Abstract
Background/Aims
Clinical guidelines recommend a diet low in sodium and high in potassium to reduce blood pressure and cardiovascular events. Little is known about the relationship between dietary sodium and potassium intake and chronic kidney disease (CKD).
Methods
13,917 participants from the National Health and Nutrition Examination Survey (2001–2006) were examined. Sodium and potassium intake were calculated from 24-hour recall and evaluated in quartiles. CKD was defined as eGFR <60 mL/min, or eGFR ≥ 60mL/min with albuminuria (>30mg/g creatinine).
Results
The mean (SE) age and eGFR of participants was 45.0 ± 0.4 years and 88.0 ± 0.60 ml/min/1.73m2, respectively. 2333 (14.2%) had CKD: 1146 (7.3%) had an eGFR < 60 ml/min/1.73m2 and 1514 (8.4%) had an eGFR ≥ 60 ml/min/1.73 m2 and albuminuria. After adjustment for age, sex, race, body mass index, diabetes, hypertension, cardiovascular disease and congestive heart failure subjects in the highest quartile of sodium intake had a lower odds of CKD compared to subjects in the lowest quartile (adjusted OR 0.79, 95% CI, 0.66 to 0.96; p<0.016). Compared to the highest quartile, participants in the lowest quartile of potassium intake had a 44% increased odds of CKD (adjusted OR 1.44, 95% CI 1.16–1.79, p=0.0011).
Conclusions
Higher intake of sodium and potassium is associated with lower odds of CKD among US adults. These results should be corroborated through longitudinal studies and clinical trials designed specifically to examine the effects of dietary sodium and potassium intake on kidney disease and its progression.
Keywords: Chronic Kidney Disease, Dietary sodium intake, Dietary potassium intake
INTRODUCTION
Chronic Kidney Disease (CKD) is an epidemic and a worldwide public health problem. The prevalence of CKD is the US alone has increased from 10% in 1988–94 to 13.1% in 1999–2004 [1]. The increasing incidence and prevalence can be attributed to changing demographics of the general population coupled with earlier detection of CKD. However, increases in the prevalence of obesity, diabetes [2–4] and hypertension [5, 6], known traditional cardiovascular risk factors, accounts for the majority of increase in the prevalence of CKD. The presence of kidney disease is associated with higher morbidity and mortality and increased health care utilization. Control of blood pressure, strict glycemic control and blocking of the renin angiotensin aldosterone axis are some of the proven strategies in preventing and slowing the progression of CKD [7–11]. However, in most cases, even with adoption of these strategies, the incidence and prevalence of CKD continues to rise. Thus the strategy of adopting traditional risk factor modifications alone is not sufficient. This emphasizes the need for different therapeutic targets, such as dietary sodium and potassium intake, to prevent CKD and slow its progression.
Extrapolations from observational studies and intervention trials suggest that population-wide moderation of sodium intake and increase of potassium intake might reduce cardiovascular events and prevent the onset of high blood pressure. The National Heart, Lung, and Blood Institute (NHLBI) promote the DASH (Dietary Approaches to Stop Hypertension) diet for the prevention and of control hypertension. DASH diet has targets of 2300 and 4700 mg for sodium and potassium, respectively and is rich in fruits, vegetables, and low-fat dairy foods with reduced amounts of saturated fat, total fat, and cholesterol. Blood pressure lowering effects of DASH diet is seen in subjects with high normal BP as well as with established hypertension [12]. Because risk factors for cardiovascular disease and CKD often overlap, it is tempting to speculate that lower sodium and higher potassium intake may also be protective against kidney disease and kidney disease progression. However, the relationship between dietary sodium and potassium intake and chronic kidney disease (CKD) has not been examined in the general US population. We performed a cross-sectional study using the National Health and Nutrition Examination Survey (2001–2006) and included 13,917 participants to test the hypothesis that high dietary sodium intake and low dietary potassium intake is associated with an increased odds of CKD in US adults.
METHODS
Study population
The National Health and Nutrition Examination Survey (NHANES) is a population-based survey designed to collect information on the health and nutrition of adults and children in the US, and is unique in that it combines interviews and physical examinations. A stratified, multistage sampling design was used, with over-sampling of African Americans, Hispanics and persons over the age of 60 in order to produce reliable statistics. In this analysis, we used data from 31,507 participants from NHANES 2001–2006. Data was weighted using the dietary weights as described in the statistical analysis for NHANES data. Participants were excluded if they did not have positive weights for the analysis (n=3,243), if they lacked data on sodium intake, potassium intake, and urinary albumin excretion or were missing data for the calculation of eGFR by the abbreviated Modification of Diet in Renal Disease formula (MDRD) [13] (n=14397). The final sample used in this study included 13,917 adult participants.
