Abstract
We tested the hypothesis that neuropathic tissue is more sensitive to stimulation by intense focused ultrasound (iFU) than control tissue. We created a diffusely neuropathic paw in rats via partial ligation of the sciatic nerve, whose sensitivity to iFU stimulation we compared with sham-surgery and normal control paws. We then applied increasing amounts of iFU (individual 0.2 second pulses at 1.15 MHz) to the rats’ paws, assaying for their reliable withdrawal from that stimulation. Neuropathic rats preferentially withdrew their injured paw from iFU at smaller values of iFU intensity (176 W/cmˆ2 +/- 56) than did sham surgery (217 W/cmˆ2 +/- 25) and normal control (>280 W/cmˆ2) animals, with greater sensitivity and specificity (85% for neuropathic rats and 50% each of sham surgery and normal control rats). These results directly support our hypothesis as well as Gavrilov’s idea that doctors may some day use iFU stimulation to diagnose patients with neuropathies.
Keywords: intense focused ultrasound, pain, neuropathy, pain diagnosis
Introduction
Several groups of researchers have shown that sufficiently intense focused ultrasound (iFU) can generate sensations in healthy test subjects when applied to tissue both superficially and at depth (Dalecki et al. 1995; Dickey et al, 2012; Gavrilov et al. 1977a,b; Gavrilov et al. 1996; Gershuni et al. 1980; and Wright et al. 1993, 2002) likely through mechanical stimulation, at least at threshold values of iFU (reviewed in Gavrilov et al, 1996). Therefore, as hypothesized originally by Gavrilov and colleagues, it is plausible that iFU stimulation of neuropathic tissue could generate diagnostically useful sensations because those sensations may differ in quality or intensity compared to sensations generated by iFU stimulation of normal tissue.
Here we address Gavrilov’s hypothesis indirectly, working with an animal model of neuropathic pain and asking not what the animals felt due to iFU stimulation but rather: is their neuropathic tissue preferentially sensitive to stimulation by iFU? To do so we created a diffusely neuropathic in jury in the right hind paws of rats, then applied iFU to each hind paw in a serial fashion, assessing the minimum amount of iFU necessary to cause the rat to reliably withdraw from the iFU stimulation. We found that compared to control tissue (sham surgery; no surgery) and contralateral tissue, neuropathic tissue required the least amount of iFU to cause the animal to withdraw. These results confirm our hypothesis and also support Gavrilov’s.
Methods
All animal procedures were approved by the Institutional Animal Care and Use Committees of the University of Washington and the Veterans Administration of Puget Sound.
Animal model
We partially ligated the sciatic nerve within the right hind paws of 33 Sprague–Dawley rats (180–200 mg, Taconic) following the surgical protocol of Seltzer et al (1990). This produced diffuse, neuropathic pain throughout the paw, as supported by the rat’s guarding of that paw, consistent with the criterion of Seltzer et al (1990). Hereafter we refer to these animals as the pSNL rats (pSNL = partial sciatic nerve ligation). Specifically, deep anesthesia and analgesia was induced through use of isoflurane inhalant system. After subcutaneous injection of lidocaine (0.3 ml/kg), the sciatic nerve of the right hind paw was exposed, temporarily dissected from the surrounding tissue and then partially sutured using 7-0 sofsilk™ sutures (Syneture, Norwalk, CT) ⅓ to ½ of the way through (Figure 1). Buprenorphine (0.05 mg/kg) was dripped into the muscle bed before closing with surgical staples (Roboz Surgical Instrument Co., Inc., Rockville, MD) and the animal was allowed to recover from surgery.
Figure 1. Ligation of the exposed sciatic nerve.
