Abstract
Purpose
Anemia is an expected consequence of intensive chemotherapy regimens administered to acute leukemia patients. This study was designed to determine if epoetin alfa would decrease the number of transfusion events and units of packed red blood cells (PRBCs) transfused, and secondarily, to study its effects on quality of life (QOL) and complete remission (CR) rates.
Patients and Methods
Patients with acute lymphoblastic leukemia (ALL), lymphoblastic lymphoma (LL), or Burkitt’s lymphoma (BL) receiving frontline myelosuppressive chemotherapy were randomized to receive epoetin alfa or no epoetin during the first 6 cycles of their planned chemotherapy. QOL was assessed by Edmonton Symptom Assessment Scale (ESAS) and FACT-Anemia questionnaires.
Results
Fifty five patients were randomized to epoetin alfa and 54 to no epoetin. Transfusion data was available in 79 of the 81 (98%) evaluable patients who completed the treatment/observation period. The trial was stopped early due to poor accrual before the target of 123 evaluable patients was met. A mean of 10.6 units of PRBCs over 5 months were administered to those receiving epoetin alfa compared to 13 units for those who did not (p=0.04). There was no significant difference in QOL as assessed by FACT-Anemia or ESAS. The CR rate and 3-year CR duration were not adversely affected by use of epoetin alfa.
Conclusion
Epoetin alfa decreases the number of PRBC transfusions and does not appear to negatively impact remission duration. No difference in QOL was observed.
Keywords: Anemia, epoetin, leukemia
INTRODUCTION
Anemia is one of the most common manifestations of acute lymphoblastic leukemia (ALL), lymphoblastic lymphoma (LL), and Burkitt’s lymphoma (BL). A variety of symptoms can occur depending on the degree of anemia, including fatigue, weakness, hypersensitivity to cold, dyspnea, tachycardia, dizziness, and acute coronary syndromes.1 In addition, the induction and consolidation phases of chemotherapy for ALL, LL and BL are significantly myelosuppressive, further contributing to the severity of the anemia.2 Thus, transfusion support of packed red blood cells (PRBCs) becomes critical in minimizing the potential complications of severe anemia.
Although screening techniques have been improved substantially in recent years, transfusion of PRBCs still represents a risk of morbidity for the recipient, mainly related to infection.3–6 In addition, other potential complications include volume overload (predominantly in elderly patients or those with underlying congestive heart failure), and iron overload (in instances where multiple transfusions are administered).7,8 On rare occasions frequent transfusions can result in development of alloantibodies which ultimately limit or delay availability of compatible PRBCs. Interventions which could lead to even a modest decrease in transfusion requirements would represent a major advantage for patients receiving myelosuppressive chemotherapy for ALL, LL, or BL.
We hypothesized that epoetin alfa, an erythropoeisis-stimulating agent (ESA), could benefit patients with ALL, LL or BL by decreasing PRBC transfusion requirements after frontline induction and consolidation chemotherapy. We therefore designed a randomized study comparing outcome measures between the two groups (epoetin alfa versus no epoetin alfa) with respect to transfusions of PRBCs, response, response duration, and quality of life (QOL).
PATIENTS, MATERIALS AND METHODS
Eligibility criteria
Patients receiving induction chemotherapy at M.D. Anderson Cancer Center (MDACC) with either the hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternating with high dose methotrexate and cytarabine) or augmented Berlin-Frankfurt-Muenster (BFM) regimens for treatment of newly diagnosed ALL, LL, or BL were eligible.9–13 Patients in first relapse with remission duration for at least 12 months were also eligible. There were no age restrictions. Deficiencies of vitamin B12, folate, or iron were allowed provided that replacement therapy was initiated. Enrollment was not allowed if the baseline hemoglobin was > 10 g/dL or erythropoietin had been administered within the prior 3 months. Uncontrolled hypertension, prior thrombotic event and/or poorly controlled or new onset seizure disorder were contraindications to participation. The enrollment period included the first 14 days from the start of induction chemotherapy. Patients had to give written informed consent for participation.
