Abstract
Reports of human infections with highly pathogenic H5N1 avian influenza viruses in many countries in Asia and Africa with varying case fatality rates highlight the pandemic potential of these viruses. In order to contain a rapidly spreading influenza virus in a pandemic scenario, a vaccine which can induce rapid and robust immune responses, preferably in a single dose, is necessary. Murine beta-defensin 2 (Mbd2), a small molecular weight protein expressed by epithelial cells, has been shown to enhance antigen-specific immune responses by recruiting and activating professional antigen presenting cells to the site of vaccination. This study assessed the potential of Mbd2 to enhance the immunogenicity and protective efficacy of a human adenovirus (HAd)-based vaccine expressing the hemagglutinin (HA) and nucleoprotein (NP) [HAd-HA-NP] of an H5N1 influenza virus. A single inoculation of mice with both HAd-HA-NP and a HAd vector expressing Murine β-defensin 2 (HAd-Mbd2) resulted in significantly higher levels of both humoral and cell-mediated immune responses compared to the groups vaccinated only with HAd-HA-NP. These responses were evident even at Day 7 post-immunization. Furthermore, the HAd-HA-NP+HAd-Mbd2-immunized group receiving the lowest vector dose (2 × 107 + 1 × 107) was completely protected against an rgH5N1 virus challenge on Day 7 post-vaccination. These results highlight the potential of Mbd2 as a genetic adjuvant in inducing rapid and robust immune responses to a HAd-based vaccine.
Keywords: adenovirus, avian influenza, adenovirus vector-based vaccines, influenza, pandemic influenza vaccine, murine beta-defensin 2
1. Introduction
Highly pathogenic avian influenza (HPAI) H5N1 viruses remain a public health threat around the world (Katz et al., 2009; Vemula and Mittal, 2010). Since the initial emergence of H5N1 influenza virus in humans following poultry outbreaks in Hong Kong in 1997, H5N1 viruses have so far spread to over sixty countries in Asia, Africa and Europe resulting in more than five hundred cases of human infections with a case fatality rate of over 60% (World Health Organization, 2013). Although human-to-human transmission has been infrequent and limited, it is widely believed that genetic reassortment between a human and avian influenza virus or mutations in the H5N1 virus genome could result in the generation of a novel H5N1 strain that could initiate a pandemic if it acquired the ability to undergo sustained transmission in the immunologically naive human population (Imai et al., 2012; Russell et al., 2012). The 2009 H1N1 pandemic influenza virus is thought to have originated due to a complex reassortment process (Parrish and Kawaoka, 2005). Although the 2009 pandemic was not as deadly compared to previous pandemics, its worldwide spread in a short period highlighted the public health threat posed by novel influenza viruses originating from non-human reservoirs. Vaccination remains the most effective and economical way to combat an influenza pandemic (Pandey et al., 2010; Pandey et al., 2012). The ideal vaccine for a pandemic influenza should induce rapid and robust immune responses resulting in effective protection.
Defensins are a family of small cationic proteins known to have antimicrobial activity (Oppenheim et al., 2003; Yang et al., 2002; Yang et al., 2007). Murine β-defensin 2 (Mbd2) which belongs to the β-defensin class of defensins is mainly expressed by epithelial cells and has been shown to play an important role in mediating both innate and adaptive immune responses (Morrison et al., 1999). By interacting with Chemokine C-C-Motif Receptor 6 (CCR6), Mbd2 has been shown to recruit/chemo-attract immature dendritic cells (DC) to the site of an infection thereby facilitating better antigen uptake and presentation (Biragyn et al., 2002b). Moreover, Mbd2 has also been shown to induce DC maturation in a toll-like receptor (TLR) 4-dependent manner (Biragyn et al., 2002b). This study evaluated the ability of Mbd2 to enhance the immunogenicity and efficacy of a human adenovirus (HAd) vector-based vaccine (HAd-HA-NP) expressing hemagglutinin (HA) and nucleoprotein (NP) of a H5N1 influenza virus in a mouse model. The results demonstrated that a single dose of HAd-HA-NP in combination with HAd-Mbd2 (a HAd vector expressing Mbd2) significantly enhanced influenza-specific humoral and cellular immune responses even on Day 7 post-immunization resulting in complete protection against challenge with a rgH5N1 virus compared to the groups immunized only with HAd-HA-NP.