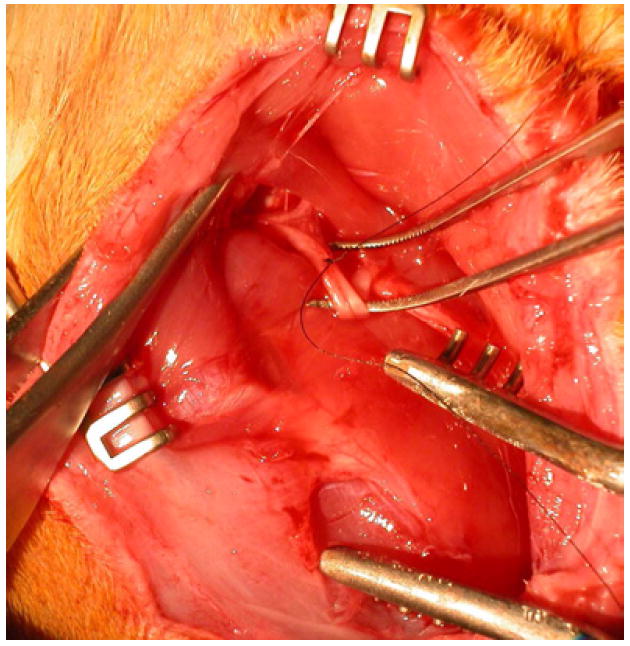
Here, the sciatic nerve in a rat’s leg has been exposed and a suture has been tied through 1/3 to 1/2 of the nerve, in order to create a sensitive paw.
We also performed sham surgery on 30 additional Sprague–Dawley rats in which the same surgical procedure was carried out except the actual ligation of the sciatic nerve. We call these animals the sham surgery control animals. Finally, we gathered an additional 12 Sprague-Dawley rats to act as normal, non-surgical controls. Because diet can cause significant differences in an animal’s experience of neuropathic pain (Shir et al. 2001) we fed all our rats a non-soy diet for at least one week prior to surgery as well as throughout the testing period.
Ultrasound device
We used a commercial piezo-electric, flat transducer built into a custom solid, cylindrical cone-shaped aluminum housing (described in Miao et al, 2005 and Dickey et al, 2012) to determine the amount of iFU necessary to cause the rats to withdraw their paws in response to iFU stimulation. We created the iFU signal (individual ultrasound pulses with a center frequency of 1.15 MHz, each lasting for 0.2 seconds) with two function generators (HP33120A, Hewlett-Packard, Palo Alto, CA), amplified by a power amplifier (ENI A150, ENI, Rochester, NY) whose signal we transmitted directly to the transducer. We monitored the input voltage to the transducer using a Wave Runner LT 322 oscilloscope (LeCroy Corporation, Chestnut Ridge, NY).
We translated this voltage to the spatially averaged and temporally averaged intensity (ISATA) emitted by the iFU device via a ‘force balance’ technique (Hill et al., 1994). In essence, the effective weight caused by iFU beamed into an acoustic absorber placed on a scale, along with a measure of the radial extent of the iFU emitted from our device (see below), translates into a temporally averaged intensity itself averaged over the area enclosed by the half-pressure-maximum contour in the focal plane. To assay the spatial characteristics of the iFU focus we used a needle hydrophone (NTR, Seattle WA) in degassed water and measured the axial and radial variation of the pressure field (for these beam plots see Figures 2 and 3, respectively, in Dickey et al, 2012). The primary focus of the iFU device occurred within 500 microns of the proximal surface of the device, with a secondary focus of one fourth the intensity at 0.75 cm from that proximal surface, as measured in degassed water.
Figure 2. Application of iFU to rat’s paw for behavioral testing.

Here, the iFU device is shown during application of ultrasound to the plantar surface of the rat’s paw. The wire mesh cage allows the transducer to be adequately coupled with the rat’s paw using ultrasound gel.
Figure 3. Paw withdrawal thresholds for pSNL, sham, and control rats.