Study Design and Therapy
Details of the treatment regimens (hyper-CVAD inclusive of rituximab for CD20 positive ALL or BL and imatinib for Philadelphia chromosome (Ph) positive ALL, or augmented BFM) are as detailed previously.9–13 Once enrolled, patients were randomized to either epoetin alfa or no epoetin alfa during the first 6 cycles of their planned chemotherapy. One to one randomization was performed and balanced with respect to the treatment in each stratum using the Pocock and Simon algorithm14 for three age categories (≤18, 19–59, and ≥ 60 years). Epoetin alfa was administered at a starting dose of 40,000 units subcutaneously once weekly. If, after 4 weeks of therapy, an increase of ≥ 1 gm/dL in the hemoglobin level from the baseline value was not achieved, the dose of epoetin alfa was increased to 60,000 units subcutaneously/week. If <18 years of age, the starting dose of epoetin alfa was 600 units/kg/week subcutaneously to a maximum dose of 40,000 units/week. Doses were escalated to 900 units/kg/week (maximum of 60,000 units/week) for patients who did not achieve a ≥ 1 gm/dL increase in hemoglobin level. If after 4 weeks on maximal dose of epoetin alfa, an increase of ≥ 1 gm/dL in hemoglobin was not achieved, study participation was discontinued. Failure to achieve complete remission (CR) after 2 courses of induction chemotherapy or disease recurrence after initial CR also resulted in withdrawal from the trial. Epoetin alfa was held if the hemoglobin was ≥10 gm/dL and resumed when the hemoglobin fell below 10 gm/dL. All epoetin alfa injections were administered by a health care provider. Neither patients nor physicians were blinded to treatment. Hemoglobin was evaluated on at least weekly basis in all patients. PRBC transfusions were administered as per MDACC institutional standards in both arms (table 1). For patients who received interim care outside MDACC, the local physicians were requested to adhere to the MDACC PRBC transfusion guidelines as feasible, although deviations were allowed at the physician’s discretion if deemed in the best interest of the patient. All transfusions of PRBCs were verified.
Table 1.
Institutional standards for PRBC transfusions
| Adult | Pediatric |
|---|---|
| Hemoglobin ≤8 g/dL | Hemoglobin ≤6.5 g/dL |
| Symptomatic anemia | Symptomatic anemia |
| Hemoblobin ≤9 g/dL and sepsis, pulmonary, cardiovascular, or neurovascular disease | Hemoblobin ≤8 g/dL and sepsis, pulmonary, cardiovascular, or neurovascular disease |
Quality of life (QOL) assessments were performed using the Edmonton Symptom Assessment Scale (ESAS)15,16 and Functional Assessment of Cancer Therapy-Anemia Quality of life (FACT-An)17 questionnaires at study entry and prior to each chemotherapy cycle in patients who agreed to participate in this optional part of the study. The ESAS assesses symptoms of pain, fatigue (tired), depression, well-being, and shortness of breath, among others. The FACT-An evaluates well-being and fatigue.
Statistical methods
The primary endpoint was to compare the number of PRBC transfusions after at least 5 weeks of treatment, in patients receiving epoetin alfa versus the control arm. The secondary endpoint was to investigate any possible adverse effect of epoetin alfa on the CR rate and progression-free survival (PFS). The sample size needed to determine superiority statistically was projected at 164 patients. However, a significant decrease in accrual was observed after an adverse influence of epoetin alfa on survival in solid tumor malignancies was reported. The study was therefore terminated early in October 2008 after 109 patients had been enrolled.
Baseline demographics and clinical characteristics were summarized as descriptive statistics including mean and median (including range and standard deviation) or frequency for each of the continuous or categorical variables, respectively.