2. Materials and Methods
2.1. Cell lines
MDCK (Madin-Darby canine kidney), 293 (human embryonic kidney cells expressing HAd5 E1 gene products), 293Cre (293 cells that constitutively expresses Cre-recombinase enzyme, a gift from Merck Inc, Whitehouse Station, NJ), and BHH2C (bovine-human hybrid clone 2C cells. which express HAd5 E1 gene products) (van Olphen and Mittal, 2002) cell lines were grown as monolayer cultures in Eagle’s minimum essential medium (MEM) (Life Technologies, Gaithersburg, MD) and supplemented with 10% reconstituted bovine serum (Fetal Clone III; Hyclone, Logan, UT) and 50 μg/ml gentamycin.
2.2. Adenovirus vectors
The Cre-recombinase-mediated site-specific recombination system was used to construct a replication-defective HAd5 vector expressing Mbd2 (HAd-Mbd2) (Vemula et al., 2013). The construction and characterization of HAd-ΔE1E3 (an empty HAd vector) (Noblitt et al., 2004) and HAd-HA-NP [a HAd vector expressing the HA and NP of A/Vietnam/1203/04 (H5N1) virus] (Pandey et al., 2012) has been previously described. The HAd vectors were purified by cesium chloride density-gradient centrifugation and titrated by plaque assay on BHH2C cells as described earlier (Noblitt et al., 2004).
2.3. Animal inoculation and protection studies
All animal studies were conducted following guidelines and approval from Institutional Biosafety Committee and Institutional Animal Care and Use Committee at Purdue University. Groups of six-to-eight-week old female BALB/c mice (Harlan Sprague Dawley Inc., Indianapolis) (N=11 per group) were inoculated intramuscularly (i.m.) with 2 × 107, 1 × 108, or 5 × 108 plaque-forming units (pfu) of HAd-HA-NP alone or in combination with 1 × 107 pfu of HAd-Mbd2. Control groups received 1 × 108 pfu of HAdΔE1E3 or 1 × 108 pfu of HAd-ΔE1E3+ 1 × 107 pfu of HAd-Mbd2. At Days 7 and 14 post-immunization, blood samples were collected by retro-orbital puncture to evaluate the development of HA-specific humoral responses by hemagglutination inhibition (HI) and virus neutralization (VN) assays. Six animals from each group were euthanized, and the spleens were collected to evaluate the induction of the HA- and NP-specific cell-mediated immune responses by HA or NP pentamer staining and interferon-γ ELISpot assays. The remaining five mice from each group were challenged by i.n. administration of 5000 egg infectious dose 50 (EID50) [equivalent to 100 mouse infectious dose 50 (MID50)] of reverse genetics-derived A/Puerto Rico/8/1934 (H1N1) [PR8] containing HA and NA gene fragments of A/Vietnam/1203/04 (H5N1) [VNH5N1-PR8/CDC-RG] (Subbarao et al., 2003). Since this virus is not lethal and does not produce clinical disease and weight loss in mice, protective efficacy was monitored by virus clearance in the lungs. Day 3 post-challenge, the mice were euthanized and the lungs were collected to determine virus titers to evaluate protective efficacy.
2.4. Virus titration
Briefly, lung tissues collected Day 3 following challenge were homogenized in 1 ml of sterile phosphate buffered saline (PBS), and then 10-fold serially diluted lung homogenates were used to infect MDCK cells seeded in 96-well plates. After 72 hours (h) of incubation at 37°C, the HA activity of the culture supernatants was determined by the hemagglutination of turkey red blood cells (TRBC). The limit of virus detection was 0.5 log10 50% tissue culture infectious dose (TCID50) per ml.
2.5. Hemagglutination inhibition Assay
Sera from all mice were treated with a receptor-destroying enzyme from Vibrio cholera (Denka Seiken, Tokyo, Japan) at 37°C for 16 h to destroy nonspecific serum inhibitor activity. The presence of HI antibody was determined using four hemagglutination units of each influenza virus and 0.5% TRBC as described (Hoelscher et al., 2007).
2.6. Micro-neutralization assay
The micro-neutralization assay was performed using MDCK cells and 100 TCID50 of VNH5N1-PR8/CDC-RG. Serial two-fold dilutions of heat-inactivated serum samples were mixed with 100 TCID50 of VNH5N1-PR8/CDC-RG and incubated at room temperature for 1 h. The virus antibody mixture was then added to the monolayer of MDCK cells, and the plates were incubated for 72 h at 37°C. After incubation, the HA activity of the supernatant was assessed by hemagglutination assay with 0.5% TRBC. The VN titer was defined as the reciprocal of the highest dilution of serum which showed complete absence of TRBC agglutination (Sambhara et al., 2001). The assay was done in triplicate.