Primary Predictor and Outcome
The primary predictor or independent variables were dietary sodium and potassium intake. Dietary sodium and potassium intake were specifically calculated from 24-hour dietary recalls that were requested from all NHANES examinees. All dietary interviews were conducted at the mobile examination centers (MEC) using in-person mode of interview. Data was collected on individual foods and total nutrient intakes twice by trained dietary interviewers fluent in Spanish and English. Each MEC examination room contained a standard set of measuring guides. These measuring guides were used to help the participant estimate portion sizes. Information on added salt (frequency and type) were obtained apart from detailed descriptions about food reported (i.e. type, form, brand name, amount consumed) and nutrients from each food.
The primary outcome of interest was CKD defined as estimated glomerular filtration rate (eGFR) < 60ml/min/1.73m2 or eGFR ≥ 60ml/min/1.73m2 with albuminuria. Estimated GFR was calculated using the 4-variable Modification of Diet and Renal Disease (MDRD) equation [13]. Albuminuria was assessed by the urinary albumin to creatinine ratio and was defined as a ratio of > 30 mg/g creatinine. Subjects with a GFR=60 ml/min or greater were only classified as CKD if they had albuminuria. The eGFR <60 ml/min category included all subjects with an eGFR <60 ml/min regardless of whether they had albuminuria.
Baseline Demographic and Clinical Data
Questionnaire data included self-reported age and gender. Race/ethnicity was broken into four categories: Non-Hispanic white, Non-Hispanic black, Mexican-American and other. Cardiovascular disease (CVD) and congestive heart failure (CHF) were self-reported by participants. CHF was diagnosed if the participant reported ever being told by a physician they had CHF. CVD was diagnosed if participants reported being told by a doctor they have coronary heart disease, angina, a heart attack or stroke. Hypertension was diagnosed if the participant was taking antihypertensive medications, reported being told by a physician that they have high blood pressure, or the average of three blood pressure readings was a systolic blood pressure > 140 mmHg or a diastolic blood pressure >90 mmHg. Systolic and diastolic blood pressure was measured in a standard fashion. Participants were defined as having diabetes when they reported taking medication for diabetes, had a fasting glucose concentration ≥ 126 mg/dL (to convert to SI units multiply by 0.05551) or reported being told by a physician they have diabetes. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
Statistical Analysis
For the purpose of this analysis, dietary sodium and potassium intake was examined in quartiles. Quartiles of sodium intake 1 through 4 were ≤ 2116, 2117–3061, 3062–4267, > 4267 mg/day, respectively and quartiles of potassium intake 1 through 4 were ≤ 1737, 1738–2455, 2456–3341, > 3342 mg/day, respectively. For this analysis, the lowest quartile was used as the reference group for sodium intake and the highest quartile was used as the reference group for potassium intake. Demographic and clinical data were compared across quartiles of dietary sodium and potassium intake through the use of chi-square test for categorical data and ANOVA for continuous variables. Multivariate logistic regression models were used to examine the association between sodium and potassium intake and CKD. We considered a priori variables that may be associated with dietary sodium and potassium intake and CKD as potential confounders in multivariate models. To permit the most useful interpretation of our results in addition to unadjusted models, we used the following concentrations of adjustment. Model 1 included age, gender, race/ethnicity, BMI, diabetes and hypertension status. Model 2 included covariates in Model 1 plus CHF and CVD. We also examined the relationship of combinations of sodium and potassium intake with CKD. Median intake of sodium and potassium were used to determine “high” and “low” intake. Four groups of sodium and potassium intake were defined as follows: high sodium/high potassium intake; high sodium/low potassium intake; low sodium/high potassium intake and low sodium/low potassium intake. Two-tailed values of P<0.05 were considered statistically significant. All statistical analyses were performed with SAS software, version 9.13 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
The mean (SE) age and estimated glomerular filtration rate (eGFR) of the participants was 45.0 ± 0.4 years and 88.0 ± 0.60 ml/min/1.73m2, respectively. The mean (SE) dietary sodium and potassium intake were 3520 ± 26 mg/day and 2760 ± 22 mg/day, respectively (Figure 1). In this cohort, 2333 (14.2%) had CKD: 1146 (7.3%) had an eGFR < 60 ml/min/1.73m2 and 1514 (8.4%) had albuminuria ≥ 30mg/g. Among those with albuminuria, 1187 had a GFR ≥ 60 ml/min. Of the patients with CKD, the mean (SE) dietary sodium and potassium intake were 3053 ± 40 mg/day and 2495 ± 34 mg/day, respectively (Figure 1). Baseline characteristics of the participants across dietary sodium and potassium intake are shown in Tables 1 and 2, respectively. Participants in the highest quartile of sodium intake were more likely to be younger, to be male, to have a lower prevalence of hypertension and albuminuria, lower systolic blood pressure and to have higher BMI and eGFR than participants in the lower quartiles of sodium intake (Table 1). Participants in the highest quartile of potassium intake were more likely to be younger, to be male, to have a lower prevalence of hypertension, diabetes and albuminuria, to have lower BMI, and lower systolic blood pressure than participants in the lower quartiles of potassium intake (Table 2).
