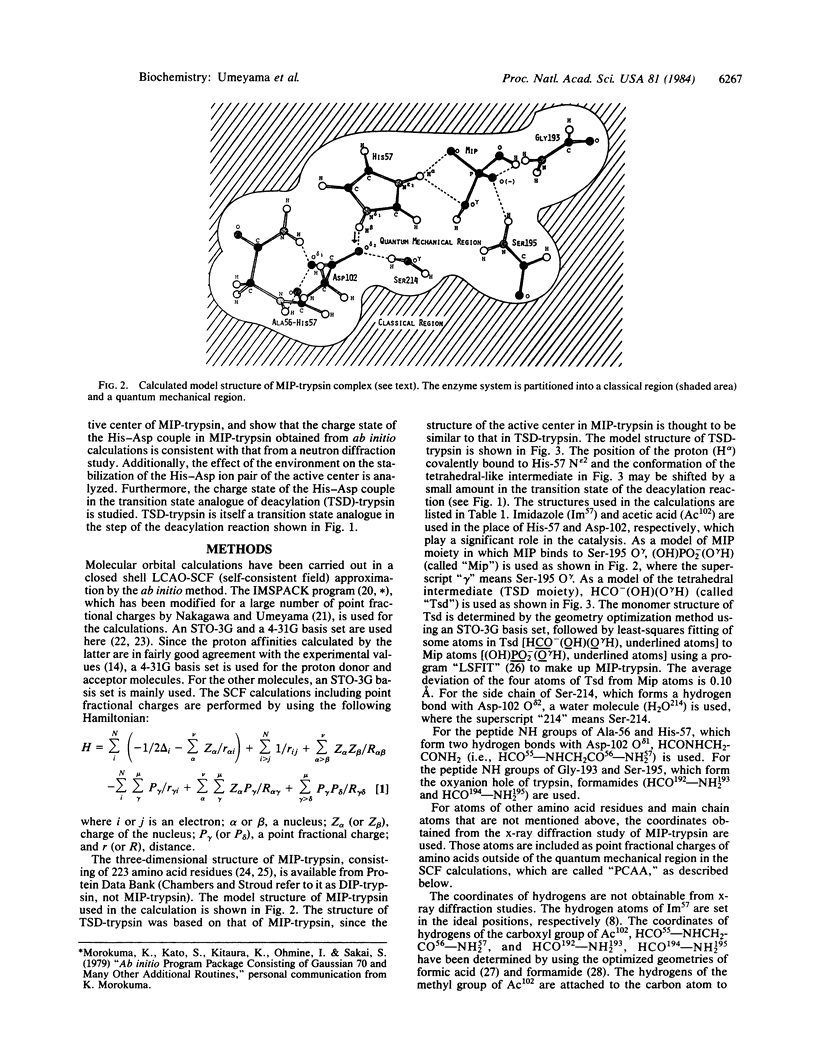
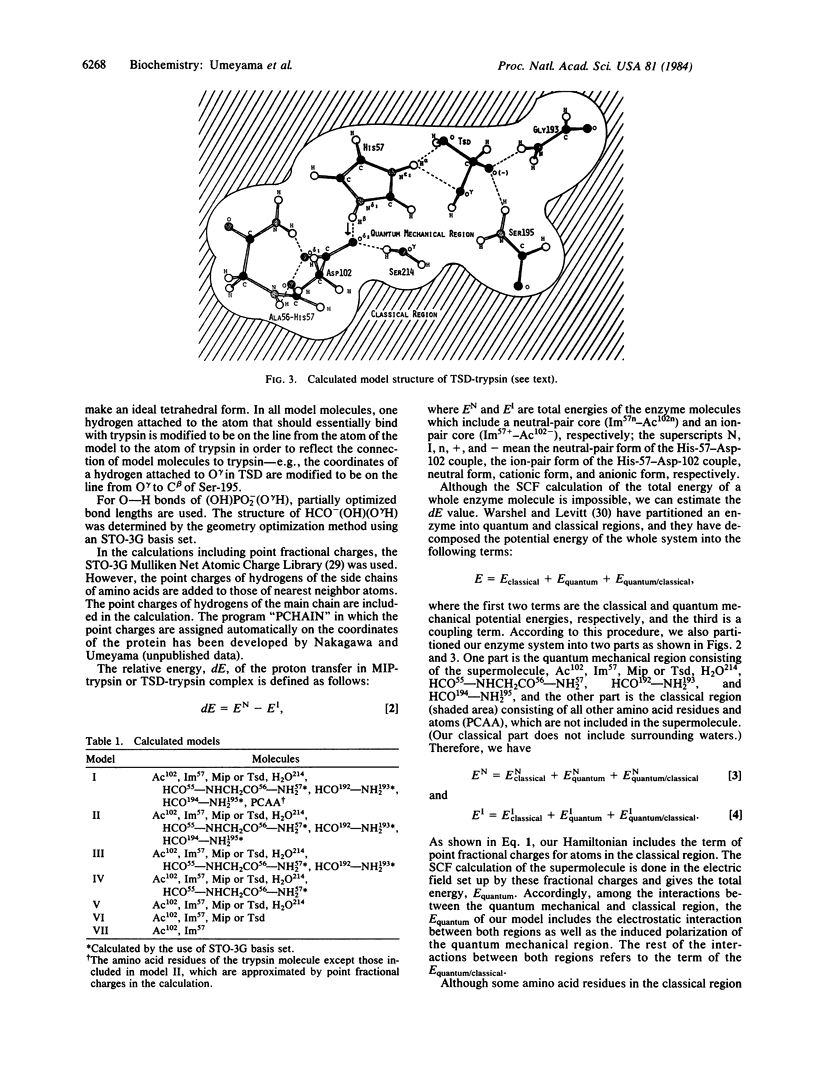
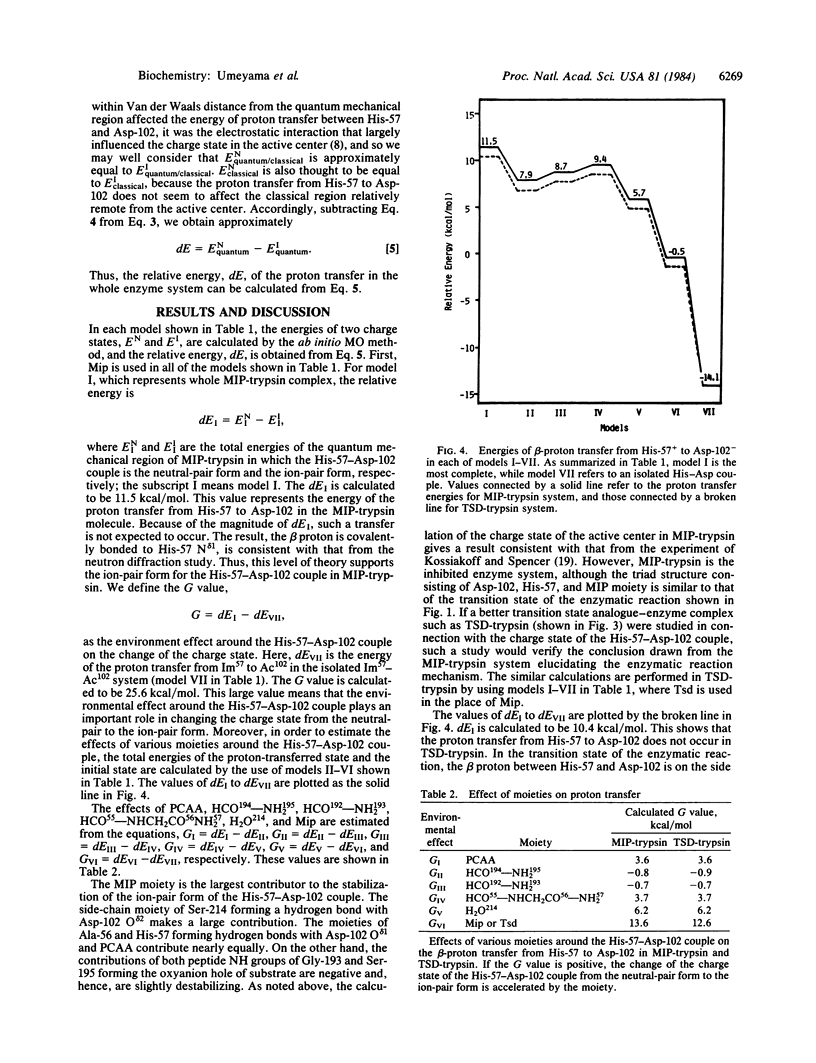
Abstract
Ab initio molecular orbital studies have been made as a model for the deacylation step of trypsin. Ser-195 is modeled by H2O in which one H is replaced either by--PO2(OH)- (monoisopropyl phosphoryl, MIP) or by--CHO(OH)- (a transition state analogue, TSD). The quantum mechanical region includes imidazole+ and acetate- as models for His-57+ and Asp-102-, two hydrogen bonds from two formamide molecules to the oxyanion MIP or TSD, and three hydrogen bonds to Asp-102. The remainder of the enzyme is treated classically as a fractional charge model. The effect of proton transfer from His-57+ to Asp-102- is very similar for the MIP and TSD models, and the proton transfer is energetically unfavorable for all models that include at least the hydrogen bond from an H2O that models Ser-214. Thus, the several hydrogen bonds to the models of the catalytic unit (substrate, Ser-195, His-57, and Asp-102) stabilize the His-57+/Asp-102- salt link, and this indicates that proton transfer does not occur from His-57+ to Asp-102-. (Also, the similarities of energy of transfer of this proton transfer for the various models show that the model substrate analogue behaves very similarly to the MIP inhibitor.)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. C. The catalytic function of active site amino acid side chains in well-characterized enzymes. Ann N Y Acad Sci. 1981;367:383–406. doi: 10.1111/j.1749-6632.1981.tb50580.x. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Smallcombe S. H., Whitaker D. R., Richards J. H. Carbon nuclear magnetic resonance studies of the histidine residue in alpha-lytic protease. Implications for the catalytic mechanism of serine proteases. Biochemistry. 1973 Nov 6;12(23):4732–4743. doi: 10.1021/bi00747a028. [DOI] [PubMed] [Google Scholar]
- Kossiakoff A. A., Spencer S. A. Neutron diffraction identifies His 57 as the catalytic base in trypsin. Nature. 1980 Nov 27;288(5789):414–416. doi: 10.1038/288414a0. [DOI] [PubMed] [Google Scholar]
- Krieger M., Koeppe R. E., 2nd, Stroud R. M. pH dependence of tritium exchange with the C-2 protons of the histidines in bovine trypsin. Biochemistry. 1976 Aug 10;15(16):3458–3464. doi: 10.1021/bi00661a010. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Porubcan M. A. The charge-relay system of serine proteinases: proton magnetic resonance titration studies of the four histidines of porcine trypsin. J Mol Biol. 1976 Apr 15;102(3):487–509. doi: 10.1016/0022-2836(76)90330-2. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Umeyama H., Kitaura K., Morokuma K. A molecular orbital study on the zinc-water-glu 270 system in carboxypeptidase A. Chem Pharm Bull (Tokyo) 1981 Jan;29(1):1–6. doi: 10.1248/cpb.29.1. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Umeyama H. Role of local induced-fit of Ser 195 in beta-trypsin: a molecular orbital study. FEBS Lett. 1982 Mar 22;139(2):181–184. doi: 10.1016/0014-5793(82)80846-6. [DOI] [PubMed] [Google Scholar]