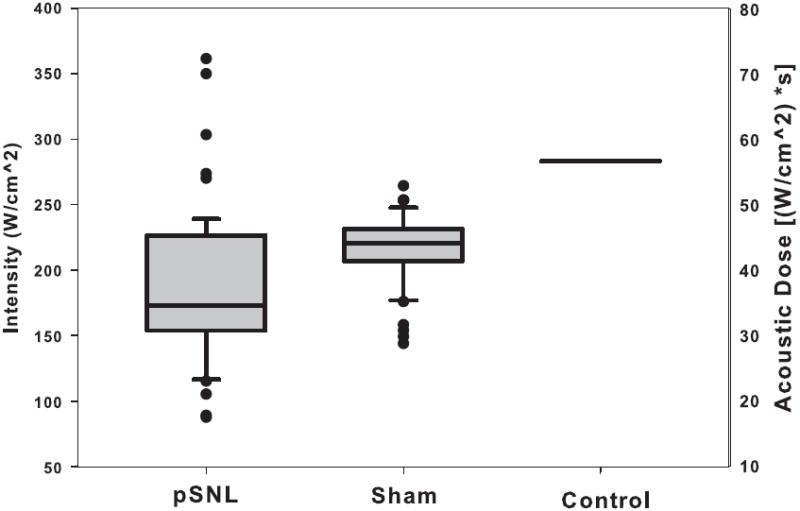
Here we show the iFU threshold values for each group of animals, with the intensity of iFU on the left side and the acoustic dose on the right. Note that each group’s iFU threshold value differed from that of the others in a statistically significant fashion, with p < 0.0001.
Behavioral test for iFU stimulation
On days 5 and 7 after surgery, we habituated sets of three rats to their free-ranging presence within a cage containing three separate enclosures, with a wire-mesh bottom. We also habituated the rats to the touch, through the wire mesh, of the proximal face of the iFU transducer coated with acoustic gel to the plantar aspect of the rat’s paw (Figure 2). After habituation we again placed the proximal surface of the device through the bottom of the wire mesh cage until it touched the bottom of one of the rat’s hind paws, as before. This time we applied individual acoustic pulses of length 0.2 seconds to the plantar surface of the paw, using ultrasound gel (Aquasonic 100 Ultrasound Transmission gel, Parker Laboratories Inc.) to ensure adequate coupling. (Note that if during the iFU test procedure a rat began to withdraw its paw in response to contact with the transducer but without iFU application, it was re-habituated to the touch of the device before re-starting iFU threshold testing.) During and immediately after iFU application we looked for an immediate and rapid withdrawal of the stimulated hind paw, before returning to testing the other hind paw in each of the three rats after a minimum of 30 seconds. In the absence of a hind paw withdrawal response the intensity of ultrasound was increased in increments beginning at approximately 30% of the previous intensity, tapering to a 10%, starting with an initial acoustic intensity of approximately 50 W/cm2. Rats showing only one out of two withdrawal responses to a given level of iFU stimulation at a given power by a given paw were considered negative tests and the intensity was increased as described until the minimum iFU threshold intensity at a given duration was achieved that induced two consecutive withdrawal responses by a given paw to two consecutive iFU applications. We define this minimum intensity as the iFU threshold value. If rats withdrew both hind paws at a given iFU intensity the acoustic intensity was decreased and iFU was reapplied until only one paw withdrawal response was observed twice after each of two consecutive applications of iFU to that paw or we determined that we could not identify a single sensitive paw.
After identification of the iFU threshold value, we then tested both hind paws in a serial fashion for their withdrawal responses to that amount of iFU for an additional six iFU applications (with at least 30 seconds between each iFU application). We used this data (a total of eight withdrawal tests for each paw) to determine the sensitivity and specificity of the iFU threshold for that rat, defined below in the Data Analysis section.
Behavioral test for sensitivity to heat
Rats were also tested for hind paw withdrawal to thermal stimulation from a standardized heat lamp via the ‘Hargreaves’ test (Hargreaves et al. 1988). Each rat underwent one Hargreaves test followed by the iFU test, followed by another Hargreaves test, with approximately one hour between tests.
Sets of three rats were placed in a Plexiglas enclosure (with three separate compartments) with an open top and a plexiglass floor placed 20 cm above a radiant heat source (Model 33 Tail Flick Analgesia Meter, IITC Life Science Instruments Inc.). We first habituated the rats to the enclosure for approximately 5 minutes and then aimed the heat lamp at the plantar surface of each hind paw in succession. We measured the time it took for the rat to withdraw its paw in response to the heat, known as the ‘latency’ time. A maximum of 20 seconds of light exposure was set to avoid tissue damage. We performed six withdrawal latency trials for each paw for each rat.
