Abstract
Respiratory dysfunction is one of the most common causes of death associated with premature birth (Barton et al., 1999). In the United States, 7–10% of pregnant women receive antenatal glucocorticoid (GC) therapy (Matthews et al., 2004), while approximately 19% of very low birth weight infants receive postnatal GC therapy (Jobe, 2009). Clinical research suggests that GC treatment causes permanent neuromotor and cognitive deficits (Yeh et al., 2004) and stunts cerebellar growth (Parikh et al., 2007; Tam et al., 2011). We previously reported that GC-mediated neural progenitor cell (NPC) apoptosis may be responsible for cerebellar neuropathology (Maloney et al., 2011; Noguchi et al., 2008; Noguchi et al., 2011). The goal of the current study was to determine whether lithium protects NPCs from GC neuroapoptosis in vivo and in vitro. Given that it protects against a range of brain insults, we hypothesized that lithium would significantly attenuate GC induced NPC toxicity. We report that acute lithium pretreatment provides potent, cell-intrinsic neuroprotection against GC induced NPC toxicity in vivo and in vitro.
Keywords: Apoptosis, cerebellum, dexamethasone, external granule layer, glucocorticoid, lithium, neuroprotection, neural progenitor cell
1. Introduction
Respiratory dysfunction is one of the most common causes of death associated with premature birth (Barton et al., 1999). As a result, perinatal glucocorticoid (GC) therapy is widely used to mature the lungs and improve respiratory function in prematurely born infants. Opinions on the safety of GC therapy vary dependent on when (antenatally or postnatally) or how long (acutely or chronically) it is administered. In the United States, approximately 7–10% of pregnant women receive antenatal GC therapy (Matthews et al., 2004), and there is a broad consensus that the benefits of a single treatment greatly outweigh the risks (National Institutes of Health Consensus Development Panel [NIHCDP], 1994). However, there are concerns that GC therapy stunts growth when multiple antenatal treatments are given (NIHCDP, 1994). Similarly, approximately 19% of very low birth weight infants receive postnatal GC therapy (Jobe, 2009). Unfortunately, clinical research suggests that this treatment produces permanent neuromotor and cognitive deficits (Yeh et al., 2004) and stunts cerebellar growth (Parikh et al., 2007; Tam et al., 2011). Initially, the American Academy of Pediatrics (2002) recommended that postnatal GC therapy not be used outside of randomized controlled clinical trials. However, in 2010, a revised policy recommended that the clinician balance potential adverse and beneficial effects when deciding whether to administer postnatal GC therapy (Watterberg, 2010). Currently, this treatment remains highly individual/institutional specific (Tin and Wiswell, 2009), suggesting that there is no clear consensus for its use.
Despite these concerns, surprisingly little is known about how GC stimulation disrupts cerebellar development. Recently, we reported that neonatal GC exposure in mouse pups produces rapid (within 4 hours) and selective apoptosis (programmed cell death) in the neural progenitor cells (NPC) of the external granule layer (EGL) (Noguchi et al., 2008). The EGL is a transient proliferative region occupying the outermost layer of the developing neonatal cerebellum. During ontogenesis, the EGL produces granule cell neurons in the outer EGL that mature in the inner EGL before migrating past the molecular and Purkinje cells layers to populate the internal granule cell layer (Figure 1A). While almost all other neural proliferative layers disappear prenatally, the human and rodent EGL continues proliferating during the postnatal period. In rodents, the EGL proliferates until around two weeks of age at which point it rapidly disappears once neurogenesis is no longer needed (Carletti and Rossi, 2008). The importance of the EGL in normal brain development and function is underscored by the fact that it produces 90% of the neurons in the cerebellum which represent over half the neurons in the brain (Andersen et al., 1992; Harvey and Napper, 1988; Herculano-Houzel, 2009). Due to the large number of neurons a single NPC can produce, NPC apoptosis can magnify cerebellar pathology when compared to neuronal apoptosis (Noguchi et al., 2011). Consistent with this concept, a single neonatal injection of GCs permanently reduces the number of granule cell neurons in the cerebellum by 18% in rodents (Noguchi et al., 2008).
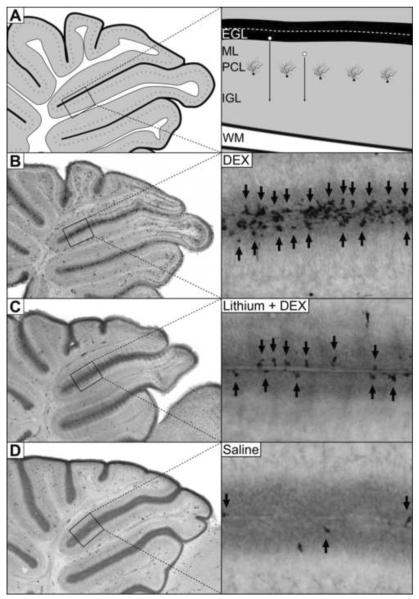
Figure 1. Lithium protects against NPC apoptosis in the EGL.
[A] The external granule layer (EGL) is a proliferative layer responsible for producing a massive number of granule cell neurons during cerebellar development. Once an NPC produces a granule cell neuron, it migrates (white circles with black arrows) past the molecular (ML) and Purkinje cell layers (PCL) before populating the internal granule cell layer (IGL) adjacent to the cerebellar white matter (WM). [B] A 3.0 mg/kg injection of dexamethasone (DEX) dramatically increases neural progenitor cell apoptosis in the EGL as measured by activated caspase-3 immunolabeling (black arrows). [C] Pretreatment with lithium chloride 15 minutes prior to DEX injection significantly lowers EGL apoptosis. [D] Limited amounts of physiological apoptosis can be seen in the saline control group.