2.7. ELISpot assay
96-well flat-bottom polyvinyl chloride micro-titer plates (Millipore, Billerica, MA) were coated overnight at 4°C with an anti-mouse IFN-γ antibody (BD Bioscience, San Jose, CA). Splenoctyes (5 × 105 or 1 × 106 cells/well) isolated from inoculated mice were cultured in the presence of either a HA-518 or a NP-147 peptide in RPMI medium (GIBCO, Grand Island, NY) supplemented with 10% reconstituted FBS for 60 h and developed according to an ELISpot protocol (Singh et al., 2008).
2.8. Statistical analysis
The Kruskall-Wallis test was used for calculation of significance. The significance was set at P <0.05.
3. Results
3.1. Effect of Mbd2 on humoral immune responses induced by HAd-HA-NP
To determine whether Mbd2 could augment humoral immune responses induced by HAd-HA-NP, BALB/c mice (6 animals/group) were i.m. immunized with 2 × 107, 1 × 108, or 5 × 108 of HAd-HA-NP alone or in combination with 1 × 107 pfu of HAd-Mbd2. Control animals received either 1 × 108 P pfu of HAd-ΔE1E3 or 1 × 108 pfu of HAd-ΔE1E3+ 1 × 107 pfu of HAd-Mbd2. Serum samples were collected on Days 7 and 14 after immunization, and the influenza-specific antibody response was determined using HI and VN assays. There was a dose dependent increase in HI and VN titers at Day 7 post-immunization. These titers were slightly higher in the HAd-HA-NP+HAd-Mbd2 groups compared to the HAd-HA-NP groups although the differences were not statistically significant (Figs. 1a & 1b). However, at Day 14 post-vaccination, considerably higher levels of both HI and VN titers were observed in all vaccinated groups compared to the control group (Figs. 1a & 1b). At Day 14 post-immunization, mice immunized with either 2 × 107 pfu or 1 × 108 pfu of HAd-HA-NP+HAd-Mbd2 resulted in a 2–3 fold increase of HI antibody titers compared to those immunized with only HAd-HA-NP (Fig 1a). However, the HI titers in the group receiving the highest dose (5 × 108 pfu) of HAd-HA-NP+HAd-Mbd2 vaccine were slightly higher compared to those vaccinated with the same dose of HAd-HA-NP. Similar results of enhancement of humoral immune response in the presence of Mbd2 were obtained when the serum samples were analyzed for the VN antibody titers (Fig 1b). Overall, these results indicate that Mbd2 enhances the humoral immune responses induced by the HAd-HA-NP vaccine and that the effect was noticeable at a wide range of the vaccine doses tested.
Figure 1. Effect of Mouse β-defensin 2 (Mbd2) on the humoral immune responses induced by HAd-HA-NP.
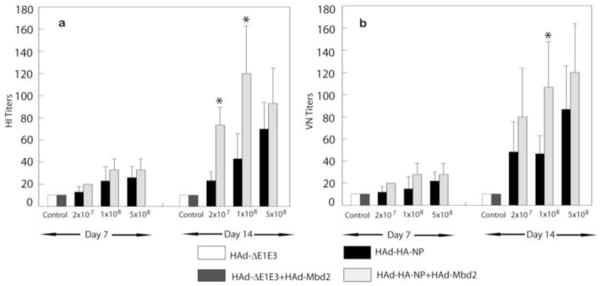
Groups of mice (N=11) were intramuscularly vaccinated with 2 × 107, 1 × 108, or 5 × 108 plaque forming units (pfu) of HAd-HA-NP with or without 1 × 107 pfu of HAd-Mbd2. Animals that similarly received 1 × 108 pfu of the HAd-ΔE1E3 or 1 × 108 pfu of HAd-ΔE1E3 + 1 × 107 pfu of HAd-Mbd2 served as controls. At Days 7 or 14 post-immunization, serum samples were obtained and analyzed for hemagglutination inhibition (HI) (1a) and virus neutralizing (VN) antibody titers (1b) by HI and microneutralization assays, respectively. The data represent mean antibody titers ± standard deviation (SD) from six animals per group. *, P≤0.05 compared to mice vaccinated with a similar dose of HAd-HA-NP.