Data Analysis
We entered our data into an Excel (Microsoft, Redmond, WA) spreadsheet where the intensity and dose (intensity multiplied by the duration of ultrasound application, here 0.2 seconds) at each acoustic protocol were calculated, reported as aggregates in terms of an average +/− standard deviation. Differences in acoustic intensity or Hargreaves latency between groups were evaluated by analyses of variance with Tukey’s tests for appropriate post-hoc comparisons (GB Stat; Dynamic Microsystems; Silver Springs, Maryland) followed by one-tailed, unpaired t-tests assuming unequal variance, within Excel, for individual comparisons. Differences between two groups of data are reported as statistically significant if the p-value < 0.05 (though most of our p-values were no greater than 0.001).
We calculated the sensitivity and specificity of iFU application to the paws using data from all eight FU applications of a given iFU threshold value to each of the rat’s rear paws. We then averaged the sensitivity and specificity values across all rats within a given group, reporting their average values along with their standard deviation. We define the sensitivity of iFU stimulation as the number of withdrawal responses by the injured paw at the iFU threshold intensity divided by the total number of applications of iFU to that paw at that intensity. Similarly, we define the specificity of iFU stimulation as the number of times the rat did not withdraw its contralateral paw at the iFU threshold intensity divided by the total number of applications of iFU to that paw at that intensity.
With regard to our box plot figures: the line in the box gives the median value, the box encompasses the position of 75% of the values, the whiskers bound 95% of values and individual points represent individual values that extend beyond the 95% boundary.
Results
Hargreaves heat lamp results
We did not find any variation in our data with the day of testing or whether or not the Hargreaves testing occurred before or after iFU testing (data not shown). We therefore pooled our Hargreaves heat lamp data across testing days and before and after iFU testing.
We performed a total of 124 Hargreaves heat lamp tests (6 data points per test) for the pSNL (64 tests), sham surgery control (54 tests) and normal control (6 tests) animals. The average Hargreaves latency value for the pSNL rats measured 10.66 ± 5.3 seconds, while that of the sham surgery control rats measured 11.45 ± 5.1 seconds and that of the normal control rats measured 17.25 ± 3.5 seconds. We found that all groups (pSNL, sham surgery control, normal control) had Hargreaves latency values that were statistically significantly (p<0.001) different from each other. Statistical analysis also showed no difference between latency values of the ipsilateral and contralateral paws within each group of rats.
iFU withdrawal test results
We did not find any variation in our data with the day of testing (data not shown). We therefore pooled our data across testing days.
We found that the upper bound on the intensity (283 W/cm2) and dose (56.6 [W/cm2]*s) achievable by the device was insufficient to cause the normal control rats to withdraw their paws to iFU stimulation in a reliable fashion. Specifically, of the 12 rats tested, paired withdrawal responses after iFU application were observed for only three rats on day 1 and two rats on day 2. these five positive responses were associated with the left paw only, one with the right paw only, and one with both paws. Moreover, we observed only occasional withdrawal responses at these iFU threshold values for these animals for the standard six additional iFU applications. Taken together, this series of iFU studies produced a total of 12 withdrawals by the left paw and 11 withdrawals by the right paw. Essentially, the normal control animals were not likely to withdraw their paw from iFU at the largest values of iFU stimulation we could produce, but when they did, they were equally as likely to withdraw their right as their left paw.
iFU application to the sham surgery control animals elicited a withdrawal response of the right paw in 21 of 58 total iFU tests, 20 tests resulted in withdrawal by the left paw, 7 tests by both paws at the same iFU threshold value, and 10 tests did not produce a response with even the largest iFU stimulation we could produce (Table 1). In our threshold value analysis for the sham surgery control group, we included all rats from these groups, using the highest value attempted for the 10 tests where no response was elicited in our calculations, as a proxy for the (assumed higher) true threshold. For this group we found an iFU threshold intensity and dose of 217± 25 W/cm2 and 43.4 ± 5[W/cm2]*s, respectively. Essentially, the sham surgery control animals were equally as likely to withdraw their right or left paw from iFU stimulation of sufficient intensity and dose.
Table 1.