Interestingly, the ability of GCs to cause NPC apoptosis may be related to their role in normal neurodevelopment. For instance, we reported endogenous GC release, GC metabolism, and GC receptor expression are carefully orchestrated to increase GC stimulation selectively in the EGL as this proliferative region is naturally eliminated from the cerebellum (Noguchi et al., 2011). As a result, this selective toxicity may be a byproduct of the natural role of GC stimulation in apoptotically eliminating the EGL once neurogenesis is no longer needed. This concept is supported by research showing adrenalectomy (which eliminates endogenous GC release) dramatically delays the natural disappearance of the EGL (Meyer, 1983; Yehuda et al., 1989; Yehuda and Meyer, 1991). Since the cerebellum is involved in a variety of neuromotor and cognitive functions (Schmahmann, 2004), GC induced EGL apoptosis may be responsible for cerebellar stunting and behavioral deficits reported clinically in humans exposed to GCs (Parikh et al., 2007; Tam et al., 2011; Yeh et al., 2004). Indeed, in a recent study, we found postnatal GC exposure led to permanent neuromotor deficits and selective cerebellar stunting in mice (Maloney et al., 2011). If GC induced EGL apoptosis is responsible for these deficits, the use of neuroprotective agents may prevent the iatrogenic effects of GC therapy, while retaining beneficial effects on lung maturation. Interestingly, lithium protects against neuronal apoptosis produced by a wide variety of insults Jorda et al., 2004; Straiko et al., 2009; Young et al., 2008). In addition, it has been shown to delay NPC apoptosis in the EGL following radiation exposure (Inouye et al., 1995). Based on these findings, the neuroprotective effect of lithium on GC induced apoptosis was examined.
2. Results
2.1 Lithium carbonate protects against GC induced EGL apoptosis
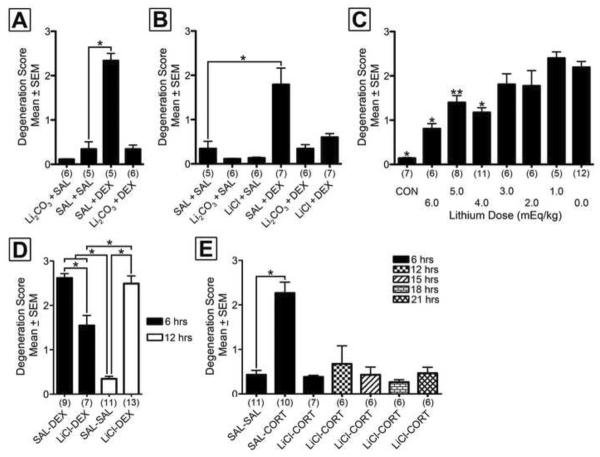
In previous research, we established that DEX exposure produces rapid apoptosis (as measured by activated caspase-3 immunolabeling) in the EGL of ICR mice at six hours postinjection (Noguchi et al., 2011). In order to examine the neuroprotective abilities of lithium, a 6 mEquivalent (mEq)/kg (i.e., 221.67 mg/kg) intraperitoneal injection of lithium carbonate or saline was administered 15 minutes prior to 3.0 mg/kg DEX or saline. Animals were isolated from the mother at 30°C until perfusion 6 hours later. Following semiquantitative analysis, one-way ANOVA revealed a statistically significant effect of drug treatment on EGL apoptosis, F(3,18) = 77.87, p < 0.0001. A Bonferroni planned comparison versus control group revealed DEX alone significantly increased EGL apoptosis but this effect was prevented by lithium pretreatment (Figure 2A). Lithium carbonate exposure alone had no significant effect on degeneration scores.
Figure 2. Lithium permanently prevents GC induced apoptosis in vivo.
[A] Semiquantitative degeneration scores reveal lithium carbonate (Li2CO3) potently blocks dexamethasone (DEX) induced apoptosis compared to saline (SAL) treatment. [B] Lithium chloride (LiCl) and Li2CO3 both potently protect against GC induced apoptosis. [C] Lithium pretreatment significantly reduces DEX induced apoptosis at 4.0 mEq/kg and above in a dose dependent manner. A control (CON) group exposed to no DEX or lithium exhibits low physiological levels of apoptosis which is significantly lower than DEX treatment alone. [D] Lithium protects against DEX induced EGL apoptosis at 6 hours however this toxicity returns at 12 hours. [E] Lithium neuroprotection against corticosterone (which has a shorter half-life than DEX) permanently blocks EGL apoptosis. Numbers in parentheses represent number of animals per group. Bonferroni post-hoc significance levels: *p < 0.001, **p < 0.01.
2.2 Lithium chloride is as neuroprotective as lithium carbonate
In the clinical setting, lithium is typically taken orally in the form of lithium carbonate. Once ingested, hydrochloric acid in the stomach converts lithium carbonate to lithium chloride (2 HCl + Li2CO3 = 2 LiCl + H2 + CO2). Since an intraperitoneal injection of lithium carbonate would bypass stomach acid exposure, we also tested the neuroprotective effect of a 6 mEq/kg dose of lithium chloride (253.8 mg/kg) and compared it to the same mEq/kg dose of lithium carbonate. Lithium carbonate, lithium chloride, or saline was administered 15 minutes prior to a 3.0 mg/kg DEX or saline injection. Animals were perfused 6 hours later for activated caspase-3 immunohistochemistry. Following semiquantitative analysis, one-way ANOVA revealed a statistically significant effect of drug treatment, F(5,31) = 12.04, p < 0.0001 (Figure 2B). A Bonferroni planned comparison versus control group revealed DEX alone significantly increased EGL apoptosis which was prevented by both lithium chloride and lithium carbonate (Figures 1B–D). Administration of either form of lithium alone had no significant effect on degeneration scores. These results reveal that lithium carbonate is as neuroprotective as lithium chloride. Since lithium carbonate needs acidification prior to injection and would necessitate the addition of hydrochloric acid in our cell culture work, lithium chloride was used in all subsequent experiments.
