batten disease”. We also asked parents if genetic testing for other diseases had been sought. In addition, if parents or a family member had either considered or completed genetic testing, they were asked how they had learned about it, and if they recalled having discussed any of 8 selected topics likely to be part of a genetic counseling session (e.g., risks and benefits of genetic testing, reproductive risk, and follow-up options after genetic testing). Finally, parents were asked if they had disclosed their child’s genetic status beyond their immediate family. Parents also completed true/false questions to test knowledge about Batten disease medical facts, general genetics information, and Batten-specific genetics information. The ‘knowledge’ items allowed exploration of possible associations between respondents’ knowledge base related to genetics, and their opinions and experience with GTIC.
Because of the sensitive nature of some survey data, respondents’ anonymity was preserved. Investigators provided the survey to the Batten Disease Support and Research Association (BDSRA), which distributed it to the approximately 1,100 households on its mailing list. The BDSRA is the major North American Batten disease support organization. All parents of affected children (alive or deceased) were eligible to participate. The survey did not collect information that could inadvertently identify participants or affected children. We did not confirm parent-reported clinical diagnoses, nor did we ask for any other identifying information such as number of affected children, the name, age or sex of affected children, specific mutations, age at diagnosis, or number of years since diagnosis. All study activities were approved by the University of Rochester’s Research Subjects Review Board (RSRB). The RSRB provided an exemption from the written informed-consent process, to maintain participant anonymity. The survey included a RSRB-approved cover letter explaining to prospective respondents that returning the completed survey indicated agreement to participate in the study.
2.2 Data Analysis
Frequency counts were calculated for categorical response items. Nonparametric tests were performed on the true/false ‘knowledge’ items because the distribution of these scores (percent accurate) was non-normal. Spearman rank-order correlation tests were performed to evaluate bivariate relationships between sociodemographic variables (age, gender, educational level), and knowledge accuracy. A series of Mann-Whitney tests were performed to evaluate knowledge differences between those who had versus had not participated in genetic testing. If items were skipped, they were treated as incorrect in calculating accuracy scores ([number correct/15] *100). Student’s t test for dependent samples was used to evaluate pairwise difference between subsets of the fact-based questions dealing with different content. An a priori alpha level = .05 was used for all statistical tests. All analyses were performed with Statistica, version 6.1.
3. Results
3.1 Demographics
17% (N=184/1,100) surveys were returned. Of these, we excluded 5 surveys that were returned but not completed. Among the remaining 179 surveys, some respondents skipped one or more questions, thus the sample size varies for the data reported below. 146 respondents reported their age; we excluded two participants for this analysis only because of suspicion that the affected child’s age (18 and 21 years, respectively) was reported instead; these surveys were each from parents of children with juvenile Batten disease. Thus, the average age of respondents was 49.3± 11.7 years (N = 144; range 23–79). The most common diagnosis reported was juvenile NCL (CLN3), reported by 46% (N = 82/179). Most respondents (84.9%) had received genetic confirmation of the clinical diagnosis. Table 1 presents all demographic data. Nearly all parents (94%; N = 169/179) had shared their child’s genetic status with at least one person other than an immediate family member. We note that more participants endorsed sharing genetic status than had endorsed a genetically confirmed Batten diagnosis in their child.
Table 1.