Tabulation of the number of tests of rats that in response to stimulation by intense focused ultrasound (iFU) withdrew their ipsilateral (Ipsi) paw versus contralateral (Contra) paw versus both paws in a consistent fashion as a function of surgery type or as right vs left for the control (no surgery) animals. Here pSNL refers to those rats whose sciatic nerve received a partial ligation while ‘sham’ refers to those animals whose sciatic nerve was surgically exposed, but not ligated. ‘NT’ refers to those animals for which we could not find a threshold value of iFU stimulation.
| pSNL | Sham | Control | |||
|---|---|---|---|---|---|
| Ipsi | 58 | Ipsi | 21 | Right | 1 |
| Contra | 0 | Contra | 20 | Left | 3 |
| Both | 1 | Both | 7 | Both | 1 |
| NT’s | 0 | NT’s | 10 | NT | 7 |
| Total: | 59 | Total: | 58 | Total: | 12 |
| # tests used for t-test | 58 | # tests used for t-test | 58* | # tests used for t-test | 12* |
Note that during some tests our device reached the maximum intensity it could emit yet did not allow us to obtain an iFU threshold value. For this circumstance we therefore used this maximum value in the t-tests as a proxy for the (assumed higher) true threshold.
Twenty-seven of the thirty-three pSNL rats guarded their right paw on both test days (5 and 7 days after surgery), while six guarded their right paw on only one of the two days. We therefore performed fifty-nine tests of the withdrawal response to iFU stimulation of those pSNL rats that guarded their right paw. As one measure of the robustness of iFU stimulation and its ability to differentiate neuropathic tissue from contralateral tissue, we found that the rats withdrew their ipsilateral paws at the iFU threshold value for 58 of our 59 tests (98%) while only one rat withdrew both paws at its iFU threshold value for only one of the 59 tests (2%). In our calculations we did not use data from the test where the rat withdrew both paws at threshold intensity. For the pSNL group we found an iFU threshold intensity and dose of 176 ± 56 W/cm2 and 37.4 ± 11[W/cm2]*s, respectively.
The sensitivity and specificity of iFU stimulation for each of the three groups of animals gives a measure of the robustness of our procedure. The sensitivity and specificity values for the pSNL surgery rats measured approximately 85% compared to 50% for sham surgery and control animals – Table 2.
Table 2.
Sensitivity and specificity values for animals subjected to one of sham or pSNL surgeries.
| Extent of Surgery | Sensitivity | Specificity |
|---|---|---|
| Sham | 46.2 ± 29 | 52.2 ± 28 |
| pSNL | 84.7 ± 16 | 85 ± 16 |
Figure 3 and Table 3 bring together the iFU threshold intensity and dose values for our normal control, sham surgery control, and pSNL animals. Statistical analysis of this data shows that the iFU threshold values for each of the three groups of animals (pSNL, sham surgery control, normal control) differed significantly from each other (p < 0.001). Specifically, the threshold values for the pSNL animals measured significantly lower than that measured for each of the sham surgery and normal control animals. Also, the results for the each of the pSNL animals and sham surgery control animals measured lower than that of the maximum values of iFU intensity and dose we observed for the normal control animals.
Table 3.
Mean and standard deviation of values of iFU threshold intensity and dose associated with each group of experimental animals associated with Figure 3.
| Groups | Control | Sham | pSNL |
|---|---|---|---|
| iFU threshold intensity | 283 W/cm2 | 217± 25 W/cm2 | 176 ±56 W/cm2 |
| iFU threshold dose | 56.6 [W/cm2]*s | 43.4 ± 5[W/cm2]*s | 37.4 ± 11 [W/cm2]*s |
Discussion
We tested the hypothesis that diffusely neuropathic tissue is preferentially sensitive to iFU stimulation compared to contralateral tissue from the same rat as well as compared to model tissue from sham surgery and normal control rats. Animals that experienced the full pSNL procedure – the pSNL rats – withdrew their neuropathic paws after application of lower values of iFU stimulation than that required to elicit hind paw withdrawal from sham surgery control rats and normal control rats. Moreover, the pSNL rats withdrew their neuropathic paw much more often than their contralateral paw, while the sham surgery and normal control rats withdrew each hind paw with comparable frequency. Our results therefore support our hypothesis.