With respect to the primary endpoint of the study, the overall number of PRBC units transfused (including frequency per week) were tallied during the study period (starting at 5 weeks through 5 months from study enrollment). In addition, the maximum change in hemoglobin level from baseline was also computed. The Wilcoxon rank sum test was used to compare these computed values between the two treatment groups. Fisher’s exact test was performed to compare the proportion of relapses between the two treatment groups. The Kaplan-Meier method was applied to estimate the probabilities of PFS and overall survival (OS) with differences analyzed by the log-rank test.
With respect to the QOL assessments, a fitting linear mixed model was used to estimate the effect of treatment with or without epoetin alfa on QOL outcomes. Spearman correlation coefficients were used to assess the correlation of hemoglobin with fatigue scales (both questionnaires) at the baseline, mid-point and end of study time points.
RESULTS
Clinical characteristics
From September 2003 to July 2008, 109 patients were randomized to receive epoetin alfa (n=54) or no epoetin alfa (n=55) (Figure 1). Of these, 28 withdrew early for the following reasons: 7 subsequently declined to participate, 7 developed toxicity which mandated removal from study (discussed later), 6 developed disease-related complications prohibiting continuation of epoeitin alfa therapy, 4 randomized to the control arm received at least 2 doses of epoetin alfa or darbepoetin alfa, 3 proceeded to allogeneic stem cell transplantation, and 1 relapsed (within 5 weeks). Baseline pretreatment characteristics were similar between the two groups (Table 2), except for higher baseline erythropoietin (EPO) plasma levels in the group randomized to receive epoetin alfa (p=0.05) and trend for higher proportion of Ph positive ALL in the control group (p=0.09).
Figure 1. Randomization schema.
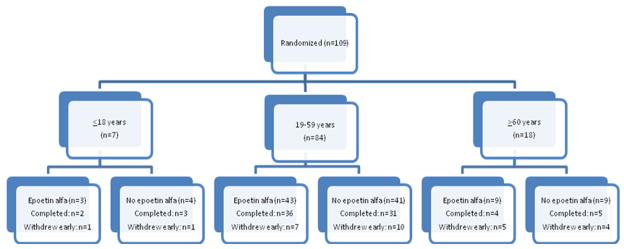
Randomization schema for epoetin alfa versus no epoetin. Patients were stratified by age groups (≤ 18, 19–59, and ≥ 60 years old).
Table 2.
Baseline demographics and clinical characteristics of 109 randomized patients
| Epoetin (n=55) | No-Epoetin alfa (n=54) | P-value | |
|---|---|---|---|
|
| |||
| Sex, n (%) | 0.13 | ||
| Male | 33 (58) | 24 (42) | |
| Female | 22 (42) | 30 (58) | |
|
| |||
| Race, n (%) | 0.01 | ||
| Caucasian | 27 (43) | 36 (57) | |
| African American | 1 (25) | 3 (75) | |
| Hispanic | 27 (69) | 12 (31) | |
| Asian | 0 (0) | 3 (100) | |
|
| |||
| Age (years) | 0.63 | ||
| Mean (SD) | 41 (16.7) | 42 (17.3) | |
| Median (range) | 39 (18–76) | 42 (15–84) | |
|
| |||
| Diagnosis, n (%) | 0.15 | ||
| B lymphoblastic leukemia/lymphoma | 38 (47) | 43 (53) | |
| T lymphoblastic leukemia/lymphoma | 8 (73) | 3 (27) | |
| Burkitt leukemia/lymphoma | 9 (60) | 6 (40) | |
| Biphenotypic | 0 (0) | 2 (100) | |
|
| |||
| Bone marrow involvement, n (%) | 0.58 | ||
| Yes | 46 (49) | 48 (51) | |
| No | 9 (60) | 6 (40) | |
|
| |||
| Cytogenetics, n (%) | 0.09 | ||