Sample background characteristics (N = 179)
| N | % | |
|---|---|---|
| Parent’s Sex | ||
| female | 122 | 68 |
| male | 38 | 21 |
| not reported | 19 | 11 |
| Parent’s Education | ||
| some high school or GED | 8 | 5 |
| high school diploma | 22 | 12 |
| some college | 55 | 31 |
| college degree | 60 | 34 |
| some post-graduate studies | 2 | 1 |
| post-graduate degree | 27 | 15 |
| not reported | 5 | 3 |
| Child’s Batten disease Diagnosis | ||
| CLN1 / Infantile Batten disease | 25 | 14 |
| CLN2 / Late Infantile Batten disease | 47 | 26 |
| CLN3 / Juvenile Batten disease | 82 | 46 |
| Other | 7 | 4 |
| Not reported | 18 | 10 |
3.2 Genetic testing opinions
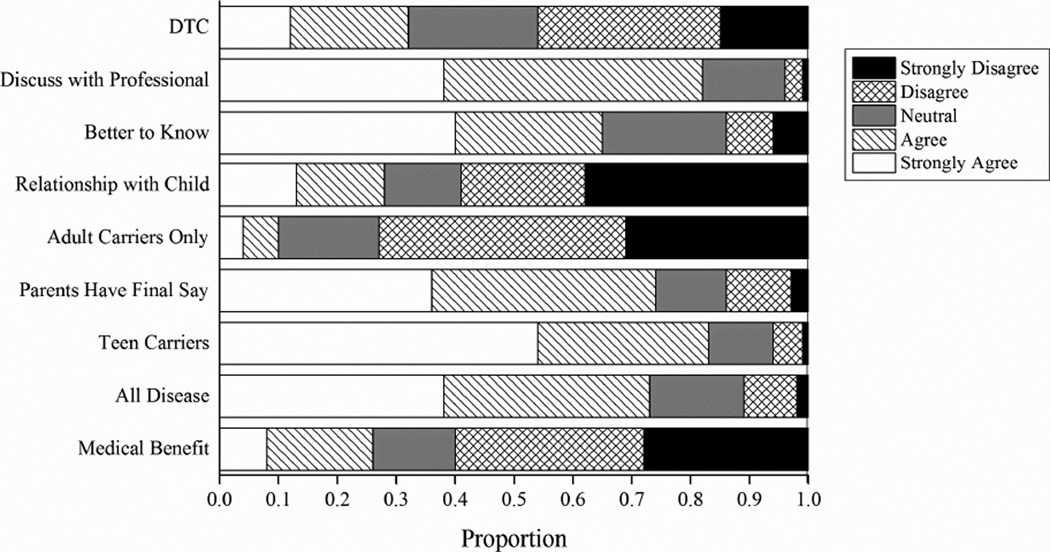
Parents rated their agreement or disagreement with ‘opinion’ statements related to GTIC (Figure 1). Four respondents did not complete this section and 15 did not answer at least one question. The number of participants completing each item is indicated in the Figure. 73% (126/172) felt predictive genetic testing for an asymptomatic person should be available, regardless of medical benefit. 74% (128/173) agreed or strongly agreed with the statement, “Parents should have the final say on whether or not their healthy child gets genetic testing to determine if he/she is a carrier or will develop Batten Disease”. In our survey, the final two ‘opinion’ statements helped us explore whether parents might pursue direct-to-consumer genetic testing (DTC). Most participants (82%) agreed or strongly agreed that people should always discuss genetic testing plans and results with a professional, yet almost a third (32%) also agreed or strongly agreed that, “It should be possible for people to have genetic testing on their own, without input from a doctor or genetic counselor.” There were no significant associations between opinions and parent gender or age. There were two weak associations between opinions and parent educational level. Higher educational level was weakly negatively associated with, “Predictive testing should be available for all diseases, regardless of medical benefit” (Spearman ρ = −.15, p < .05), and weakly positively correlated with, “Knowing a child’s genetic status will change how a parent relates to that child” (Spearman ρ = .19, p < 05).
Figure 1.
Parents’ Opinions About Genetic Testing in Children
DTC: “It should be possible for people to have genetic testing on their own, without input from a doctor or genetic counselor.” (N = 170)
Discuss Professional: “People who have genetic testing should always discuss their plans for testing, and the test results, with a professional.” (N = 175)
Better to Know: “Knowing ahead of time that a child will develop Batten disease is better than not knowing this information.” (N = 174)
Relationship With Child: “Knowing a child’s genetic status will change how a parent relates to that child” (N = 171)
Adult Carrier: “Carrier testing should only be done when an individual reaches adulthood.” (N = 175)
Parents Have Final Say: “Parents should have the final say whether or not their healthy child gets genetic testing to determine if s/he is a carrier or will develop Batten disease.” (N = 173)
Teen Carriers: “Teenagers should be told whether or not they are Batten carriers.” (N = 175)
All Disease: “Predictive testing should be available for all diseases regardless of medical benefit.” (N = 172)
Med Benefit: “Predictive testing (testing an asymptomatic person for a gene that causes a genetic disease) should be available only when there is medical benefit from doing the test.” (N = 173)
3.3 Experience with genetic testing and genetic counseling
54% (N = 97/179) reported an immediate family member other than the affected child had ever received genetic testing. 46% (N = 82/179) endorsed family experience with carrier testing, which could have involved child or adult testing. 13% (N = 24/179) reported predictive testing for Batten disease. Seven percent (N = 13/179) reported family experience with both carrier and predictive testing. Four percent (N = 7/179) reported family experience with genetic testing for non-Batten reasons, including for conditions likely considered in the diagnostic workup for Batten disease (retinitis pigmentosa, Hurler-Scheie, adrenoleukodystrophy) and for other diseases (hemophilia type-B, and breast cancer). Four parents reported family experience with genetic testing, but did not know if the testing was performed to establish carrier status or a predictive diagnosis.