Our iFU stimulation test was able to distinguish between ipsilateral and contralateral paws for the pSNL rats. In contrast the Hargreaves heat-lamp test could not. We also observed a decrease in iFU threshold values for both the paw associated with the sham operation as well as the corresponding contralateral paw, relative to the response of normal control animals. This occurred despite the fact that we did not perform surgery on the sciatic nerve enervating the contralateral paw. Together these results suggests the spinal cord of these animals changed their ability to process peripheral signals from both paws after ipsilateral neuropathic injury through a process known as central sensitization (Tracey and Mantyh, 2007), especially with regard to mechanical stimulation, the main mechanism of iFU stimulation, as reviewed in Gavrilov et al (1996). To test this hypothesis, future studies could include behavioral studies that compare application of iFU optimized to generate heat versus generate palpation, and inclusion of von calibrated mechanical stimulation.
With regard to the safety of this procedure, we note that Gavrilov, Wright, Dalecki, and colleagues applied comparable amounts of ultrasound to themselves and a few test subjects without incident. We have as well, to a larger cohort, again without incident (Dickey et al, 2012). Finally, Foley et al. (2008) found that an intensity of 7,890 W/cm2 and duration 5 seconds [hence a dose of almost 40,000 (W*s)/(cm2)] was required to cause acute damage of peripheral nerves. This is well beyond the intensity and dose values we (or anyone else, to our knowledge) have used to generate sensations with iFU in a safe manner.
Limitations
Our device has a primary, cutaneous focus and a much weaker, subcutaneous focus. Note that this secondary, weak focus appears because the design of our device, specifically use of a solid aluminum housing over the transducer, allows a portion of the ultrasound waves propagating from the transducer out the distal surface of the device to reflect inside the aluminum housing before finally propagating out the distal interface. While this design suffices for testing the central hypotheses of our study, the presence of this secondary focus does raise the following question: “in what tissue within the foot did iFU generate a sensation, tissue associated with the cutaneous focus, or with the subcutaneous focus, or both?” We can answer this question by repeating our studies with a different device that has a subcutaneous focus as well as the present device with and without EMLA cream, a known cutaneous anesthetic. We intend to follow up on this question in future studies.
We also note that our normal control animals did not undergo anesthesia. This should not have affected our results since those animals put under anesthesia returned to normal behavior soon after they awoke. Finally, our study assessed the ability of iFU to cause rats to withdraw their paws as a means of testing whether or not iFU application can differentiate between neuropathic and control tissue. Note, however, that we could not ask the rats what they are feeling, of course. We therefore don’t know if the rat’s paw withdrawal represents a reflexive response to iFU stimulation, or a conscious response to irritation or pain caused by iFU stimulation, or a combination of the two. Our human studies (Dickey et al, 2012) with the same ultrasound device applied to normal tissue of volunteers (their finger-tip pads) demonstrated that the sensations they experienced were generally only irritating. However, we never applied sufficient ultrasound to cause the volunteers to withdraw their fingers. The experience of the rats therefore remains an open question.
Potential clinical significance
Physicians may one day use intense focused ultrasound as a tool for locating and assessing deep painful pathology. For example, in an amputee patient with stump pain, physicians could use iFU under image guidance (‘ig-iFU’ – reviewed in ter Haar and Coussios, 2007) to locate and assay neuromas that may contribute to the patient’s pain. As another example, in a patient with back pain, ig-iFU could be used to non-invasively search for the presence of pain-causing structures (an edematous disc; an inflamed or distended dorsal root; a strained ligament, etc) at a given level of the spine associated with patient’s symptoms, compared to an adjacent level of the spine. In each case, if a given threshold of iFU generates a painful or uncomfortable feeling in one area but not in another, the physician could use this information as an indication of both the presence as well as the location of damage.
Conclusion
We have successfully tested the hypothesis that diffusely neuropathic tissue is more sensitive to stimulation by intense focused ultrasound (iFU) than control tissue. This is supportive of Gavrilov’s original hypothesis that focused ultrasound could one day find use diagnosing neuropathic injury through induction of abnormal, perhaps irritating sensations relative to sensations generated by iFU when applied to normal tissue. Direct testing of humans with known neuropathic injury (for example: metastatic bone cancer; diabetes; residual limbs), likely under image guidance as described above, represents the next step towards realizing Gavrilov’s vision.