| Diploid | 25 (58) | 18 (42) | |
| t(9;22) | 6 (29) | 15 (71) | |
| Other | 18 (51) | 17 (49) | |
|
| |||
| Chemotherapy, n (%) | 1.0 | ||
| Hyper-CVAD based regimens | 54 (98) | 52 (96) | |
| Augmented BFM | 1 (2) | 2 (4) | |
|
| |||
| Baseline hemoglobin (g/dl) | .88 | ||
| Mean (SD) | 9 (1.5) | 8.9 (1.5) | |
| Median (range) | 8.8 (6.6–12.5) | 8.7 (6–12.5) | |
|
| |||
| Baseline erythropoietin level | .04 | ||
| Mean (SD) | 473 (570) | 326 (514) | |
| Median (range) | 316 (9.1–3397) | 161 (6.9–3048) | |
|
| |||
| Observation period (weeks) | .72 | ||
| Mean (SD) | 19 (7) | 19 (7.3) | |
| Median (range) | 20 (1–33) | 21 (0–36) | |
|
| |||
| Number of courses of chemotherapy completed | .88 | ||
| Mean (SD) | 5.5 (2.1) | 5.6 (2) | |
| Median (range) | 7 (0–7) | 7 (1–7) | |
Efficacy results
Number of RBC transfusions and hemoglobin outcomes
Among the remaining 81 evaluable patients, 79 (98%) had transfusion data available from the fifth week until end of 5 months from the time of enrollment. There was no significant difference in the number of PRBC transfusion events per week between the two treatment groups (p=0.089) (Table 3). However, the mean number of PRBC units transfused in the epoetin alfa group was significantly lower than in the control group (p=0.04). No significant difference was detected in the maximum change in hemoglobin from baseline between the two treatment groups (p=0.21). The majority of patients (75%) required at least 1 dose escalation to 60,000 units of epoetin alfa per week.
Table 3.
Number of transfusion and units using data of 5 weeks after the start of the treatment to the end of the 5 months for 79 evaluable patients
| Mean Maximum Hemoglobin Increase from baseline, g/dl (SD) | Mean Number of Transfusion Events(SD) | Mean Number of Units Transfused (SD) | |
|---|---|---|---|
| Epoetin (n=41) | 2.7 (1.9) | 6.22 (3.87) | 10.63 (6.29) |
| No Epoetin (n=38) | 2.2 (1.6) | 7.44 (3.28) | 13.11 (5.18) |
| p-value | 0.21 | 0.089 | 0.035 |
SD, standard deviation
Quality of Life
Seventy-four patients participated in the QOL endpoint, of which 40 (54%) were receiving epoetin alfa. No significant differences between the two treatment groups were detected in the ESAS scales or in the FACT-An. There were no significant associations between hemoglobin and fatigue scores on either scale.
Disease Response Outcomes
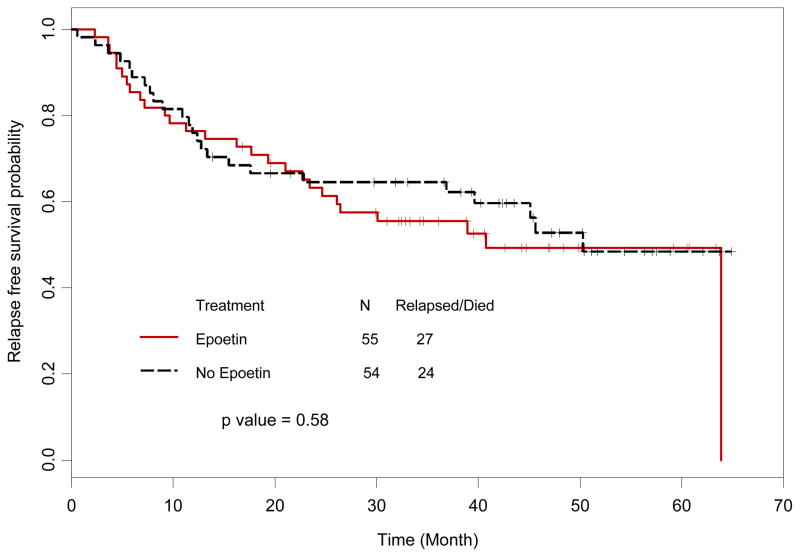
The CR rate was 95% for those treated with hyper-CVAD (n=106) and 100% for those treated with BFM (n=3). In the epoetin alfa group, 2 patients treated with hyper-CVAD had a partial response (both LL with imaging suggestive of persistent adenopathy) and 1 failed to respond. In the control group, 1 patient had a partial response (LL with residual adenopathy) and 1 patient died before response could be assessed. There were no statistical differences in CR rates between the treatment and control groups (p=0.62). The median PFS was 41% (95% CI: 24.6-NA) for the epoetin alfa group versus 50.3% (95% CI: 39.6, NA) for the control group (p=0.58, Figure 2).