145 respondents indicated how they had first learned about genetic testing. Among these respondents, the three most common sources were a neurologist: 53% (N = 77/145), the BDSRA or other support group: 43% (N = 63/145), and a genetic counselor: 23% (N = 34/145). 75% of all respondents (135/179) recalled discussion of at least one topic likely to be covered in a genetic counseling session. (Table 2). An average of 4.8 topics were discussed (SD = 2.29, Range = 1 to 8).
Table 2.
Typical topics in a genetic counseling session (N = 135)
| “If you or your child have either considered or completed genetic testing, did anyone discuss with you...? | Percent (N) |
|---|---|
| Your risk or family’s risk for an inherited condition. | 84 (113) |
| Your reproductive risks, e.g., the risk of passing the condition on to future children. | 83 (112) |
| The relationship between the presence of a gene mutation and the development of disease. | 67 (90) |
| What is a gene? | 59 (80) |
| Your medical history. | 58 (78) |
| The risk, benefits, limitations, and other possible consequences of being tested. | 49 (66) |
| Any implications of the test results for insurability, reproduction, or health. | 45 (61) |
| Any follow-up options e.g., if any additional testing, monitoring, or research options were available. | 36 (48) |
3.4 Knowledge about Batten disease and genetics
175 of 179 respondents completed this section. 53 respondents skipped at least one item, which was handled via the method described in section 2.2. Respondents scored high on knowledge questions, with a mean total score of 84% correct (SD = 11%, Range = 53%–100%). Accuracy was positively but only slightly correlated with educational level (ρ = .29, p < .001). Mean accuracy was significantly higher for Batten-specific medical and genetic questions than for general genetic questions (Batten genetics vs. general genetics: t = 4.70, p<0.001, df = 174; Batten medical vs. general genetics: t = 4.12, p < 0.001, df = 174; 95% Confidence Interval −0.18, −0.04). Respondents who had participated in any genetic counseling, regardless of the number of topics covered in a counseling visit, received a significantly higher score on both question sets than did those who had not completed any genetic counseling (Table 3). Among respondents who participated in genetic counseling, the proportion of topics discussed (of 8 total) was not significantly correlated with accuracy on any of the three sets of knowledge questions, or the total score.
Table 3.
Exposure to Genetic Counseling and Proportion of Correct Responses on True/False Items (Mann-Whitney U test)
| Exposure to Genetic Counseling | ||||
|---|---|---|---|---|
| Any (N = 132) | None (N = 43) | |||
| % Correct Mean (SD) |
% Correct Mean (SD) |
Zadj | p | |
| 1. Batten disease occurs in approximately 1 out of 1,000 people in the U.S. | 80 (40) | 81 (40) | 0.09 | 0.93 |
| 2. Vision loss is always the first symptom to develop in Batten disease. | 79 (41) | 72 (45) | 0.90 | 0.37 |
| 3. Batten disease symptoms are linked to the buildup of lipopigments in the body’s tissues. | 71 (45) | 81 (40) | 1.24 | 0.21 |
| 4. Stem cell transplants are an experimental, unproven treatment for Batten disease. | 89 (32) | 84 (37) | 0.84 | 0.40 |
| 5. Batten disease can be slowed down with “natural” treatments such as diet, exercise, and over-the-counter herbal remedies. | 88 (33) | 84 (37) | 0.70 | 0.49 |
| 81 (19) | 80 (20) | |||
| Medical knowledge of Batten disease (Items #1–5) | Median = 80 | Median = 80 | 0.58 | 0.58 |
| Range: 20–100 | Range: 40–100 | |||
| 6. A gene is composed of a group of proteins. | 32 (47) | 30 (46) | 0.19 | 0.85 |
| 7. It is possible to have an abnormal gene without having symptoms of disease. | 96 (19) | 81 (39) | 3.20 | <0.01 |
| 8. Genetic diseases always begin in childhood. | 93 (25) | 84 (37) | 1.86 | 0.06 |
| 9. Genes are found in nerve cells but not egg and sperm cells. | 81 (39) | 65 (48) | 2.15 | <0.05 |
| 76 (19) | 65 (27) | |||
| Median = 75 | Median = 75 | 2.03 | <0.05 | |
| Range: 25–100 | Range: 0–100 | |||
| 10. All people affected with Batten disease have a mutation in the same gene. | 77 (43) | 63 (49) | 1.76 | 0.079 |
| 11. Someone with one copy of a Batten gene mutation will not develop Batten disease. | 85 (36) | 77 (43) | 1.22 | 0.22 |