Figure 1.
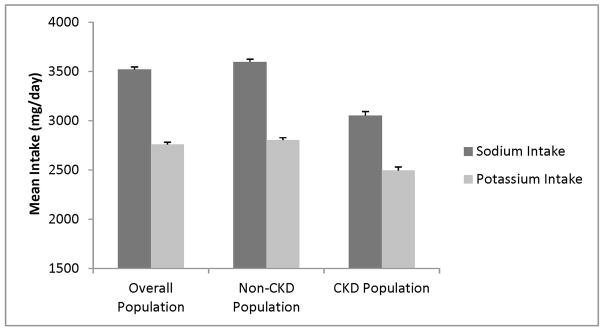
Mean Dietary Sodium and Potassium Intake in Study Participants.
Table 1.
Baseline Characteristics of Study Participants across Quartiles of Dietary Sodium Intake
| Characteristics | Sodium Intake (mg/day) | P-Value | |||
|---|---|---|---|---|---|
| ≤ 2116 | 2117–3061 | 3062–4267 | > 4267 | ||
|
| |||||
| Age (years) | 49 ± 0.5** | 47 ± 0.6** | 45 ± 0.4** | 41 ± 0.5 | < 0.0001 |
|
| |||||
| Sex (% Male) | 30.9 | 38.6 | 48.7 | 72.3 | < 0.0001 |
|
| |||||
| Race (%) | |||||
| Non-Hispanic Black | 13.5 | 11.9 | 8.8 | 10.3 | < 0.0001 |
| Non-Hispanic White | 68.6 | 71.3 | 74.5 | 75.0 | |
| Mexican-American | 8.6 | 7.8 | 8.0 | 7.2 | |
|
| |||||
| Diabetes (%) | 7.9 | 8.0 | 7.0 | 6.1 | 0.064 |
|
| |||||
| Hypertension (%) | 33.0 | 30.5 | 25.4 | 25.3 | < 0.0001 |
|
| |||||
| Albuminuria (>30mg/g) (%) | 10.6 | 9.5 | 6.8 | 7.3 | < 0.0001 |
|
| |||||
| BMI (kg/m2) | 28.0 ± 0.2** | 28.0 ± 0.2** | 28.2 ± 0.2 | 28.6 ± 0.2 | 0.025 |
|
| |||||
| eGFR (ml/min/1.73m2) | 85.0 ± 0.7** | 86.5 ± 0.8** | 89.0 ± 0.6* | 90.7 ± 0.7 | <0.0001 |
|
| |||||
| SBP (mmHg) | 123.2 ± 0.6** | 122.1 ± 0.5* | 121.1 ± 0.6 | 120.3 ± 0.5 | 0.002 |
|
| |||||
| DBP (mmHg) | 71.0 ± 0.4 | 71.3 ± 0.5 | 71.3 ± 0.3 | 71.6 ± 0.4 | 0.74 |
Values are expressed as mean ± standard error unless otherwise specified. BMI= body mass index; eGFR= estimated glomerular filtration rate; SBP= systolic blood pressure; DBP= diastolic blood pressure
P < 0.05 Compared to the 4th Quartile,
P < 0.001 Compared to the 4th Quartile.
Table 2.