2.3 Lithium neuroprotection is dose responsive
Next we produced a neuroprotection dose response curve for lithium chloride. Animals were injected with 6, 5, 4, 3, 2, 1, and 0 mEq/kg (i.e., 254, 212, 170, 127, 84, 41, or 0 mg/kg) lithium chloride 15 minutes prior to a 3.0 mg/kg DEX injection and perfused for activated caspase-3 immunolabeling 6 hours later. A control group injected twice with saline 15 minutes apart and perfused 6 hours later was also tested. The results revealed that lithium chloride is neuroprotective at doses 4 mEq and above (Figure 2C). Data were further examined by correlating the dose of lithium with degeneration score. The results revealed a statistically significant negative linear correlation (r = −0.6924, p < 0.0001), indicating that lithium's protection is dose responsive.
2.4 Lithium prevents rather than delays EGL apoptosis
In previous research, lithium was shown to delay (rather than prevent) radiation induced EGL apoptosis (Inouye et al., 1995). If the same were true for GC induced apoptosis, lithium would have limited value as a neuroprotectant. In order to test this hypothesis, we first tested lithium's neuroprotective ability against dexamethasone over time. PND7 mouse pups were pretreated with 6 mEq/kg dose of lithium chloride or saline 15 minutes before a 3.0 mg/kg dexamethasone or saline injection. Animals were perfused 6 or 12 hours after dexamethasone injection for activated caspase-3 immunolabeling and semiquantification. A significant ANOVA and all pairwise Bonferroni posthoc comparisons revealed that while lithium was neuroprotective at 6 hours, DEX induced toxicity returned 12 hours post-treatment, F(3,36) = 54.95, p < 0.0001 (Figure 2D). These results suggest one of two possibilities. The first is that lithium simply delays, rather than prevents, GC induced apoptosis. The second possibility is that, due to drug metabolism, the apoptotic effects of dexamethasone extend beyond lithium's neuroprotection. In order to test this hypothesis, we examined lithium neuroprotection versus corticosterone, the endogenous rodent GC. The rodent half-life of corticosterone is only 25 minutes (Vachon and Moreau, 2001) whereas the half-life of lithium is 3.5 hours (Wood et al., 1986). As a result, lithium's neuroprotection should extend far beyond corticosterone's ability to produce apoptosis. A significant ANOVA and planned comparison versus control showed that lithium prevented, rather than delayed corticosterone induced apoptosis at all time points, F(6,45) = 15.74, p < 0.0001 (Figure 2E). Based on this finding, we concluded that lithium prevents GC induced apoptosis.
2.5 DEX induced apoptosis is cell intrinsic at therapeutic concentrations
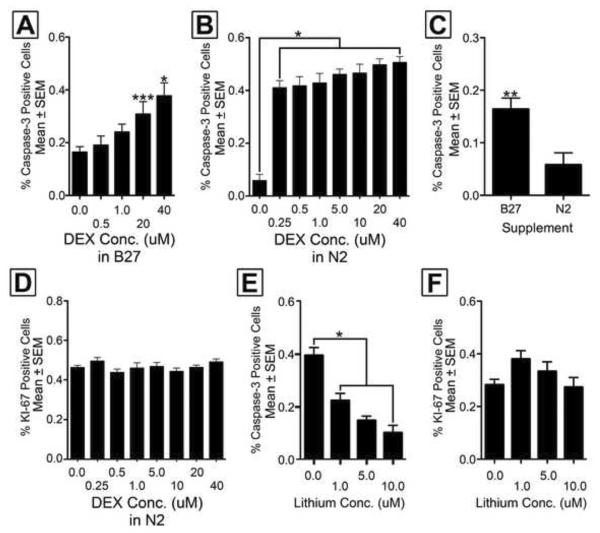
Next we examined the apoptotic and proliferative effects of DEX in primary EGL NPC cultures to determine whether DEX induced apoptosis is mediated directly at the cell (cell-intrinsic), a secondary response caused by effects on neighboring cells, or indeed another region of the body. We first compared the N2 and B27 supplements to see which should be used in our experiments. While numerous experiments have used B27 in EGL cell cultures (Collins et al., 2008; Lee et al., 2005; Rios et al., 2004) this supplement contains corticosterone (Brewer et al., 1993). While the exact concentration of corticosterone in Life Technologies' B27 is proprietary, its inclusion suggests this GC is present at a physiologically relevant concentration. As a result, it was critical to determine if simply using this supplement increases EGL apoptosis, which may mask the effect of DEX on NPCs. First, we generated a DEX concentration response curve for both supplements to see if B27 may mask any significant effects. With B27, plated cells were allowed to incubate overnight and DEX was added the following morning at 0.0, 0.5, 1.0, 20.0, or 40.0 μM. One-way ANOVA revealed a statistically significant difference between groups (F[4,74] = 5.530, p < 0.001) and a subsequent planned comparison versus control (0.0 μM) found only the 20.0 and 40.0 μM concentrations produced a significant increase in apoptosis (Figure 3A). Unfortunately, since the highest pharmacologically relevant concentration of DEX is considered 1 μM (Liberman et al., 2009), this finding has limited clinical relevance.
Figure 3. Dexamethasone induced apoptosis and lithium neuroprotection is cell intrinsic.
[A] A concentration response curve shows EGL NPCs cultured in B27 media exhibit a significant increase in the percentage apoptotic cells at 20 μM and above. [B] When EGL NPCs are cultured in N2 media, DEX increases the percentage of apoptotic NPCs at 0.25 μM and above. [C] EGL NPCs cultured in B27 supplement alone show significant increases in the percentage of activated caspase-3 positive cells when compared to N2. [D] DEX concentration has no significant effect on proliferation (as measured by the percentage of KI-67 positive NPCs) in N2 media. [E] Lithium chloride protects against DEX induced apoptosis in a concentration dependent manner in cell culture. [F] The same cells show lithium has no effect on the percentage of KI-67 positive cells. Bonferroni post-hoc significance levels: *p < 0.001, ** p < 0.01, ***p < 0.05.