| 12. When someone has Batten disease, his or her siblings have a 1 in 4 chance of also developing Batten disease. | 95 (21) | 86 (35) | 2.09 | <0.05 |
| 13. The function of the proteins encoded by the Batten gene or genes is well understood at this point in time. | 84 (37) | 72 (45) | 1.72 | 0.09 |
| 14. Girls and boys equally likely to get Batten disease. | 92 (27) | 77 (43) | 2.80 | <0.01 |
| 15. A Batten gene that is normal during a person’s childhood can develop a mutation as the person ages. | 72 (45) | 56 (50) | 1.96 | <0.05 |
| 84 (18) | 72 (26) | |||
| Genetic knowledge of Batten disease (Items #10–15) | Median = 83 | Median = 83 | 2.79 | <0.01 |
| Range: 33–100 | Range: 0–100 | |||
| 81 (14) | 73 (19) | |||
| Total (all items) | Median = 87 | Median = 80 | 2.60 | <0.01 |
| Range: 33–100 | Range: 13–100 | |||
4. Discussion
This study evaluated experience, knowledge, and opinions related to GTIC, among parents of children with Batten disease. Most families had received a genetic diagnosis for their Batten-affected child, and slightly over half of families had sought genetic testing for non-affected family members – chiefly to determine if an at-risk child would phenoconvert (predictive testing) or to establish carrier status for a healthy family member. Earlier studies suggest parents wish to establish their child’s future risk for inherited diseases irrespective of an immediately beneficial treatment, for reasons including wanting certainty about their child’s health status and wanting to prepare for the future [19–23].
In the current study, joint themes of parental choice and informed-decision making were expressed in participants’ responses to the ‘opinions about genetic testing’ statements. Notably, the increased availability of DTC genetic testing has facilitated independent access, which was favored by almost a third of our sample. Policies vary among DTC providers but at least 20 may offer direct access to patients/families, many do not provide or require genetic counseling or physician consultation, and predictive diagnosis and risk assessment for some pediatric-onset or non-treatable conditions is available [8, 24–26]. Because of concerns about test quality, oversight, and inconsistent policies about genetic counseling, the AAP/ACMG joint statement strongly advises against DTC genetic testing of children [4, 5]. Currently, one DTC company that we know of (23andMe, Inc.) explicitly tests for selected mutations causing two Batten variants[27, 28] ; at least one other company offers whole genome sequencing (Illumina), which conceivably could include identification of Batten-causing mutations [29].
Several prior studies suggest that knowing a child’s genetic status may not impact parenting behaviors [13–15, 30]. Over half of respondents disagreed with the statement, “Knowing a child’s genetic status will change how a parent relates to that child”. Psychological adjustment to a genetic diagnosis for a future-developing condition is another concern. Children learning their genetic risk for FAP experienced only mild anxiety/depression, isolated to worries about the diagnosis. Women undergoing childhood carrier testing for one of two recessive conditions (Duchenne muscular dystrophy; hemophilia A) later felt the diagnosis did not negatively impact their lives [31].
Higher scores on some knowledge questions was modestly associated with having participated in genetic counseling. This is supported by a previous study that found genetic counseling for familial cancer increased knowledge about cancer genetics [32]. However, we recognize that in this retrospective survey, we cannot determine any causal direction for the relationship between genetics knowledge and genetics counseling, and/or that other unknown factors impacted these variables. We did not assess whether knowledge was correlated with the number of affected children in a family; perhaps families with multiple affected children have more opportunities to receive genetic counseling. Finally, the BDSRA and other support groups were the second most common initial source of information about genetic testing, suggesting a role for advocacy organizations in providing information and facilitating access to genetic testing and genetic counseling resources.