Baseline Characteristics of Study Participants across Quartiles of Dietary Potassium Intake
| Characteristics | Potassium Intake (mg/day) | P-Value | |||
|---|---|---|---|---|---|
| ≤ 1737 | 1738–2455 | 2456–3341 | > 3342 | ||
|
| |||||
| Age (years) | 44 ± 0.50 | 47 ± 0.5** | 47 ± 0.5** | 44 ± 0.5 | < 0.0001 |
|
| |||||
| Sex (% Male) | 29.5 | 41.7 | 50.8 | 68.5 | < 0.0001 |
|
| |||||
| Race (%) | |||||
| Non-Hispanic Black | 17.7 | 12.2 | 8.0 | 7.3 | < 0.0001 |
| Non-Hispanic White | 65.3 | 69.3 | 76.7 | 77.3 | |
| Mexican-American | 7.7 | 8.3 | 7.3 | 8.2 | |
|
| |||||
| Diabetes (%) | 6.9 | 8.7 | 7.2 | 6.1 | 0.002 |
|
| |||||
| Hypertension (%) | 30.3 | 31.0 | 28.3 | 24.3 | < 0.0001 |
|
| |||||
| Albuminuria (>30mg/g) (%) | 10.1 | 9.7 | 7.3 | 7.0 | < 0.0001 |
|
| |||||
| BMI (kg/m2) | 28.6 ± 0.2 | 28.3 ± 0.2 | 28.2 ± 0.3 | 27.8 ± 0.2* | 0.006 |
|
| |||||
| eGFR (ml/min/1.73m2) | 89.1 ± 0.7 | 87.4 ± 0.8 | 87.2 ± 0.7 | 88.4 ± 0.7 | 0.056 |
|
| |||||
| SBP (mmHg) | 121.6 ± 0.6 | 122.9 ± 0.6 b | 122.0 ± 0.5 a | 120.3 ± 0.4 | 0.007 |
|
| |||||
| DBP (mmHg) | 70.8 ± 0.4 | 71.2 ± 0.4 | 71.6 ± 0.4 | 71.6 ± 0.3 | 0.22 |
Values are expressed as mean ± standard error unless otherwise specified. BMI= body mass index; eGFR= estimated glomerular filtration rate; SBP= systolic blood pressure; DBP= diastolic blood pressure
P < 0.05 Compared to the 1st Quartile,
P < 0.001 Compared to the 1st Quartile.
P < 0.05 Compared to the 4th Quartile,
P < 0.001 Compared to the 4th Quartile.
Dietary Sodium and Potassium Intake and CKD
The logistic regression analysis examining the relationship between sodium intake and CKD is shown in Table 3. In unadjusted analysis, higher sodium intake was associated with decreased odds of CKD. Subjects with sodium intake in the highest quartile had a 54% decreased odds of CKD compared to subjects in the lowest quartile. After adjusting for age, sex, race, BMI, diabetes, hypertension, CVD and CHF higher sodium intake was still associated with decreased odds of CKD. Participants with sodium intake in the second, third and fourth quartiles had 15%, 32% and 21% lower odds of CKD compared to subjects with sodium intake in the lowest quartile. The odds of CKD did not change when subjects with CHF were excluded or when subjects with macroalbuminuria (urine albumin to creatinine ratio >300mg/g) were excluded (data not shown). The relationship between dietary potassium intake and CKD is shown in Table 4. In unadjusted analysis, participants in the first and second quartiles of potassium intake had an increased odds of CKD compared to participants in the highest quartile. After adjustment for age, sex, race, BMI, diabetes, hypertension, CVD and CHF subjects in the lowest quartile of potassium intake still had a 44% increased odds of CKD compared to participants in the highest quartile (adjusted OR 1.44, 95% CI 1.16–1.79, p=0.0011). The second and third quartiles of potassium intake were not associated with increased odds of CKD. The odds of CKD did not change when subjects with CHF or macroalbuminuria were excluded from the analysis (data not shown). Results were unchanged when diuretic use was included in the fully adjusted model (results not shown). When the association of dietary sodium and potassium intake was examined with eGFR <60 ml/min and eGFR ≥60 ml/min with microalbuminuria as separate dependent variables, the association of dietary sodium and potassium intake was only present with eGFR < 60 ml/min (results not shown).
Table 3.