Next, we used the corticosterone-free supplement N2 to generate a concentration response curve. Using N2 medium, one-way ANOVA revealed DEX increases apoptosis at concentrations 0.25 μM and above, which is well within the therapeutic range, F(7,232) = 26.33, p = 0.0035 (Figure 3B). Notably, the percentage of caspase-3 positive cells when no DEX is added is twice as high for the B27 group when compared to the N2 group, t(47) = 3.142, p < 0.01(Figure 3C). Finally, we examined the effects of DEX concentration on proliferation (as measured by the percentage of KI-67 positive NPCs). Results revealed DEX concentration had no effect on NPC proliferation, F(7,232) = 1.263, p > 0.05 (Figure 3D).
2.6 Lithium's neuroprotection is cell intrinsic
We then tested whether lithium's neuroprotective effects are also mediated directly at the NPC. Plated cells immersed in N2 supplement were allowed to incubate overnight and then exposed to 0, 1, 5, or 10 μM of lithium chloride one hour before exposure to 40 μM DEX. Twenty-four hours later, cells were quantified after fixation and immunolabeled for activated caspase-3, KI-67, and DAPI. A significant one-way ANOVA with Bonferroni correction revealed lithium chloride significantly reduced the percentage of activated caspase-3 positive cells at 1 μM and above, F(3,114) = 24.60, p < 0.0001 (Figure 3E). Data were further examined by correlating the concentration of lithium with percent of activated caspase-3 positive cells. The results revealed a statistically significant negative linear correlation (r = −0.5341, p < 0.0001), indicating the lithium's protection is dose responsive. A similar analysis on KI-67 positive cells showed no significant differences between groups indicating lithium has no effect on proliferation, F(3,114) = 2.326, p > 0.05 (Figure 3F).
3. Discussion
Here we report that lithium pretreatment is neuroprotective against GC induced EGL apoptosis in a dose responsive manner. This finding is consistent with numerous studies which show that lithium protects against a range of central nervous system insults including brain ischemia (Nonaka and Chuang, 1998), tauopathy (Hong et al., 1997), GABAergic induced apoptosis (Straiko et al., 2009), β-amyloid neurotoxicity (Phiel et al., 2003), and ethanol induced apoptosis (Young et al., 2008). In vivo, both lithium carbonate and its primary metabolite lithium chloride are neuroprotective for NPCs in developing cerebellum. Neuroprotection was evident at 4 mEq/kg and above which is similar to the doses used by our colleagues to protect against anesthesia- and ethanol-induced neuronal apoptosis (Straiko et al., 2009; Zhong et al., 2006).
While lithium exerts anti-apoptotic properties against a variety of central nervous system insults its neuroprotective mechanisms are still being investigated. During intrinsic apoptosis, BAX and BAK disrupt mitochondrial membrane permeability, leading to the release of cytochrome c. This, in turn, allows cytochrome c to bind with APAF-1 and caspase-9, which proteolytically cleaves pro-caspase-3 into its activated form. Activated caspase-3 is a critical executioner caspase, participating in proteolytic cleavage of numerous proteins, DNA fragmentation, and chromatin condensation (Slee et al., 2000). Mounting evidence suggests that lithium regulates important components of the apoptotic cascade. For instance, exposure to ethanol is thought to promote apoptosis by inhibiting the activation of extracellular-signal-regulated kinases 1 and 2. However, lithium can block this inhibition, thus enhancing cell survival (Young and Olney, 2008). In addition, after cerebral ischemia, lithium significantly increased levels of the anti-apoptotic protein Bcl-2 (Sheng et al., 2011). Bcl-2 inhibits relocation of BAX and BAK to the mitochondrial membrane, thus reducing membrane permeability and inhibiting cytochrome c release. Furthermore, although its precise apoptotic mechanism is poorly understood, glycogen synthase kinase-3β (GSK3β) promotes intrinsic apoptosis. Lithium is a potent inhibitor of GSK3β by maintaining GSK3β in a dephosphorylated, inactivated state (De Sarno et al., 2002; Klein and Melton, 1996; Mora et al., 2011). Of particular relevance to the current study, Mora et al. (2001) reported that lithium suppressed the activation of caspase-3 in potassium-deprived cultured cerebellar granule neurons. These observations suggest that lithium is a potent neuroprotectant against DEX-induced NPC apoptosis by promoting cellular survival signaling pathways and inhibiting the apoptotic cascade.
Additionally, while previous reports have found lithium delays, but not does not prevent, radiation induced EGL apoptosis (Inouye et al., 1995), we found this same neuroprotectant prevents GC induced apoptosis. This may be related to the different intracellular signaling mechanisms regulating radiation and GC induced apoptosis. For instance, since EGL NPCs are mitotically active, they are highly susceptible to the genotoxic effects of ionizing radiation. The p53 protein (a tumor suppressor) can detect this damage and activate DNA repair proteins. However, if a cell is irreparable, p53 initiates apoptosis to remove the cell and prevent proliferation of mutant progeny (Herzog et al., 2007). While lithium can block downstream apoptotic signaling, it cannot repair genetic damage. Therefore, as lithium becomes metabolized and neuroprotection declines, p53 signaling may eventually be able to re-initiate apoptosis. Unlike apoptosis produced by genotoxins, we have found GC induced apoptosis is p53 independent (Noguchi et al., 2008). Therefore, if lithium pretreatment is still neuroprotective by the time GCs levels decline below the apoptotic threshold, cell death may be prevented rather than delayed.