Figure 2. Progression-free survival.
Progression-free survival in patients randomized to epoetin alfa versus no epoetin alfa. There was no statistically significant difference in progression-free survival between the two groups (p=0.40).
Overall survival
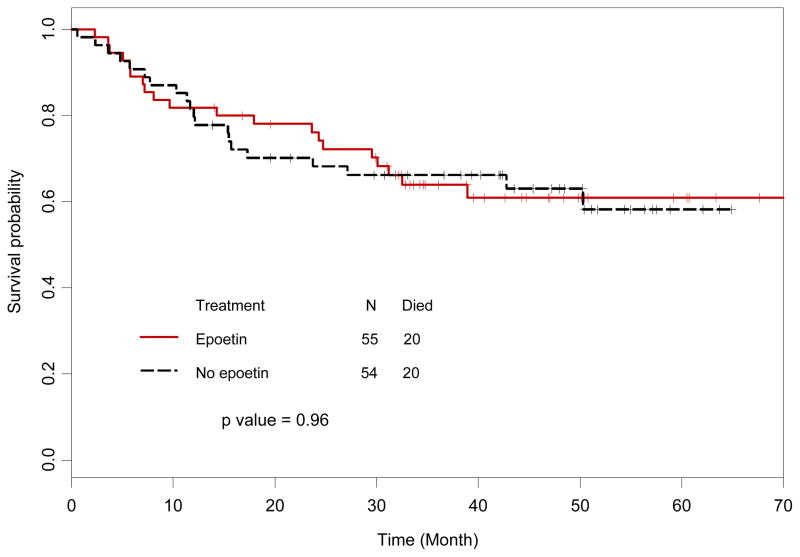
Analysis of OS included all 109 randomized patients, with 40 (37%) deaths overall, 20 (37%) in the control arm and 20 (36%) in the epoetin alfa arm. The median OS has not been reached. There were no significant differences in OS between the two treatment arms (p=0.96, Figure 3).
Figure 3. Overall survival.
Overall survival in patients randomized to epoetin alfa versus no epoetin alfa. There was no statistically significant difference in overall survival between the two groups (p=0.67).
Safety
Eight patients in the epoetin alfa arm had events, 6 were attributed to epoetin therapy (5 thrombotic events, 1 seizure). Only 1 of these 5 patients with thrombotic events (lower extremity deep vein thrombosis) had been dose escalated to 60,000 units per week. Six patients in the no epoetin alfa arm also had similar serious adverse events (including 2 thrombotic events, 2 seizures). There was no statistical difference in the rate of thrombotic events between the two groups (p=0.44).
DISCUSSION
In this study using epoetin alfa during intensive induction and consolidation chemotherapy (hyper-CVAD or augmented BFM) in patients with newly diagnosed ALL, LL or BL, we found a modest but statistically significant decrease in the number of units of PRBCs transfused per week compared with the no epoetin alfa arm. For 79 evaluable patients who completed the 5 month observation period, the mean number of units transfused was 10.6 in the epoetin alfa arm compared with 13 in the no epoetin alfa group (p=0.04).
Several studies have demonstrated a beneficial effect of ESAs on the QOL of patients receiving chemotherapy.18–23 In our study, we did not find a statistically significant, beneficial effect of epoetin alfa on QOL, however, the number of patients studied for this endpoint was small and the study was not powered for this endpoint.