Acknowledgments
This work received financial support from the Life Sciences Discovery Fund of Washington State, the NIH (UL1 RR025014, R41 NS 049719-01), the Veterans Administration, and direct funding from PhysioSonics, Inc.
Footnotes
The author’s contributions are as follows: experimental design (Kliot, Jarvik, Loeser, McClintic, Mourad, Pederson, Sparks, Tych); data collection (Garcia, McClintic, Ollos, Pederson, Sparks, Tych); data analysis (Gofeld, McClintic, Mourad, Terman, Tych); write-up (Gofeld, Jarvik, Kliot, Loeser, McClintic, Mourad, Terman). The following authors have a significant financial interest in the research described in this paper: Kliot, and Mourad.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dalecki D, Child SZ, Raeman CH, Carstensen EL. Tactile perception of ultrasound. The Journal of the Acoustical Society of America. 1995;97:3165–70. doi: 10.1121/1.411877. [DOI] [PubMed] [Google Scholar]
- Dickey TC, Tych R, Kliot M, Loeser JD, Pederson K, Mourad PD. Intense focused ultrasound can reliably induce sensations in human test subjects in a manner correlated with the density of their mechanoreceptors. Ultrasound in medicine & biology. 2012;38:85–90. doi: 10.1016/j.ultrasmedbio.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JL, Little JW, Vaezy S. Effects of high-intensity focused ultrasound on nerve conduction. Muscle & nerve. 2008;37:241–50. doi: 10.1002/mus.20932. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR, Gersuni GV, Ilyinski OB, Tsirulnikov EM, Shchekanov EE. A study of reception with the use of focused ultrasound. I. Effects on the skin and deep receptor structures in man. Brain research. 1977;135:265–77. doi: 10.1016/0006-8993(77)91030-7. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR, Gersuni GV, Ilyinsky OB, Tsirulnikov EM, Shchekanov EE. A study of reception with the use of focused ultrasound. II. Effects on the animal receptor structures. Brain research. 1977;135:279–85. doi: 10.1016/0006-8993(77)91031-9. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR, Tsirulnikov EM, Davies IA. Application of focused ultrasound for the stimulation of neural structures. Ultrasound in medicine & biology. 1996;22:179–92. doi: 10.1016/0301-5629(96)83782-3. [DOI] [PubMed] [Google Scholar]
- Gershuni GV, Tsirul’nikov EM, Gavrilov LR, Pudov VI, Rozenblium AS. Effect of exposure to focused megahertz-range ultrasound on the structure of the otic labyrinth during the process of auditory perception. Doklady Akademii nauk SSSR. 1980;251:763–6. [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hill CR, Rivens I, Vaughan MG, ter Haar GR. Lesion development in focused ultrasound surgery: a general model. Ultrasound in medicine & biology. 1994;20:259–69. doi: 10.1016/0301-5629(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Miao CH, Brayman AA, Loeb KR, Ye P, Zhou L, Mourad P, Crum LA. Ultrasound enhances gene delivery of human factor IX plasmid. Human gene therapy. 2005;16:893–905. doi: 10.1089/hum.2005.16.893. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–18. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Shir Y, Sheth R, Campbell JN, Raja SN, Seltzer Z. Soy-containing diet suppresses chronic neuropathic sensory disorders in rats. Anesthesia and analgesia. 2001;92:1029–34. doi: 10.1097/00000539-200104000-00042. [DOI] [PubMed] [Google Scholar]
- ter Haar G, Coussios C. Hing intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23:89–104. doi: 10.1080/02656730601186138. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–91. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Wright A, Graven-Nielsen T, Davies II, Arendt-Nielsen L. Temporal summation of pain from skin, muscle and joint following nociceptive ultrasonic stimulation in humans. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2002;144:475–82. doi: 10.1007/s00221-002-1062-4. [DOI] [PubMed] [Google Scholar]
- Seltzer Ze’ev, D R, Shir Yoram. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–18. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]