We also examined the apoptotic effects of DEX and lithium using primary EGL cell cultures. It should be noted that DEX induced apoptosis (as measured by activated caspase-3 immunohistochemistry) occurs 4–8 hours after exposure in vivo yet we reported this toxicity occurs at 24 hours in vitro. Interestingly, the delayed time course is consistent with numerous studies examining apoptosis in vitro. For instance, Heine and Rowitch (2009) found DEX increases EGL NPC apoptosis 24 hours after exposure in cell culture. In addition, ethanol increases neuronal apoptosis 4–8 hours after in vivo exposure (Young and Olney, 2006) but takes 24 hours to produce in vitro (Zhong et al., 2006). Zhong et al. (2006) also reported that lithium pretreatment can prevent this toxicity over the same time period. As a result, the time course we chose is consistent with previous studies examining both apoptosis and lithium protection. It has been suggested that the extended delay in apoptogenesis in cell culture might be related to the absence of apoptosis modulating factors in vitro (Moulder et al., 2002). Indeed, ethanol induced apoptosis in neonatal wild-type mice is detectable 4 hours after exposure in vivo but toxicity is dramatically delayed to 24 hours following caspase-3 knockout (Young et al., 2005).
Due to the inclusion of corticosterone, the endogenous rodent GC that itself produces NPC apoptosis (Noguchi et al., 2008; Noguchi et al., 2011), it was important to determine whether B27 was an appropriate supplement for culturing EGL NPCs in vitro. We found B27 increases background apoptosis and masks DEX induced EGL apoptosis. Thus, while this supplement may be appropriate for other cell types not susceptible to GC induced apoptosis, we cannot recommend its use for primary EGL culture experiments. The importance of this finding is underscored by the fact that several previous studies have studied primary EGL NPCs using B27 media (Collins et al., 2008; Lee et al., 2005; Rios et al., 2004). Finally, it should be mentioned that due to the complex composition of B27 and its proprietary concentration information, we cannot rule out that other components of the medium may induce NPC apoptosis.
We next produced a concentration response curve for DEX induced apoptosis in vitro. While previous research has reported DEX increases NPC apoptosis at 40 μM (Heine and Rowitch, 2009), we were concerned that this concentration was far above DEX's maximum in vitro therapeutic range of 1 μM (Liberman et al., 2009). Our concentration response curve revealed that DEX indeed increases NPC apoptosis at therapeutic doses (0.25 μM and above) when NPCs are cultured in corticosterone-free N2 media. To our knowledge, this is the first study to find that therapeutic concentrations of DEX can produce NPC apoptosis cell intrinsically. We also found lithium dose dependently protects against this toxicity. This is consistent with numerous other studies showing lithium's ability to protect against apoptosis is cell intrinsic (Jorda et al., 2004; Nonaka et al., 1998; Zhong et al., 2006).
Notably, DEX treatment did not affect the percentage of KI-67 positive cells, suggesting this drug does not attenuate NPC proliferation. Using supratherapeutic concentrations of DEX (40 μM), Heine and Rowitch (2009) concluded GC stimulation causes downstream inhibition of the Sonic Hedgehog Pathway (ShhP). Since activation of the ShhP potently increases proliferation in the EGL, the authors concluded the reduced neurogenesis following DEX exposure was mediated by ShhP inhibition (rather than increased NPC apoptosis). However, results from studies by two independent groups testing the effects of GCs on the ShhP contradict this interpretation (Wang et al., 2010; Wang et al., 2012). Both groups reported that numerous select GCs potentiate or stimulate the ShhP in EGL NPCs and other cell types, suggesting that ShhP inhibition is not an inherent downstream effect of GC stimulation. Furthermore, they found GCs which are ShhP agonists competitively bind to the Smoothened receptor that normally stimulates the ShhP. These results suggest that ShhP effects are mediated by a completely independent receptor site (Wang et al., 2010; Wang et al., 2012). Importantly, select GCs agonize the ShhP at doses at least 10 times higher than the GC effects (Wang et al., 2012). Therefore, in the EGL, one would expect any dose high enough to influence the ShhP to also initiate apoptosis. If true, any changes in proliferation would be eliminated by the apoptotic death of the NPC. It was also reported that a much smaller number of GCs can inhibit the ShhP. While neither directly tested the effects of DEX on the ShhP, this drug did produce ciliary accumulation (a prerequisite for ShhP stimulation). Since all tested GCs which increase ciliary accumulation also potentiated the ShhP (Wang et al., 2012), this suggests DEX may actually enhance ShhP stimulation. These results may explain why Heine and Rowitch (2011) have been unable to replicate DEX induced ShhP inhibition in vitro and failed to reproduce this effect in vivo (Heine and Rowitch, 2010). Nevertheless, numerous papers have reported primary detrimental effects of GCs on the cerebellum are due to reductions in proliferation due to ShhP inhibition (Baud and Gressens, 2011; Gulino et al., 2009; Kosmac et al., 2013; Tam, 2013).
4. Materials and Methods
4.1 Animals and Drugs
Lithium chloride, lithium carbonate, and the synthetic GC dexamethasone (DEX) were obtained from Sigma Aldrich (St. Louis, MO, USA). To generate lithium chloride, lithium carbonate was acidified drop-wise with 2N hydrochloric acid until sublimation of gas bubbles was complete. The solution was then neutralized with sodium hydroxide prior to injection. DEX Sodium Phosphate USP (Hawkins, Incorporated Pharmaceuticals Group, Minneapolis, MN) is the phosphate ester of DEX and is highly soluble in water, making it the preferred form used clinically. Once injected, this pro-drug is rapidly converted to DEX. In all in vivo experiments, DEX sodium phosphate was dissolved in saline with doses expressed as equimolar equivalents to DEX. Since the plasma enzymes that hydrolyze DEX sodium phosphate into DEX may be absent in vitro, DEX was used in all cell culture experiments. ICR mice (Harlan, IN, USA) were used in all experiments. Previous research established that gender has no effect on DEX induced EGL apoptosis (Noguchi et al., 2008) and, as a result, was not used as a factor in any analyses. All animal care procedures were in accordance with procedures approved by the Washington University Animal Studies Committee and consistent with the NIH Guidelines for the Care and Use of Laboratory Animals. After injection, mouse pups were separated from their mother and maintained in a V1200 Mediheat Veterinarian Recovery Chamber (Harvard Apparatus, Holliston, MA, USA) at 30°C until perfusion unless otherwise stated.