3.2. Effect of Mbd2 on cellular immune responses induced by HAd-HA-NP
To assess the ability of MBd2 to enhance cellular immune responses induced by a H5N1 vaccine, mice were immunized either with HAd-HA-NP or HAd-HA-NP+HAd-Mbd2. On Days 7 and 14 post-vaccination, six mice from each group were euthanized, and the splenoctyes were analyzed for the HA- or NP-specific cell mediated immune (CMI) response using an interferon-gamma (IFN-γ)-specific ELISpot assay. Significantly higher (P<0.001) numbers of both HA-518 and NP-147-specific IFN-γ secreting CD8+ T cells were detected in the spleen cells of all HAd-HA-NP vaccinated mice (with or without HAd-Mbd2) compared to the control groups (HAdΔE1E3 or HAdΔE1E3+HAd-Mbd2) both at Days 7 and 14 post-vaccination (Fig 2). Co-administration of HAd-HA-NP with HAd-Mbd2 (HAd-HA-NP+ HAd-Mbd2) significantly (P≤0.001) enhanced the number of both HA-518 and NP-147-specific IFN-γ secreting CD8+ T cells compared to the HAd-HA-NP immunized group at both Day 7 and Day 14 post-vaccination. The adjuvant effect of Mbd2 was clearly visible at all doses of the HAd-HA-NP vaccine. Surprisingly, mice vaccinated with 2 × 107 pfu of HAd-HA-NP+ HAd-Mbd2 had a significantly higher number of HA and NP-specific IFN-γ secreting CD8 T cells both at Days 7 and 14 post-vaccination even compared to the group receiving the higher dose of 5 × 108 pfu of HAd-HA-NP without HAd-Mbd2 (HAd-HA-NP). Similar results were obtained when pooled cells from inguinal lymph nodes of vaccinated mice were analyzed for the presence of HA-518 and NP-147-specific IFN-γ secreting CD8+ T cells at Day 7 post-vaccination (data not shown).
Figure 2. Effect of Mouse β-defensin 2 (Mbd2) on the frequency of HA-518 and NP-147 epitope-specific IFN-γ secreting CD8+ T cellsin the spleens of HAd-HA-NP immunized mice.
Groups of mice (N=11) were intramuscularly vaccinated with 2 × 107, 1 × 108, or 5 × 108 plaque forming units (pfu) of HAd-HA-NP with or without 1 × 107 pfu of HAd-Mbd2. Animals that similarly received 1 × 108 pfu of the HAd-ΔE1E3 or 1 × 108 pfu of HAd-ΔE1E3 + 1 × 107 pfu of HAd-Mbd2 served as controls. At Days 7 or 14 post-immunization, six animals/group were euthanized, and the spleens were collected. Half million splenocytes from immunized mice were cultured in the presence of the HA-518 or NP-147 peptides on an anti-IFN-γ antibody-coated 96-well filter plates and developed according to an ELISpot protocol. The ELISpot plates were read using a Bioreader 5000 (BIOSYS, Miami, FL). The data represent mean ± standard deviation (SD) from six animals per group. *, P≤0.05 compared to mice vaccinated with a similar dose of HAd-HA-NP.