- Robillard G., Shulman R. G. High resolution nuclear magnetic resonance studies of the active site of chymotrypsin. II. Polarization of histidine 57 by substrate analogues and competitive inhibitors. J Mol Biol. 1974 Jul 5;86(3):541–558. doi: 10.1016/0022-2836(74)90179-x. [DOI] [PubMed] [Google Scholar]
- Scheiner S., Kleier D. A., Lipscomb W. N. Molecular orbital studies of enzyme activity: I: Charge relay system and tetrahedral intermediate in acylation of serine proteinases. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2606–2610. doi: 10.1073/pnas.72.7.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner S., Lipscomb W. N. Molecular orbital studies of enzyme activity: catalytic mechanism of serine proteinases. Proc Natl Acad Sci U S A. 1976 Feb;73(2):432–436. doi: 10.1073/pnas.73.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeyama H., Imamura A., Nagata C. A molecular orbital study on the enzymic reaction mechanism of alpha-chymotrypsin. J Theor Biol. 1973 Oct;41(3):485–502. doi: 10.1016/0022-5193(73)90057-x. [DOI] [PubMed] [Google Scholar]
- Umeyama H., Nakagawa S., Kudo T. Role of Asp102 in the enzymatic reaction of bovine beta-trypsin. A molecular orbital study. J Mol Biol. 1981 Aug 15;150(3):409–421. doi: 10.1016/0022-2836(81)90556-8. [DOI] [PubMed] [Google Scholar]
- Umeyama H., Nakagawa S. The pKa value of His 57-Asp 102 couple in the active site of bovine pancreatic beta-trypsin: a molecular orbital study. J Theor Biol. 1982 Dec 21;99(4):759–775. doi: 10.1016/0022-5193(82)90196-5. [DOI] [PubMed] [Google Scholar]
- Warshel A., Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976 May 15;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]