4.2 Immunohistochemistry and semiquantitative analysis
For in vivo experiments, postnatal day (PND) 7 animals were deeply anesthetized and transcardially perfused with 4% paraformaldehyde in 0.1 M Tris buffer. Brains were removed and postfixed for several days before sagittal sectioning at 75 um on a vibratome. For activated caspase-3 immunolabeling, sections were quenched using methanol with 3% hydrogen peroxide for 15 minutes, blocked using 2% BSA, 0.2% milk, 0.1% Triton-X in PBS for an hour, and incubated overnight in activated caspase-3 primary antibody (Cell Signaling Technology, Danvers, MA, USA) diluted at 1:1000. The next morning, sections were incubated in goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA, USA), reacted with Vectastain ABC Elite Kit (Vector Laboratories), and visualized using the Vector VIP chromogen reagent (Vector Laboratories).
Semiquantitative analysis of EGL apoptosis was performed as described previously (Noguchi et al., 2008; Noguchi et al., 2011). Briefly, a rater blind to treatment scored each cerebellum by examining several midsagittal sections per animal and semiquantitatively estimating the amount of activated caspase-3 positive cells in the EGL based on the following scale: 0 = no apoptotic profiles in all EGL regions, 1 = apoptotic profiles are seen in a minority of EGL regions, 2 = apoptotic profiles are seen in a majority of EGL regions, and 3 = apoptotic profiles are seen in all regions of the EGL.
4.3 Cell culture
Cerebella were isolated from PND6-PND8 mouse pups and papain digested with DNase at 37°C for 30 minutes. EGL NPCs were isolated from dissociated tissue using a 35%/65% Percoll fractionation using a 12 minute centrifugation at 2500 rpm as described previously (Dougherty et al., 2005). Cells were then resuspended using 5 mL neurobasal with supplement, 1% sodium pyruvate, 1% L-glutamine, and 1% penicillin/streptomycin supplemented with Sonic Hedgehog (R&D Systems, Minneapolis, MN, USA) at 0.625 μg/mL. Either 2% B27 (Life Technologies, Grand Island, NY, USA) or 2.25% N2 (Life Technologies, Grand Island, NY, USA) were used as described in the Results section. Finally, 333 μL of resuspended cells were plated at 800,000 cells per well on poly-d-lysine coated glass coverslips in 24 well plates. Once plated, primary EGL cultures were allowed to incubate overnight in B27 or N2 supplement and treated the next morning with drug(s). At 24 hours after drug exposure, cells were fixed with 4% paraformaldehyde and triple fluorescently labeled using the proliferative cell marker KI-67 (BD Biosciences, San Jose, CA, USA; 1:800), apoptotic marker activated caspase-3, and DAPI (a cell nucleus stain) as described previously (Dougherty et al., 2005). The 24 hour post-exposure time point was chosen since previous research has shown DEX significantly increases EGL apoptosis at this time (Heine and Rowitch, 2009). Quantification involved using three coverslips for each treatment condition. Fluorescently labeled coverslips were then imaged using a Perkin Elmer UltraView Vox spinning disk confocal on a Zeiss Axiovert microscope at 40X. Ten random images were taken from each coverslip for activated caspase-3, KI-67, and DAPI. A rater, blind to treatment, used NIH imageJ (Version 1.47h) to count the number of activated caspase-3, KI-67, and DAPI positive cells for each image. Measures of apoptosis or proliferation were expressed as percentage of DAPI labeled cells that are activated caspase-3 or KI-67 positive, respectively. All cell counts were statistically analyzed using a t-test or ANOVA with significant effects examined using Bonferroni posthoc.
5. Conclusions
Since GCs stimulate lung maturation and surfactant production, they are commonly employed as the first line of defense in treating respiratory dysfunction associated with premature birth. Yet, clinical research has associated postnatal GC therapy with decreased neonatal cerebellar volume (Parikh et al., 2007; Tam et al., 2011) and stunted neuromotor and cognitive development lasting well into adolescence (Yeh et al., 2004). We previously suggested that EGL apoptosis may be the mechanism driving GC induced pathology and behavioral deficits (Noguchi et al., 2008; Noguchi et al., 2011). Consistent with this hypothesis and with the clinical literature, acute neonatal DEX exposure in mice leads to selective cerebellar apoptosis (Noguchi et al., 2008) and permanent neuromotor deficits (Maloney et al., 2011). Given its ability to protect against NPC apoptosis, lithium may limit the iatrogenic effects of cerebellar stunting and behavioral deficits commonly reported after GC treatment for preterm infant respiratory dysfunction. Nevertheless, while lithium may be neuroprotective in the cerebellum, we did not examine whether it may cause adverse effects on the rest of the body. Clinically, there has been limited research examining the safety of lithium during the perinatal period. Early studies indicated that lithium is a teratogen if taken chronically by pregnant mothers (Weinstein and Goldfield, 1975). However, more recent evidence calls these findings into question, reporting no significant differences in the occurrence of fetal abnormalities between neonates exposed to lithium versus those who were not (Jacobson et al., 1992). Even less is known about lithium's effects postnatally, but case studies have reported incidences of lethargy, hypothermia, and hypotonia (Gentile, 2006).
However, our results suggest that a neuroprotective dose of lithium can be administered acutely, avoiding the steady state levels used to treat bipolar disorder and potential teratogenic effects. As a result, one might expect any adverse effects to be more limited following an acute neuroprotective dose of lithium. Nevertheless, we strongly suggest caution be used when administering lithium in the perinatal clinical setting.