Given the potentially sensitive nature of genetic information, it was surprising to learn how broadly parents shared their family’s genetic status. But we also note that this study was conducted after implementation of the Genetic Information Non-Discrimination Act (GINA) which would conceivably protect parents from some of the negative consequences of disclosure, particularly to employers [33]. In retrospect, it would have been interesting to ask respondents about their knowledge of GINA and whether this law influences their genetic status disclosures.
Non-response bias is another limitation - fewer than 20% of the surveys were returned. We do not know whether respondents were representative of the full BDSRA membership. We also focused only on parents affiliated with the BDSRA. Furthermore, because the survey was distributed to the entire BDSRA membership, many non-parents received it. Therefore, the response rate does not truly reflect the proportion of parents in the BDSRA membership. We acknowledge that direct contact would have boosted participation and reduced missing data, but we chose to preserve participants’ anonymity due to the sensitive nature of some questions. In retrospect, survey questions about genetic testing could have been more specifically worded to clarify who (child or adult) had undergone carrier testing or non-Batten genetic testing; we would likely revise these items in future studies with the survey. Finally, respondents were highly educated. While this bias exists in many studies, we acknowledge that educational background could impact attitudes and knowledge about genetic testing.
5. Conclusions
Although this study focused on Batten disease, we believe the results provide a springboard to discuss GTIC topics relevant to many childhood onset diseases, including predictive diagnostic testing, carrier testing, and genetic counseling. These topics are especially pertinent in light of emerging clinical trials, which often require genetic confirmation of the disease before study enrollment, and where the therapeutic window for intervention might occur before clinical signs of disease are apparent. Responses to survey items addressing opinions regarding GTIC suggest that overall, parents wish to assert their autonomy in decisions regarding genetic testing of their children. This wish for autonomy also highlights the need for genetic counseling and education so parents can make informed decisions regarding the benefits and risks of childhood genetic testing.
Acknowledgments
Funding Source: This research was supported by NIH grant 5K23 NS058756-01 from the National Institute of Neurological Disorders and Stroke
The authors thank the Batten Disease Support and Research Association (BDSRA), which provided assistance by distributing surveys to study participants, and the families who participated in this research.
Abbreviations
- AAP/ACMG
American Academy of Pediatrics/American College of Medical Genetics
- BDSRA
Batten Disease Support and Research Association
- DTC
Direct to Consumer
- FAP
Familial adenomatous polyposis
- GINA
Genetic Information Non Discrimination Act
- GTIC
Genetic testing in children
- HD
Huntington disease
- NCL
Neuronal ceroid lipofuscinosis
- RSRB
Research Subjects Review Board
- URBC
University of Rochester Batten Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None of the other authors have any financial relationships that are relevant to this article to disclose.
Conflict of Interest: Dr. Adams, Dr. Mink, and Dr. Kwon serve on the Medical Advisory Board for the Batten Disease Support and Research Association. The other authors have no conflicts of interest to disclose.
Contributor Information
Heather R. Adams, Email: heather_adams@urmc.rochester.edu.
Katherine Rose, Email: katherine_rose@urmc.rochester.edu.
Erika F. Augustine, Email: erika_augustine@urmc.rochester.edu.
Jennifer M. Kwon, Email: jennifer_kwon@urmc.rochester.edu.
Elisabeth A. deBlieck, Email: Lisa.DeBlieck@chet.rochester.edu.
Frederick J. Marshall, Email: Frederick_Marshall@urmc.rochester.edu.
Amy Vierhile, Email: amy_vierhile@urmc.rochester.edu.
Jonathan W. Mink, Email: jonathan_mink@urmc.rochester.edu.
Martha A. Nance, Email: martha.nance@parknicollet.com.
References
- 1.Santavuori P, Lauronen L, Kirveskari E, Åberg L, Sainio K, Autti T. Neuronal ceroid lipofuscinoses in childhood. Neurological sciences: official journal of. 2000;21:S35–S41. doi: 10.1007/s100720070038. [DOI] [PubMed] [Google Scholar]
- 2.Mole S. MRC Laboratory for Molecular Cell Biology. [Access date, 23 Aug 2013];NCL Mutation and Patient Database. http://www.ucl.ac.uk/ncl/mutation.shtml.