3.3. Co-administration of HAd-HA-NP and HAd-Mbd2 confers rapid protection against a H5N1 virus challenge
In order to determine whether Mbd2-mediated enhancement of humoral and cell-mediated immune responses to the HAd-HA-NP vaccine would result in early protection against a H5N1 virus challenge, BALB/c mice (5 animals/group) were i.m. immunized with 2 × 107, 1 × 108, or 5 × 108 pfu of HAd-HA-NP alone (HAd-HA-NP) or in combination with 1 × 107 pfu of HAd-Mbd2 (HAd-HA-NP+HAd-Mbd2). Control animals received 1 × 108 pfu of the empty vector (HAd-ΔE1E3) or 1 × 108 pfu of HAd-ΔE1E3+ 1 × 107 pfu of HAd-Mbd2 (HAd-ΔE1E3+ HAd-Mbd2). At Days 7 and 14 post-immunization, mice were challenged intranasally (i.n.) with 100 MID50 of VNH5N1-PR8/CDC-RG. The lung viral titers at Day 3 post-challenge were determined to assess the protective efficacy of the vaccine. As expected, significant levels of virus titers were observed in mice inoculated with HAd-ΔE1E3 or HAd-ΔE1E3+ HAd-Mbd2 and challenged on Days 7 or 14 post-inoculation (Table 1). Mice immunized either with 2 × 107 or 1 × 108 pfu of HAd-HA-NP had a reduced but significant viral load in the lungs when animals were challenged at Day 7 post-vaccination. However, all mice vaccinated either with 2 × 107 or 1 × 108 pfu of HAd-HA-NP+HAd-Mbd2 had lung virus titers below the level of detection (defined as < 0.5 log10 50% TCID50) when animals were challenged at Day 7 following vaccination. Surprisingly, mice vaccinated with a higher dose (5 × 108 pfu) of either vaccine also had lung virus titers below the detection level indicating that a high dose of an Ad-based vaccine may also provide rapid protection against a homologous virus challenge. The lung virus titers were also below the level of detection in the groups which received 2 × 107, 1 × 108, or 5 × 108 pfu of both HAd-HA-NP and HAd-HA-NP+HAd-Mbd2 and were challenged on Day 14 post-immunization. Overall, these results indicate that co-administration of HAd-HA-NP and HAd-Mbd2 provided rapid protection even at a low vaccine dose.
Table 1.
Protection against H5N1 virus challenge.
| Group | Vector/s | Vector dose | Challenge on Day Post-inoculation | Lung virus titer (mean±SD) |
|---|---|---|---|---|
| 1 | HAd-HA-NP | 2 × 107 | Day 7 | 2.53±0.33* |
| 2 | HAd-HA-NP+HAd-Mbd2 | 2 × 107 + 1 × 107 | Day 7 | ≤0.50 |
| 3 | HAd-HA-NP | 1 × 108 | Day 7 | 2.25± 0.16* |
| 4 | HAd-HA-NP + HAd-Mbd2 | 1 × 108 + 1 ×107 | Day 7 | ≤0.50 |
| 5 | HAd-HA-NP | 5 × 108 | Day 7 | ≤0.50 |
| 6 | HAd-HA-NP+ HAd-Mbd2 | 5 × 108 + 1 ×107 | Day 7 | ≤0.50 |
| 7 | HAdΔE1E3 + HA-Mbd2 | 1 × 108 + 1 ×107 | Day 7 | 4.39±0.30 |
| 8 | HAdΔE1E3 | 1 × 108 | Day 7 | 4.55±0.46 |
| 9 | HAd-HA-NP | 2 × 107 | Day 14 | ≤0.50 |
| 10 | HAd-HA-NP + HAd-Mbd2 | 2 ×107 + 1 ×107 | Day 14 | ≤0.50 |
| 11 | HAd-HA-NP | 1 × 108 | Day 14 | ≤0.50 |
| 12 | HAd-HA-NP + HAd-Mbd2 | 1 × 108 + 1 ×107 | Day 14 | ≤0.50 |
| 13 | HAd-HA-NP | 5 × 108 | Day 14 | ≤0.50 |
| 14 | HAd-HA-NP+HAd-Mbd2 | 5 × 108 + 1 ×107 | Day 14 | ≤0.50 |
| 15 | HAdΔE1E3 + HA-Mbd2 | 1 × 108 + 1 ×107 | Day 14 | 4.40±0.28 |
| 16 | HAdΔE1E3 | 1 × 108 | Day 14 | 4.32±0.07 |
Mouse β-defensin 2 (Mbd2) enhances the protective efficacy of HAd-HA-NP vaccine against H5N1 influenza virus challenge. Groups of mice (N=10) were intramuscularly vaccinated with 2 × 107, 1 × 108, or 5 × 108 plaque forming units (pfu) of HAd-HA-NP with or without 1 × 107 pfu of HAd-Mbd2. Animals that similarly received 1 × 108 pfu of the HAd-ΔE1E3 or 1 × 108 pfu of HAd-ΔE1E3 + 1 × 107 pfu of HAd-Mbd2 served as controls. At Days 7 or 14 post-immunization, five animals/group were challenged intranasally with a 100 50% mouse infectious dose (MID50) of a reverse genetics derived A/Puerto Rico/8/1934(H1N1) [PR8] virus containing hemagglutinin (HA) and neuraminidase (NA) gene fragments of A/Vietnam/1203/04 (H5N1) [VNH5N1-PR8/CDC-RG]. Day 3 after challenge, the animals were euthanized, and the lung virus titers were determined by 50% tissue culture infective dose (TCID50) analysis on MDCK cells to evaluate the protective efficacy of the vaccine. The data represent the mean virus titers ± standard deviation (SD) from five animals per group. The detection limit of the lung viral titer was 0.5 Log10 TCID50/ml (indicated as <0.50).