Highlights
We examine lithium neuroprotection on glucocorticoid-induced cerebellar pathology
Lithium protects against cerebellar neural progenitor cell apoptosis in vivo
Lithium neuroprotection is dose-responsive in vitro
Lithium prevents rather than delays neural progenitor cell apoptosis
Lithium neuroprotection is cell-intrinsic
Acknowledgements
This work was supported by: (K.N.) NIH/NIMH Grant MH083046, (N.F.) the Intellectual and Developmental Disabilities Research Center at Washington University (NIH/NICHD P30 HD062171), and (J.D.) the Children's Discovery Institute of Washington University and NINDS (5R00NS067239-03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Academy of Pediatrics Committee on Fetus and Newborn Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330–8. doi: 10.1542/peds.109.2.330. [DOI] [PubMed] [Google Scholar]
- Andersen BB, Korbo L, Pakkenberg B. A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol. 1992;326:549–60. doi: 10.1002/cne.903260405. [DOI] [PubMed] [Google Scholar]
- Barton L, Hodgman JE, Pavlova Z. Causes of death in the extremely low birth weight infant. Pediatrics. 1999;103:446–51. doi: 10.1542/peds.103.2.446. [DOI] [PubMed] [Google Scholar]
- Baud O, Gressens P. Hedgehog rushes to the rescue of the developing cerebellum. Sci Transl Med. 2011;3:105ps40. doi: 10.1126/scitranslmed.3003080. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented neurobasal, a new serum-free medium combination. J Neuroscience Res. 1993;35:567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Carletti B, Rossi F. Neurogenesis in the cerebellum. Neuroscientist. 2008;14:91–100. doi: 10.1177/1073858407304629. [DOI] [PubMed] [Google Scholar]
- Collins LL, Williamson MA, Thompson BD, Dever DP, Gasiewicz TA, Opanashuk LA. 2,3,7,8-Tetracholorodibenzo-p-dioxin exposure disrupts granule neuron precursor maturation in the developing mouse cerebellum. Toxicol Sci. 2008;103:125–36. doi: 10.1093/toxsci/kfn017. [DOI] [PubMed] [Google Scholar]
- De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3β phosphorylation by sodium valproate and lithium. Neuropharmacol. 2002;43:1158–64. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Dougherty JD, et al. PBK/TOPK, a proliferating neural progenitor-specific mitogen-activated protein kinase kinase. J Neurosci. 2005;25:10773–85. doi: 10.1523/JNEUROSCI.3207-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile S. Prophylactic treatment of bipolar disorder in pregnancy and breastfeeding: focus on emerging mood stabilizers. Bipolar Disord. 2006;8:207–20. doi: 10.1111/j.1399-5618.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- Gulino A, De Smaele E, Ferretti E. Glucocorticoids and neonatal brain injury: the hedgehog connection. J Clin Invest. 2009;119:243–6. doi: 10.1172/JCI38387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Napper RM. Quantitative study of granule and Purkinje cells in the cerebellar cortex of the rat. J Comp Neurol. 1988;274:151–7. doi: 10.1002/cne.902740202. [DOI] [PubMed] [Google Scholar]
- Heine VM, et al. A small-molecule smoothened agonist prevents glucocorticoid-induced neonatal cerebellar injury. Sci Transl Med. 2011;3:105ra104. doi: 10.1126/scitranslmed.3002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Priller M, Ling J, Rowitch DH, Schüller U. Dexamethasone destabilizes Nmyc to inhibit the growth of hedgehog-associated medulloblastoma. Cancer Res. 2010;70:5220–5. doi: 10.1158/0008-5472.CAN-10-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Rowitch DH. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11βHSD2-dependent mechanism. J Clin Invest. 2009;119:267–77. doi: 10.1172/JCI36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: A linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog K-H, Schulz A, Buerkle C, Gromoll C, Braun JS. Radiation-induced apoptosis in retinal progenitor cells is p53-dependent with caspase-independent DNA fragmentation. Eur J Neurosci. 2007;25:1349–56. doi: 10.1111/j.1460-9568.2007.05381.x. [DOI] [PubMed] [Google Scholar]
- Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem. 1997;272:25326–32. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- Inouye M, Yamamura H, Nakano A. Lithium delays the radiation-induced apoptotic process in external granule cells of mouse cerebellum. J Radiat Res. 1995;36:203–8. doi: 10.1269/jrr.36.203. [DOI] [PubMed] [Google Scholar]
- Jacobson SJ, et al. Prospective multicentre study of pregnancy outcome after lithium exposure during first trimester. Lancet. 1992;339:530–3. doi: 10.1016/0140-6736(92)90346-5. [DOI] [PubMed] [Google Scholar]
- Jobe AH. Postnatal corticosteroids for bronchopulmonary dysplasia. Clin Perinatol. 2009;36:177–88. doi: 10.1016/j.clp.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordà EG, et al. Lithium prevents colchicine-induced apoptosis in rat cerebellar granule neurons. Bipolar Disorders. 2004;6:144–9. doi: 10.1046/j.1399-5618.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–59. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmac K, et al. Glucocortiocoid treatment of MCMV infected newborn mice attenuates CNS inflammation and limits deficits in cerebellar development. PLoS Pathog. 2013;9:e1003200. doi: 10.1371/journal.ppat.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–9. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman AC, Druker J, Refojo D, Holsboer F, Arzt E. Glucocorticoids inhibit GATA-3 phosphorylation and activity in T cells. FASEB J. 2009;23:1558–71. doi: 10.1096/fj.08-121236. [DOI] [PubMed] [Google Scholar]
- Maloney SE, Noguchi KK, Wozniak DF, Fowler SC, Farber NB. Long-term effects of multiple glucocorticoid exposures in neonatal mice. Behav Sci. 2011;1:4–30. doi: 10.3390/behavsci1010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SG, et al. Fetal glucocorticoid exposure and hypothalamo-pituitary-adrenal (HPA) function after birth. Endocr Res. 2004;30:827–36. doi: 10.1081/erc-200044091. [DOI] [PubMed] [Google Scholar]