There have been significant concerns regarding the potential deleterious effects of ESAs in solid tumors. This issue surfaced in 2005, when data regarding the association of use of ESAs with poorer tumor-related outcomes in patients receiving chemotherapy for breast cancer emerged 24. The study was halted early owing to a higher mortality observed in the epoetin-treated patients. However, the dosing of epoetin alpha was designed to achieve and maintain hemoglobin levels above 12 g/dL, a practice which is not recommended with erythropoiesis-stimulating agents. In addition, some prognostic factors favored the placebo group which may have influenced the proportion of tumor progression related deaths in the ESA-treated groups. Several other studies in different tumor types have demonstrated adverse effects on survival, however, all of these studies were designed to target hemoglobins of 12 g/dL or higher.24–28 In contrast, other studies using epoetin alpha have reported either modest benefits or no differences in survival. 22,29–32 In our study, hemoglobin levels were carefully monitored, and epoetin alfa was discontinued if the hemoglobin rose above 10 g/dL. There was no significant increase in the number of thrombotic events in patients receiving epoetin alfa compared with those who did not. Importantly, none of these events were fatal. More importantly, the use of epoetin alfa had no negative effect on the rates of remission, response duration or overall survival. Thus, the possible negative effect observed with the use of erythropoiesis-stimulating agents under certain circumstances in some tumors, should not be extrapolated to all cancers considering the difference in patient populations, biology of the disease, chemotherapy agents, expected long-term outcome, and other variables.
This study is not without its limitations. First, owing to the rarity of aggressive lymphoid leukemias in the adult population, the number of patients studied was relatively small. Second, the study was not placebo-controlled, which could have introduced physician bias with respect to the frequency of transfusions and the number of units administered. Finally, the study was terminated early due to a sharp reduction in accrual once information regarding the potential adverse effect of epoetin alfa on survival was incorporated into the informed consent document for the study. This resulted in patient reluctance to participate in the study. However, there was no evidence that epoetin alfa as administered in our study had any deleterious effects on outcome (similar rates of CR, 5-year PFS and toxicity).
Although the early termination of the study does not allow for firm conclusions, our results suggest that use of epoetin alfa can decrease the requirements for transfusion of PRBCs in patients with ALL, LL and BL receiving myelosuppressive chemotherapy. With use of appropriate parameters for epoetin alfa dosing, including interruption of therapy when hemoglobin levels increased beyond 10g/dL, the risk of thrombotic events was not increased. The use of epoetin alfa had no adverse impact on disease outcomes such as response or survival. Additional prospective studies are clearly warranted in order to better define the risks and potential benefits of ESAs in specific patient populations.
Acknowledgments
The authors would like to thank Jennifer Cassat, Melodie England, Charles Stava, and Kathleen Rossi for their assistance in the data collection, analysis and preparation of this manuscript.
Research support: This study was supported by research funding from OrthoBiotech to MEC and supported in part by the National Institutes of Health through The University of Texas MD Anderson Cancer Center’s Cancer Center Support Grant CA16672.
Footnotes
Disclosures: MEC, DAT, GNM, MER, EB, LX, BNB, MCF have no financial disclosures. HK has received research support from Amgen and JEC has received research support from Wyeth, Bristol-Myers Squibb, and Novartis.