Odds Ratio (95% Confidence Interval) of Chronic Kidney Disease by Quartiles of Dietary Sodium Intake
| Sodium Intake (mg/day) | ≤ 2116 | 2117–3061 | 3062–4267 | > 4267 |
|---|---|---|---|---|
| Unadjusted | 1.00 (REF) | 0.77 (0.66 to 0.90) | 0.52 (0.44 to 0.61) | 0.46 (0.40 to 0.53) |
| Model 1 | 1.00 (REF) | 0.84 (0.71 to 0.99) | 0.67 (0.54 to 0.82) | 0.77 (0.64 to 0.92) |
| Model 2 | 1.00 (REF) | 0.85 (0.71 to 1.02) | 0.68 (0.55 to 0.85) | 0.79 (0.66 to 0.96) |
Model 1: Adjusted for age, sex, race/ethnicity, body mass index, diabetes and hypertension
Model 2: Adjusted for covariates in Model 1 plus congestive heart failure and cardiovascular disease
Table 4.
Odds Ratio (95% Confidence Interval) of Chronic Kidney Disease by Quartiles of Dietary Potassium Intake
| Potassium Intake (mg/day) | ≤ 1737 | 1737–2455 | 2455–3341 | > 3342 |
|---|---|---|---|---|
| Unadjusted | 1.72 (1.39 to 2.12) | 1.59 (1.30 to 1.94) | 1.22 (1.00 to 1.49) | 1.00 (REF) |
| Model 1 | 1.51 (1.20 to 1.88) | 1.21 (0.98 to 1.49) | 1.01 (0.81 to 1.27) | 1.00 (REF) |
| Model 2 | 1.44 (1.16 to 1.79) | 1.19 (0.97 to 1.46) | 1.00 (0.80 to 1.24) | 1.00 (REF) |
Model 1: Adjusted for age, sex, race/ethnicity, body mass index, diabetes and hypertension
Model 2: Adjusted for covariates in Model 1 plus congestive heart failure and cardiovascular disease
We also examined the relationship of combinations of potassium and sodium intake with CKD (Table 5). Median intake of sodium (3061 mg/day) and potassium (2455 mg/day) were used to determine high and low sodium and potassium intake. Regardless of the sodium intake, after multivariate adjustment, subjects with high potassium intake had a decreased odds of CKD compared to subjects with low potassium intake. Subjects with high potassium and high sodium intake had the lowest odds of CKD (OR 0.70, 95% CI 0.59–0.83, p<0.0001), whereas subjects with high potassium and low sodium intake also had decreased odds of CKD (OR 0.77, 95% CI 0.63–0.94, p=0.01). The combination of a high sodium intake with a low potassium intake was not associated with CKD. We also evaluated a serum sodium*HTN and sodium*diabetes interaction term in the final adjusted model for CKD. Similarly, we also examined a serum potassium *HTN and potassium*diabetes interaction for CKD. Results of the association between sodium and potassium with odds of CKD were similar irrespective of HTN or DM status (p for interaction > 0.20 for all).
Table 5.
Odds Ratio (95% Confidence Interval) of Chronic Kidney Disease by Combinations of Dietary Sodium and Potassium Intake
| Sodium and Potassium Intake | Low Sodium/Low Potassium | Low Sodium/High Potassium | High Sodium/Low Potassium | High Sodium/High Potassium |
|---|---|---|---|---|
| Unadjusted | 1.00 (REF) | 0.82 (0.68 to 0.98) | 0.59 (0.47 to 0.74) | 0.50 (0.43 to 0.57) |
| Model 1 | 1.00 (REF) | 0.76 (0.63 to 0.92) | 0.79 (0.60 to 1.04) | 0.68 (0.57 to 0.80) |
| Model 2 | 1.00 (REF) | 0.77 (0.64 to 0.94) | 0.83 (0.61 to 1.06) | 0.70 (0.59 to 0.83) |
Model 1: Adjusted for age, sex, race/ethnicity, body mass index, diabetes and hypertension
Model 2: Adjusted for covariates in Model 1 plus congestive heart failure and cardiovascular disease
DISCUSSION
In this cross-sectional study of 13,917 participants from NHANES 2001–2006, we found higher dietary intakes of both sodium and potassium to be associated with lower odds of CKD. To our knowledge, this is the first study examining the association between dietary sodium and potassium intake and CKD in the general US population.