- Meyer JS. Early adrenalectomy stimulates subsequent growth and development of the rat brain. Exp Neurol. 1983;82:432–46. doi: 10.1016/0014-4886(83)90415-6. [DOI] [PubMed] [Google Scholar]
- Mora A, Sabio G, Gonzalez-Polo RA, Cuenda A, Alessia DR, Alonso JC, Fuentes JM, Soler G, Centeno F. Lithium inhibits caspase 3 activation and dephosphorylation of PKB and GSK3 induced by K+ deprivation in cerebellar granule cells. J Neurochem. 2001;78:199–206. doi: 10.1046/j.1471-4159.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- Moulder KL, et al. Ethanol-induced death of postnatal hippocampal neurons. Neurobiol Dis. 2002;10:396–409. doi: 10.1006/nbdi.2002.0523. [DOI] [PubMed] [Google Scholar]
- Murphy KE, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2008;372:2143–51. doi: 10.1016/S0140-6736(08)61929-7. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Panel Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1–24. [PubMed] [Google Scholar]
- Noguchi KK, et al. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell Death Differ. 2008;15:1582–92. doi: 10.1038/cdd.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi KK, Lau K, Smith DJ, Swiney BS, Farber NB. Glucocorticoid receptor stimulation and the regulation of neonatal cerebellar neural progenitor cell apoptosis. Neurobiol Dis. 2011;43:356–63. doi: 10.1016/j.nbd.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Chuang DM. Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport. 1998;9:2081–84. doi: 10.1097/00001756-199806220-00031. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Katsube N, Chuang DM. Lithium protects rat cerebellar granule cells against apoptosis induced by anticonvulsants, phenytoin and carbamazepine. J Pharmacol Exp Ther. 1998;286:539–47. [PubMed] [Google Scholar]
- Parikh NA, et al. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics. 2007;119:265–72. doi: 10.1542/peds.2006-1354. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM-Y, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–39. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Rios I, Alvarez-Rodríguez R, Martí E, Pons S. Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signalling. Development (Cambridge, England) 2004;131:3159–68. doi: 10.1242/dev.01188. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Sheng R, Zhang L, Han R, Gao B, Liu X, Qin Z. Combined prostaglandin E1 and lithium exert potent neuroprotection in a rat model of cerebral ischemia. Acta Pharmaclog Sinica. 2011;32:303–10. doi: 10.1038/aps.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 276:7320–6. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- Straiko MMW, et al. Lithium protects against anesthesia-induced developmental neuroapoptosis. Anesthesiology. 2009;110:862–8. doi: 10.1097/ALN.0b013e31819b5eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam EWY, et al. Preterm cerebellar growth impairment after postnatal exposure to glucocorticoids. Sci Transl Med. 2011;3:105ra105. doi: 10.1126/scitranslmed.3002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam EWY. Neuroradiology. 2013. Potential mechanisms of cerebellar hypoplasia in prematurity. doi:10.1007/s00234-013-1230-1. [DOI] [PubMed] [Google Scholar]
- Tin W, Wiswell TE. Drug therapies in bronchopulmonary dysplasia: debunking the myths. Semin Fetal Neonatal Med. 2009;14:383–90. doi: 10.1016/j.siny.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Vachon P, Moreau JP. Serum corticosterone and blood glucose in rats after two jugular vein blood sampling methods: comparison of the stress response. Contemp Top Lab Anim Sci. 2001;40:22–4. [PubMed] [Google Scholar]
- Wang J, et al. Identification of select glucocorticoids as Smoothened agonists: Potential utility for regenerative medicine. Proc Natl Acad Sci USA. 2010;107:9323–28. doi: 10.1073/pnas.0910712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Glucocorticoid compounds modify Smoothened localization and hedgehog pathway activity. Chem & Biol. 2012;19:972–82. doi: 10.1016/j.chembiol.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterberg KL. Policy statement--postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics. 2010;126:800–8. doi: 10.1542/peds.2010-1534. [DOI] [PubMed] [Google Scholar]
- Weinstein MR, Goldfield M. Cardiovascular malformations with lithium use during pregnancy. Am J Psychiatry. 1975;132:529–31. doi: 10.1176/ajp.132.5.529. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Goodwin GM, De Souza R, Green AR. The pharmacokinetic profile of lithium in rat and mouse; an important factor in psychopharmacological investigation of the drug. Neuropharmacol. 1986;25:1285–88. doi: 10.1016/0028-3908(86)90149-8. [DOI] [PubMed] [Google Scholar]
- Yeh TF, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–13. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Fairman KR, Meyer JS. Enhanced brain cell proliferation following early adrenalectomy in rats. J Neurochem. 1989;53:241–8. doi: 10.1111/j.1471-4159.1989.tb07320.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Meyer JS. Regional patterns of brain growth during the first three weeks following early adrenalectomy. Physiol Behav. 1991;49:233–7. doi: 10.1016/0031-9384(91)90037-o. [DOI] [PubMed] [Google Scholar]
- Young C, Olney JW. Neuroapoptosis in the infant mouse brain triggered by a transient small increase in blood alcohol concentration. Neurobiol Dis. 2006;22:548–54. doi: 10.1016/j.nbd.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Young C, Straiko MMW, Johnson SA, Creeley C, Olney JW. Ethanol causes and lithium prevents neuroapoptosis and suppression of pERK in the infant mouse brain. Neurobiol Disease. 2008;31:355–60. doi: 10.1016/j.nbd.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, et al. Role of caspase-3 in ethanol-induced developmental neurodegeneration. Neurobiol Dis. 2005;20:608–14. doi: 10.1016/j.nbd.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Zhong J, Yang X, Yao W, Lee W. Lithium protects ethanol-induced neuronal apoptosis. Biochem & Biophysi Res Communica. 2006;350:905–10. doi: 10.1016/j.bbrc.2006.09.138. [DOI] [PubMed] [Google Scholar]