- 3.Ross L, Saal H, David K, Anderson R. American Academy of Pediatrics, American College of Medical Genetics and Genomics, Technical report: ethical and policy issues in genetic testing and screening of children. Genet. in Med. 2013;15:234–245. doi: 10.1038/gim.2012.176. [DOI] [PubMed] [Google Scholar]
- 4.Committee on Bioethics, Committee on Genetics. The American College of Medical Genetics, Genomics, Social, Ethical, and Legal Issues Committee, Ethical and Policy Issues in Genetic Testing and Screening of Children. Pediatrics. 2013;131:620–622. doi: 10.1542/peds.2012-3680. [DOI] [PubMed] [Google Scholar]
- 5.European Society of Human Genetics. Genetic testing in asymptomatic minors: recommendations of the European Society of Human Genetics. Eur. J Hum. Genet. 2009;17:720–721. doi: 10.1038/ejhg.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borry P, Evers-Kiebooms G, Cornel M, Clarke A, Dierick K on behalf of the Public and Professional Policy Committee (PPPC) of the European Society of Human Genetics (ESHG) Genetic testing in asymptomatic minors. Background considerations towards ESHG Recommendations. Eur. J. Hum. Genet. 2009;17 doi: 10.1038/ejhg.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews L, Fullarton J, Holtzman N, Motulsky A. Assessing Genetic Risks: Implications for health and social policy. Washington, DC: National Academy Press; 1994. Committee on Assessing Genetic Risks - Division of Health Sciences Policy: Institute of Medicine. [PubMed] [Google Scholar]
- 8.Borry P, Stultiens L, Nys H, Cassiman JJ, Dierickx K. Presymptomatic and predictive genetic testing in minors: a systematic review of guidelines and position papers. Clin. Genet. 2006;70:374–381. doi: 10.1111/j.1399-0004.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 9.Ross LF. Predictive genetic testing for conditions that present in childhood. Kennedy Institute of Ethics Journal. 2002;12:225–244. doi: 10.1353/ken.2002.0019. [DOI] [PubMed] [Google Scholar]
- 10.Wertz DC, Reilly PR. Laboratory policies and practices for the genetic testing of children: a survey of the Helix network. Am. J Hum. Genet. 1997;61:1163–1168. doi: 10.1086/301593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan RE, Savulescu J, Gillam L, Williamson R, Delatycki MB. An international survey of predictive genetic testing in children for adult onset conditions. Genetics in Medicine. 2005;7:390–396. doi: 10.1097/01.gim.0000170775.39092.44. [DOI] [PubMed] [Google Scholar]
- 12.Tarini B, Singer D, Clark S, Davis M. Parents' interest in predictive genetic testing for their children when a disease has no treatment. Pediatrics. 2009;124:e432–e438. doi: 10.1542/peds.2008-2389. [DOI] [PubMed] [Google Scholar]
- 13.Codori A, Petersen G, Boyd P, Brandt J, Giardiello F. Genetic testing for cancer in children. Short-term psychological effect. Arch. Pediatr. Adolesc. Med. 1996;150:1131–1138. doi: 10.1001/archpedi.1996.02170360021003. [DOI] [PubMed] [Google Scholar]
- 14.Codori A, Zawacki K, Peteresen G, Miglioretti D, Bacon J, Brensinger J, Booker S, Picarello K, Giardiello F. Genetic testing for hereditary colorectal cancer in children: Long term psychological effects. Am. J. Med. Genet. 2003;116A:117–128. doi: 10.1002/ajmg.a.10926. [DOI] [PubMed] [Google Scholar]
- 15.Michie S, Bobrow M, Marteau T. Predictive genetic testing in children and adults: a study of emotional impact. J. Med. Genet. 2001;38:519–526. doi: 10.1136/jmg.38.8.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crystal RG, Sondhi D, Hackett NR, Kaminsky SM, Worgall S, Stieg P, Souweidane M, Hosain S, Heier L, Ballon D, Dinner M, Wisniewski K, Kaplitt M, Greenwald BM, Howell JD, Strybing K, Dyke J, Voss H. Clinical protocol. Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis. Hum. Gene. Ther. 2004;15:1131–1154. doi: 10.1089/hum.2004.15.1131. [DOI] [PubMed] [Google Scholar]