Experimental data suggests that sodium intake may be an important risk factor for kidney disease. Sodium may be nephrotoxic directly by increasing oxidative stress and indirectly by increasing blood pressure and attenuating the effects of renin-angiotensin-aldosterone system (RAAS) blockers. Several rat studies have shown increased oxidative stress in the renal cortex and vascular beds in response to increased dietary salt intake [14–16]. These same experimental models also showed a benefit of sodium restriction on progression of kidney disease. High sodium consumption has also been shown to result in decreased renal blood flow and increased glomerular pressure, GFR and filtration fraction [17]. Major consequences of these changes in renal hemodynamics are an increase in urinary protein excretion and progression of kidney disease [18]. Furthermore, studies have found that a high sodium intake is linked to increased proteinuria and kidney disease progression [19–21]. Increased sodium intakes also affect the therapeutic response to antihypertensive agents in patients with salt sensitive hypertension. For example, inhibitors of the RAAS reduce intra-glomerular pressure, which in turn decreases proteinuria and risk for progression of kidney disease. Increased sodium intake abolishes these salutary effects of RAAS inhibitors. In a randomized controlled trial comparing the effect of Enalapril to the calcium channel blocker Isradipine on blood pressure control, patients treated with Enalapril had a more significant reduction in blood pressure while consuming a low-salt rather than a high-salt diet [22]. Similarly, despite the blood pressure lowering effect of the DASH diet, the largest reductions in blood pressure were obtained by a lower-salt version of the DASH diet [12]. A recent subgroup analysis of 1177 diabetic patients from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) and Irbesartan Diabetic Nephropathy (IDNT) trials found a significantly reduced risk of renal events in patients on an angiotensin receptor blocker with a low dietary sodium intake [23]. Thus, existing studies suggest that higher sodium consumption may be an important risk factor for CKD and CKD progression but data regarding the effect of sodium intake on kidney disease is scarce.
In our study, we found that a high sodium diet was associated with lower odds of CKD. These findings were unexpected as we hypothesized that a high sodium diet would be associated with a higher risk of CKD. It is possible that those with a known history of CKD were following a low sodium diet. Prior studies have demonstrated that most people with CKD are unaware of this diagnosis, but the mean dietary sodium intake in the patients with CKD in our cohort was 3053 ± 40 mg/day compared to 3597 ± 26 mg/day in subjects without CKD (p<0.0001). Alternatively, it is possible that a low sodium diet may be a risk factor for CKD. In a study of patients with type 1 diabetes and macroalbuminuria, a low sodium intake was associated with an increased risk of end stage renal disease [24]. There is evidence that low sodium intake may result in reflex activation of the sympathetic nervous system, RAAS and metabolic pathways that lead to increases in total and low density lipoprotein (LDL)- cholesterol [25]. Activation of these pathways may mitigate otherwise beneficial effects of lower sodium diets on blood pressure, kidney disease and kidney disease progression. To date, no interventional studies have examined the effect of sodium restriction on the development or progression of CKD.
Our study also showed that low dietary potassium intake was strongly associated with CKD. Several studies have shown an inverse relationship between dietary potassium intake and blood pressure and low potassium intake is considered a major contributor to the prevalence of hypertension [26–30]. Several studies indicate that a diet high in potassium reduces blood pressure levels [23–30]. In the International Study of Salt and Blood Pressure (INTERSALT), an increment in potassium intake from 1173 mg to 1564 mg was associated with a drop in systolic blood pressure by 2 to 3 mmHg [31]. In a meta-analysis of studies examining the effects of potassium intake on blood pressure, increased potassium intake resulted in a greater reduction of blood pressure in hypertensive patients (3.5 mmHg decrease in systolic blood pressure) compared to normotensive patients (0.97 mmHg decrease in systolic blood pressure) [32]. Thus, high dietary potassium intake may be protective for CKD through decreases in blood pressure. Alternatively, high dietary potassium intake may be protective for CKD through non-blood pressure pathways as in our study the association of potassium intake with CKD was independent of hypertension. Further studies are needed to elucidate the mechanisms by which high dietary potassium intake may be protective for CKD.
The combined activity of sodium and potassium in the diet may play an important biologic role as the urinary sodium to potassium ratio is a stronger predictor of blood pressure and cardiovascular events than urinary sodium or potassium excretion alone [25]. Higher sodium to potassium ratio in the diet is associated with higher blood pressure and a higher risk of CVD. We found that regardless of the sodium intake, a high potassium intake was protective for CKD. Interestingly, the group with both high potassium and high sodium intake had the greatest reduction in risk of CKD. The reason for this is unclear, but previous studies have found that potassium may lower blood pressure more in the presence of a high dietary sodium intake. For example, in a meta-analysis of trials examining the effect of potassium supplementation on blood pressure, the blood pressure lowering effect of increased potassium intake was greater in subjects consuming a diet high in sodium [30]. Interventional trials are needed to determine if high dietary potassium intake reduces the risk of chronic kidney disease.