P≤0.05 compared to mice vaccinated with a similar dose of HAd-ΔE1E3.
4. Discussion
Recurrent outbreaks of HPAI H5N1 viruses in domestic poultry accompanied by their occasional transmission to humans with varying case fatalities have highlighted the public health threat posed by these viruses. It is widely believed that an influenza pandemic could result if the HPAI H5N1 viruses were to gain the ability for efficient and sustained human-to-human transmission (Imai et al., 2012). New vaccine approaches which can induce rapid and robust protective immune responses, preferably after a single low dose of the vaccine, are needed to keep pace with a pandemic virus and also to meet the potential global vaccine demand in a pandemic scenario. This study assessed whether co-administration of a molecular adjuvant, Mbd2, with a vaccine candidate expressing the HA and NP of a H5N1 influenza virus, HAd-HA-NP, could enhance the levels of vaccine-induced immune responses and confer rapid protection against H5N1 virus challenge in a mouse model. Three different doses of the HAd-HA-NP vaccine (2 × 107, 1 × 108 and 5 × 108 pfu) were tested to monitor detectable levels of enhancement in immune responses and protection due to a HAd-Mbd2-induced adjuvant effect. Vaccination of mice with a combination of HAd-Mbd2 and HAd-HA-NP resulted in significantly enhanced antigen-specific immune responses compared to the groups which were vaccinated with only HAd-HA-NP. Furthermore, complete protection against a H5N1 virus challenge was observed at Day 7 post-vaccination in mice co-administered with low doses (2 × 107 or 1 × 108 pfu) of the vaccine HAd-HA-NP + HAd-Mbd2. Co-administration of gene-based vaccines with molecular adjuvants are known to chemo-attract and stimulate antigen presenting cells (APC) which have been shown to augment antigen-specific immune responses and mediate better protection following virus challenges in animal models (Bayer et al., 2011; Biragyn et al., 1999; Biragyn et al., 2001; Biragyn et al., 2002a; Hoffmann et al., 2007; Lietz et al., 2012; Ruffini et al., 2004)
Mbd2 has previously been demonstrated to enhance antigen-specific immune responses by recruiting and activating functional APC at the site of inoculation (Biragyn et al., 1999; Biragyn et al., 2002a; Ruffini et al., 2004). A DNA vaccine containing the Mbd2 gene linked to the human immunodeficiency virus (HIV) envelope (ENV) gene delivered into the skin by a gene gun induced enhanced ENV-specific mucosal as well as systemic immune responses in mice (Biragyn et al., 2002a). Furthermore, several recent studies have demonstrated the potential of Mbd2 to augment antitumor immune responses and mediate tumor regression when fused with tumor antigens or over expressed in irradiated tumor cells (Lapteva et al., 2009; Mei et al., 2012; Park et al., 2011). Consistent with earlier findings, mice co-immunized with HAd-HA-NP+HAd-Mbd2 had significantly higher levels of both HA-518- and NP-147-epitope–specific CD8+T cells in the spleen and regional lymph nodes compared with those vaccinated with HAd-HA-NP alone. Although, the adjuvant effect of Mbd2 was evident against both HA and NP proteins at all the doses of the vaccine tested, cellular immune responses induced against NP were considerably higher compared to HA. Significantly high levels of antigen-specific CD8+ T cells were also observed in all the groups receiving HAd-HA-NP without immunostimulation compared to the control groups. The dose-dependent increases in cellular immune responses with the HAd vector-based vaccine was not observed mainly because this effect peaks at a lower vaccine dose unlike to the humoral immune responses. The CMI responses induced against influenza virus internal proteins such as NP play an important role in the virus clearance from the lungs (Tamura et al., 1996) and, therefore, are critical for recovery especially in an influenza pandemic situation (Hoelscher et al., 2008; Ulmer et al., 1998; Yewdell et al., 1985).