- 17.Selden N, Guillaume D, Steiner R, Huhn S. Cellular therapy for childhood neurodegenerative disease. Part II: clinical trial design and implementation. Neurosurg. Focus. 2008;24:E23. doi: 10.3171/FOC/2008/24/3-4/E22. [DOI] [PubMed] [Google Scholar]
- 18.Worgall S, Sondhi D, Hackett N, Kosofsky B, Kekatpure M, Neyzi N, Dyke J, Ballon D, Heier L, Greenwald P, Christos M, Mazumdar M, Souweidane M, Kaplitt M, Crystal R. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum. Gene Ther. 2008;19:463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- 19.Geller G, Tambor E, Bernhardt B, Fraser G, Wissow L. Informed consent for enrolling minors in genetic susceptibility research: a qualitative study of at-risk children's and parents' views about children's role in decision-making. J. Adolesc. Health. 2003;32:260–271. doi: 10.1016/s1054-139x(02)00459-7. [DOI] [PubMed] [Google Scholar]
- 20.Geller G, Tambor E, Bernhardt B, Wissow L, Fraser G. Mothers and daughters from breast cancer families: A qualitative study of their perspective of the risks and benefits associated with minors' participation in genetic susceptibility research. J. Am. Med. Womens Assoc. 2000;55:280–284. [PubMed] [Google Scholar]
- 21.Gustafsson Stolt U, Liss P, Ludvigsson J. Abis Study Group, Parents want to know if their child is at high risk of getting diabetes. Ann. NY. Acad. Sci. 2003;1005:395–399. doi: 10.1196/annals.1288.066. [DOI] [PubMed] [Google Scholar]
- 22.Helgesson G, Swartling U. Views on data use, confidentiality, and consent in a predictive screening involving children. J. Med. Ethics. 2008;34:206–209. doi: 10.1136/jme.2006.020016. [DOI] [PubMed] [Google Scholar]
- 23.Twomey JG, Bove C, Cassidy D. Presymptomatic genetic testing in children for neurofibromatosis 2. Journal of Pediatric Nursing. 2008;23:183–194. doi: 10.1016/j.pedn.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Borry P, Howard H, Senecal K, Avard D. Health-related direct-to-consumer genetic testing: a review of companies’ policies with regard to genetic testing in minors. Fam Cancer. 2010;9:51–59. doi: 10.1007/s10689-009-9253-9. [DOI] [PubMed] [Google Scholar]
- 25.Howard H, Avard D, Borry P. Are the kids really all right? Direct-to-consumer genetic testing in children: are company policies clashing with professional norms? Eur J Hum Genet. 2011;19:1122–1126. doi: 10.1038/ejhg.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dvoskin R, Kaufman D. Tables of Direct-to-Consumer Genetic Testing Companies and Conditions Tested - August 2011. Genetics and Public Policy Center; 2011. [Access date, 23 Aug 2013]. http://www.dnapolicy.org/pub.reports.php?action=detail&report_id=28. [Google Scholar]
- 27.23andMe Inc. [access date 18 Oct 2013];Neuronal Ceroid Lipofuscinosis (PPT1-related) https://www.23andme.com/health/ppt1-related-ncl/
- 28.23andMe Inc. [access date 18 Oct 2013];Neuronal Ceroid Lipofuscinosis (CLN-5 related) https://www.23andme.com/health/cln5-related-ncl/
- 29. [Access date, 18 Oct 2013];Illumina, illumina home page, Illumina. http://www.illumina.com/
- 30.Michie S, McDonald V, Bobrow M, McKeown C, Marteau T. Parents' responses to predictive genetic testing in their children: report of a single case study. Journal of Medical Genetics. 1996;33:313–318. doi: 10.1136/jmg.33.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Järvinen O, Lehesjoki A-L, Lindlöf M, Uutela A, Kääriäinen H. Carrier testing of children for two X-linked diseases: A retrospective study of comprehension of test results and social and psychologoical significance of the testing. Pediatrics. 2000;106:1460–1465. doi: 10.1542/peds.106.6.1460. [DOI] [PubMed] [Google Scholar]
- 32.Braithwaite D, Emery J, Walter F, Prevost A, Sutton S. Psychological Impact of Genetic Counseling for Familial Cancer: A Systematic Review and Meta-analysis. Journal of the National Cancer Institute. 2004;96:122–133. doi: 10.1093/jnci/djh017. [DOI] [PubMed] [Google Scholar]
- 33.Genetic Information Non-Discrimination Act of 2008, Pub. L. 110-233. 122 Stat. 881. 2008. May 21, [Google Scholar]