Our study has several limitations worth noting. First, since this is an observation study, a causal relationship between dietary intake of sodium and potassium with CKD cannot be established. Second, we used dietary recall data to determine sodium and potassium intake as urinary sodium and potassium excretion was not available. A 24-hour urinary sodium and potassium excretion is the gold standard way to measure dietary sodium and potassium intake as dietary recall methods tend to underestimate sodium intakes due to difficulty characterizing the sodium content of foods, especially in foods eaten away from home and in the amount of sodium added in cooking [33]. However, some of these problems with dietary recall surveys can be overcome by using a high quality survey methodology and detailed information on the sodium content of food, which is what the NHANES survey did. Several studies have utilized the dietary recall data from NHANES to estimate sodium and potassium intake and relate these intakes to meaningful clinical outcomes [34–36]. Despite the argument that a 24-hour dietary recall gives an imprecise estimation of actual consumption, a uniform underestimation or overestimation of actual dietary intake by the 24-hour recall should not differentially bias the results. Third, dietary sodium and potassium intake may be linked to broader patterns of food consumption and it may be these foods that are responsible for observed relationships. Fourth, variability of diet across different ethnic groups could have caused residual confounding by race/ethnicity. Finally, it is possible that participants with a known history of CKD were following a low sodium diet. However, the mean sodium intake in the population with CKD was still > 3000 mg daily and the mean eGFR of the population was 88.0 ± 0.60 ml/min/1.73m2. Thus, it is likely that many of these subjects who met our definition for CKD were not aware of this diagnosis.
Notwithstanding these limitations, the present study also has several strengths. To our knowledge this is the first study reporting a link between dietary sodium and potassium intake with CKD in the general US population. Second, NHANES used uniform methods to collect data on dietary recall, serum creatinine and urinary albumin excretion. Third, the extensive and complete data on other important factors associated with CKD, allowed us to give an unbiased estimate for the relationship between dietary sodium and potassium intake and CKD. Finally, with the design of NHANES, we were able to extrapolate the results to the entire US civilian non-institutionalized population.
In conclusion, our study has shown that higher potassium and sodium intake are independently associated with lower odds of CKD in a nationally representative sample of the US adult population. These findings should be subjected to corroboration through longitudinal studies and clinical trials designed specifically to examine the effects of dietary sodium and potassium intake on kidney disease and its progression.
Acknowledgments
Support: Support came from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grants K23 DK087859-01A1, RO1 DK 081473 and RO1 DK 078112.
Footnotes
Conflict of Interest: All the authors declared no competing interests
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KM, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 4.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 8.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 9.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 10.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I Collaborative Study Group. Renoprotective Effect of Angiotension-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 11.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 12.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F Chronic Kidney Disease Epidemiology Collaboration. Using Standardized Serum Creatinine Values in the Modification of Diet in the Renal Disease Study Equation for Estimating Glomerular Filtration Rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Barton M, Vos I, Shaw S, Boer P, D’Uscio LV, Gröne HJ, Rabelink TJ, Lattmann T, Moreau P, Lüscher TF. Dysfunctional Renal Nitric Oxide Synthase as a Determinant of Salt-Sensitive Hypertension. J Am Soc Nephrol. 2000;11:835–845. doi: 10.1681/ASN.V115835. [DOI] [PubMed] [Google Scholar]
- 15.Meng S, Roberts LJ, 2nd, Cason GW, Curry TS, Manning RD., Jr Superoxide dismutase and oxidative stress in Dahl salt-sensitive and –resistant rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R732–R738. doi: 10.1152/ajpregu.00346.2001. [DOI] [PubMed] [Google Scholar]
- 16.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt Intake, Oxidative Stress, and Renal Expression of NADPH Oxidase and Superoxide Dismutase. J Am Soc Nephrol. 2003;14:2775–2782. doi: 10.1097/01.asn.0000092145.90389.65. [DOI] [PubMed] [Google Scholar]