Although CMI responses induced following vaccination play an important role in virus clearance, humoral immune responses induced against HA are essential for the prevention of influenza virus infection. Hence, vaccination approaches which can induce both rapid and robust humoral and CMI responses, preferably after a single administration, are important especially in a pandemic scenario to contain the rapidly spreading virus. In this study, a single administration of HAd-HA-NP in combination with HAd-Mbd2 elicited significantly higher levels of both HI and VN antibody titers compared to vaccination with HAd-HA-NP alone. Although the adjuvant effect of Mbd2 on humoral immune responses induced by the HAd-HA-NP vaccine was clearly visible at all three doses of the vaccine, it was statistically significant only at the lower vaccine doses (2 × 107 or 1 × 108 pfu). These results are in contrast with some previous studies of Mbd2 which demonstrated potent cell-mediated responses but only modest humoral responses of vaccines following combined vaccination with Mbd2 (Biragyn et al., 1999; Biragyn et al., 2002a; Biragyn et al., 2002b). The discrepancy between previous studies and this study could be due to the differences in the delivery method or the vaccine model.
In a pandemic scenario, vaccine approaches conferring early protection are needed to contain a rapidly spreading influenza virus. Although currently licensed H5N1 influenza vaccines have been shown to be effective in inducing protection in animal models when co-administered with suitable adjuvants, the time necessary to induce protective immunity may be a big limitation in a pandemic scenario. In this study, co-administration of HAd-HA-NP and HAd-Mbd2 conferred complete protection against H5N1 virus challenge at Day 7 post-vaccination. Surprisingly, rapid viral clearance was still observed despite the presence of low antibody titers in the groups receiving either 2 × 107or 1 × 108 pfu of HAd-HA-NP in combination with HAd-Mbd2. Although the immunological basis for this early protection is not known, it could have been due to the induction of low levels of antibodies in combination with the robust cellular immune responses against NP and HA in the presence of Mbd2. Since a single inoculation with a low vaccine dose (2 × 107 pfu) with HAd-Mbd2 provided effective protection at Day 7 post-vaccination, it seems that Mbd2 could serve as a molecular adjuvant for dose-sparing. Other studies have demonstrated a direct inhibitory effect of Mbd2 on influenza virus replication (Gong et al., 2010; Jiang et al., 2009). However, in this study, no significant reduction in challenge viral titers in mice inoculated with HAd-Mbd2 was observed.
5. Conclusion
In summary, these results highlight the potential of Mbd2 as a genetic adjuvant for enhancing rapid and robust immune responses to confer protection as a tool for influenza pandemic preparedness.
HIGHLIGHTS.
Mbd2 enhances Ad vaccine-induced immune response early post vaccination.
Mbd2 immunostimulation confers protection against H5N1 by Day 7 post vaccination.
This vaccine strategy has implications for pandemic influenza preparedness.
Acknowledgments
This work was supported by Public Health Service grant AI059374 from the National Institute of Allergy and Infectious Diseases. We are thankful to A. Pandey and N. Singh for constructing some of the vectors and to J. Kovach for her excellent secretarial assistance.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bayer W, et al. Improved vaccine protection against retrovirus infection after co-administration of adenoviral vectors encoding viral antigens and type I interferon subtypes. Retrovirology. 2011;8:75–90. doi: 10.1186/1742-4690-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biragyn A, et al. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. 2002a;100:1153–1159. doi: 10.1182/blood-2002-01-0086. [DOI] [PubMed] [Google Scholar]
- Biragyn A, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002b;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Biragyn A, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- Biragyn A, et al. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253–258. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- Gong T, et al. Recombinant mouse beta-defensin 2 inhibits infection by influenza A virus by blocking its entry. Arch Virol. 2010;155:491–498. doi: 10.1007/s00705-010-0608-1. [DOI] [PubMed] [Google Scholar]
- Hoelscher MA, et al. New pre-pandemic influenza vaccines: An egg- and adjuvant-independent human adenoviral vector strategy induces long-lasting protective immune responses in mice. Clin Pharmacol Ther. 2007;82:665–671. doi: 10.1038/sj.clpt.6100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelscher MA, et al. A broadly-protective vaccine against globally dispersed clade 1 and clade 2 H5N1 viruses. J Inf Dis. 2008;197:1185–1188. doi: 10.1086/529522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, et al. In situ tumor vaccination with adenovirus vectors encoding measles virus fusogenic membrane proteins and cytokines. World J Gastroenterol. 2007;13:3063–3070. doi: 10.3748/wjg.v13.i22.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486:420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, et al. Expression of mouse beta-defensin-3 in MDCK cells and its anti-influenza-virus activity. Arch Virol. 2009;154:639–647. doi: 10.1007/s00705-009-0352-6. [DOI] [PubMed] [Google Scholar]