References list
- 1.Ludwig H, Strasser K. Symptomatology of anemia. Semin Oncol. 2001;28:7–14. doi: 10.1016/s0093-7754(01)90206-4. [DOI] [PubMed] [Google Scholar]
- 2.Littlewood T, Mandelli F. The effects of anemia in hematologic malignancies: more than a symptom. Semin Oncol. 2002;29:40–4. doi: 10.1053/sonc.2002.33532. [DOI] [PubMed] [Google Scholar]
- 3.Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood. 2008;112:2617–26. doi: 10.1182/blood-2008-07-077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou S. Potential impact of pandemic influenza on blood safety and availability. Transfus Med Rev. 2006;20:181–9. doi: 10.1016/j.tmrv.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alter HJ, Stramer SL, Dodd RY. Emerging infectious diseases that threaten the blood supply. Semin Hematol. 2007;44:32–41. doi: 10.1053/j.seminhematol.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14:671–6. doi: 10.1097/MOH.0b013e3282e38e8a. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 8.Gabutti V, Borgna-Pignatti C. Clinical manifestations and therapy of transfusional haemosiderosis. Baillieres Clin Haematol. 1994;7:919–40. doi: 10.1016/s0950-3536(05)80131-3. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–61. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 10.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111:2548–55. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas DA, Cortes J, O’Brien S, et al. Hyper-CVAD program in Burkitt’s-type adult acute lymphoblastic leukemia. J Clin Oncol. 1999;17:2461–70. doi: 10.1200/JCO.1999.17.8.2461. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624–30. doi: 10.1182/blood-2003-12-4428. [DOI] [PubMed] [Google Scholar]
- 14.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. [PubMed] [Google Scholar]
- 15.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 16.Dudgeon DJ, Knott C, Chapman C, et al. Development, implementation, and process evaluation of a regional palliative care quality improvement project. J Pain Symptom Manage. 2009;38:483–95. doi: 10.1016/j.jpainsymman.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34:13–9. [PubMed] [Google Scholar]
- 18.Littlewood TJ, Nortier J, Rapoport B, et al. Epoetin alfa corrects anemia and improves quality of life in patients with hematologic malignancies receiving non-platinum chemotherapy. Hematol Oncol. 2003;21:169–80. doi: 10.1002/hon.722. [DOI] [PubMed] [Google Scholar]
- 19.Demetri GD, Kris M, Wade J, et al. Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. J Clin Oncol. 1998;16:3412–25. doi: 10.1200/JCO.1998.16.10.3412. [DOI] [PubMed] [Google Scholar]
- 20.Straus DJ, Testa MA, Sarokhan BJ, et al. Quality-of-life and health benefits of early treatment of mild anemia: a randomized trial of epoetin alfa in patients receiving chemotherapy for hematologic malignancies. Cancer. 2006;107:1909–17. doi: 10.1002/cncr.22221. [DOI] [PubMed] [Google Scholar]
- 21.Jones M, Schenkel B, Just J, et al. Epoetin alfa improves quality of life in patients with cancer: results of metaanalysis. Cancer. 2004;101:1720–32. doi: 10.1002/cncr.20569. [DOI] [PubMed] [Google Scholar]
- 22.Littlewood TJ, Bajetta E, Nortier JW, et al. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19:2865–74. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- 23.Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95:888–95. doi: 10.1002/cncr.10763. [DOI] [PubMed] [Google Scholar]
- 24.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–72. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 25.Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–60. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 26.Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25:1027–32. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- 27.Temkin SM, Hellmann M, Serur E, et al. Erythropoietin administration during primary treatment for locally advanced cervical carcinoma is associated with poor response to radiation. Int J Gynecol Cancer. 2006;16:1855–61. doi: 10.1111/j.1525-1438.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 28.Thomas G, Ali S, Hoebers FJ, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317–25. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aapro M, Scherhag A, Burger HU. Effect of treatment with epoetin-beta on survival, tumour progression and thromboembolic events in patients with cancer: an updated meta-analysis of 12 randomised controlled studies including 2301 patients. Br J Cancer. 2008;99:14–22. doi: 10.1038/sj.bjc.6604408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osterborg A, Brandberg Y, Hedenus M. Impact of epoetin-beta on survival of patients with lymphoproliferative malignancies: long-term follow up of a large randomized study. Br J Haematol. 2005;129:206–9. doi: 10.1111/j.1365-2141.2005.05440.x. [DOI] [PubMed] [Google Scholar]
- 31.Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94:1211–20. doi: 10.1093/jnci/94.16.1211. [DOI] [PubMed] [Google Scholar]
- 32.Glaspy J, Crawford J, Vansteenkiste J, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer. 102:301–15. doi: 10.1038/sj.bjc.6605498. [DOI] [PMC free article] [PubMed] [Google Scholar]