- 17.Weir MR, Dengel DR, Behrens MT, Goldberg AP. Salt-induced increases in systolic blood pressure affect renal hemodynamics and proteinuria. Hypertension. 1995;25:1339–1344. doi: 10.1161/01.hyp.25.6.1339. [DOI] [PubMed] [Google Scholar]
- 18.Suckling RJ, He FJ, MacGregor GA. Altered dietary salt intake for preventing and treating diabetic kidney disease. The Cochrane Library. 2010;(12) doi: 10.1002/14651858.CD006763.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Ekinci El, Thomas G, Thomas D, Johnson C, Macisaac RJ, Houlihan CA, Finch S, Panagiotopoulos S, O’Callaghan C, Jerums G. Effects of Salt Supplementation on the Albuminuric Response to Telmisartan With or Without Hydrochlorothiazide Therapy in Hypertensive Patients with Type 2 Diabetes Are Modulated by Habitual Dietary Salt Intake. Diabetes Care. 2009;32:1398–1403. doi: 10.2337/dc08-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Hu FB, Curhan GC. Associations of diet with albuminuria and kidney function decline. Clin J Am Soc Nephrol. 2010;5(5):836–843. doi: 10.2215/CJN.08001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J, Fung TT, Hu FB, Curhan GC. Association of dietary patterns with albuminuria and kidney function decline in older white women: a subgroup analysis from the Nurses’ Health Study. Am J Kidney Dis. 2011;57(2):245–254. doi: 10.1053/j.ajkd.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weir MR, Chrysant SG, McCarron DA, Canossa-Terris M, Cohen JD, Gunter PA, Lewin AJ, Mennella RF, Kirkegaard LW, Hamilton JH, et al. Influence of Race and Dietary Salt on the Antihypertensive Efficacy of an Angiotensin-Converting Enzyme Inhibitor or a Calcium Channel Antagonist in Salt-Sensitive Hypertensives. Hypertension. 1998;31:1088–1096. doi: 10.1161/01.hyp.31.5.1088. [DOI] [PubMed] [Google Scholar]
- 23.Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, de Graeff PA, de Zeeuw D. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82:330–337. doi: 10.1038/ki.2012.74. [DOI] [PubMed] [Google Scholar]
- 24.Thomas M, Moran J, Forsblom C, Harjutsalo V, Thorn L, Ahola A, WadXn J, Tolonen N, Saraheimo M, Gordin D, et al. FinnDiane Study Group. The Association Between Dietary Sodium Intake, ESRD, and All-Cause Mortality in Patients With Type 1 Diabetes. Diabetes Care. 2011;34:861–866. doi: 10.2337/dc10-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK Trials of Hypertension Prevention Collaborative Research Group. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med. 2009;169:32–40. doi: 10.1001/archinternmed.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young DB, Lin H, McCabe RD. Potassium’s cardiovascular protective mechanisms. Am J Physiol. 1995;268(4 Pt 2):R825–837. doi: 10.1152/ajpregu.1995.268.4.R825. [DOI] [PubMed] [Google Scholar]
- 27.Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319–28. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houston MC. The importance of potassium in managing hypertension. Curr Hypertens Rep. 2011;13:309–317. doi: 10.1007/s11906-011-0197-8. [DOI] [PubMed] [Google Scholar]
- 29.Cappuccio FP, MacGregor GA. Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J Hypertens. 1991;9:465–473. doi: 10.1097/00004872-199105000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of Oral Potassium on Blood Pressure. Meta-analysis of Randomized Controlled Clinical Trials. JAMA. 1997;277:1624–1632. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 31.Dyer AR, Elliott P, Shipley M. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. II. Estimates of electrolyte-blood pressure associations corrected for regression dilution bias. The INTERSALT Cooperative Research Group. Am J Epidemiology. 1994;139:940–951. doi: 10.1093/oxfordjournals.aje.a117100. [DOI] [PubMed] [Google Scholar]
- 32.Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomized trials. J Hum Hypertens. 2003;17:471–480. doi: 10.1038/sj.jhh.1001575. [DOI] [PubMed] [Google Scholar]
- 33.Espeland MA, Kumanyika S, Wilson AC, Reboussin DM, Easter L, Self M, Robertson J, Brown WM, McFarlane M TONE Cooperative Research Group. Statistical issues in analyzing 24-hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. Am J Epidemiol. 2001;153:996–1006. doi: 10.1093/aje/153.10.996. [DOI] [PubMed] [Google Scholar]
- 34.Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK. Dietary potassium intake and risk of stroke in US men and women: National Health and Nutrition Examination Survey I epidemiologic follow-up study. Stroke. 2001;32:1473–1480. doi: 10.1161/01.str.32.7.1473. [DOI] [PubMed] [Google Scholar]
- 35.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med. 2002;162:1619–1624. doi: 10.1001/archinte.162.14.1619. [DOI] [PubMed] [Google Scholar]
- 36.Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Change MH, Gwinn M, Dowling N, Khoury MJ, Hu FB. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171:1183–1191. doi: 10.1001/archinternmed.2011.257. [DOI] [PubMed] [Google Scholar]