- Katz JM, et al. The public health impact of avian influenza viruses. Poult Sci. 2009;88:872–879. doi: 10.3382/ps.2008-00465. [DOI] [PubMed] [Google Scholar]
- Lapteva N, et al. Attraction and activation of dendritic cells at the site of tumor elicits potent antitumor immunity. Mol Ther. 2009;17:1626–1636. doi: 10.1038/mt.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietz R, et al. Codelivery of the chemokine CCL3 by an adenovirus-based vaccine improves protection from retrovirus infection. J Virol. 2012;86:1706–1716. doi: 10.1128/JVI.06244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei HF, et al. beta-defensin 2 as an adjuvant promotes anti-melanoma immune responses and inhibits the growth of implanted murine melanoma in vivo. PLoS One. 2012;7:e31328. doi: 10.1371/journal.pone.0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison GM, et al. A novel mouse beta defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett. 1999;442:112–116. doi: 10.1016/s0014-5793(98)01630-5. [DOI] [PubMed] [Google Scholar]
- Noblitt LW, et al. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757–766. doi: 10.1038/sj.cgt.7700761. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, et al. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003;62(Suppl 2):ii17–ii21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A, et al. Egg-independent vaccine strategies for highly pathogenic H5N1 influenza viruses. Human Vaccine. 2010;6:178–188. doi: 10.4161/hv.6.2.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A, et al. Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine. PLoS ONE. 2012;7:e33428–e33428. doi: 10.1371/journal.pone.0033428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, et al. Induction of TLR4-dependent CD8+ T cell immunity by murine beta-defensin2 fusion protein vaccines. Vaccine. 2011;29:3476–3482. doi: 10.1016/j.vaccine.2011.02.061. [DOI] [PubMed] [Google Scholar]
- Parrish CR, Kawaoka Y. The origins of new pandemic viruses: the acquisition of new host ranges by canine parvovirus and influenza A viruses. Annu Rev Microbiol. 2005;59:553–586. doi: 10.1146/annurev.micro.59.030804.121059. [DOI] [PubMed] [Google Scholar]
- Ruffini PA, et al. Genetic fusions with viral chemokines target delivery of nonimmunogenic antigen to trigger antitumor immunity independent of chemotaxis. J Leukoc Biol. 2004;76:77–85. doi: 10.1189/jlb.1003481. [DOI] [PubMed] [Google Scholar]
- Russell CA, et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science. 2012;336:1541–1547. doi: 10.1126/science.1222526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambhara S, et al. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell Immunol. 2001;211:143–153. doi: 10.1006/cimm.2001.1835. [DOI] [PubMed] [Google Scholar]
- Singh N, et al. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol Ther. 2008;16:965–971. doi: 10.1038/mt.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K, et al. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology. 2003;305:192–200. doi: 10.1006/viro.2002.1742. [DOI] [PubMed] [Google Scholar]
- Tamura S, et al. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J Immunol. 1996;156:3892–3900. [PubMed] [Google Scholar]
- Ulmer JB, et al. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol. 1998;72:5648–5653. doi: 10.1128/jvi.72.7.5648-5653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Olphen AL, Mittal SK. Development and characterization of bovine x human hybrid cell lines that efficiently support the replication of both wild-type bovine and human adenoviruses and those with E1 deleted. J Virol. 2002;76:5882–5892. doi: 10.1128/JVI.76.12.5882-5892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula SV, Mittal SK. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin Biol Ther. 2010;10:1469–1487. doi: 10.1517/14712598.2010.519332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula SV, et al. Adenoviral vector expressing murine beta-defensin 2 enhances immunogenicity of an adenoviral vector based H5N1 influenza vaccine in aged mice. Virus Res. 2013 doi: 10.1016/j.virusres.2013.07.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) 2013 Available at http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/
- Yang D, et al. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- Yang D, et al. Defensin participation in innate and adaptive immunity. Curr Pharm Des. 2007;13:3131–3139. doi: 10.2174/138161207782110453. [DOI] [PubMed] [Google Scholar]
- Yewdell JW, et al. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci US A. 1985;82:1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]


