Abstract
HIV infection causes systemic immune inflammation, and increases the risk for cardiovascular (CVD) disease even among those on virologically suppressive anti-retroviral treatment (ART). We performed a biostatistical analysis and screen of candidate cellular and plasma biomarkers for association with carotid artery intima-media thickness (CIMT), independent of traditional CVD risk factors such as age, gender, systolic blood pressure (SBP), lipid levels, smoking and diabetes. We conducted a multi-stage analysis based on a cross-sectional study of CVD risk in HIV-infected subjects age >45 years on ART for >6 months. The goal of this analysis was to identify candidate cellular and plasma biomarkers of CIMT in HIV-1 infected adults. We further sought to determine if these candidate biomarkers were independent of traditional CVD risk factors previously identified in HIV negative adults. High-resolution B-mode ultrasound images of the right common carotid common artery (CCA) were obtained. Plasma soluble inflammatory mediators, cytokines and chemokines were detected. Monocytes were defined by CD14/CD16 expression, and CD8+ T-cell activation by CD38/HLA-DR expression. Subjects were a median of 49.5 years old, 87% male, had a CIMT of 0.73 mm, FRS of 6%, a median viral load of 48 copies/mL, and CD4+ T cell count of 479 cells/μL. Soluble VCAM-1, and expansion of CD14dimCD16− monocytes each associated with higher CIMT independently of age and SBP. These factors are distinct components of a shared atherogenic process; 1) vascular endothelial molecular expression and 2) vascular monocytes that enter into the vascular endothelium and promote atherosclerotic plaque.
Keywords: HIV, Carotid intima-media, CIMT, Cardiovascular disease, Framingham risk score, Biomarker, Screen, Regression, CD14, Monocytes, VCAM-1, Cytokines
1. Introduction
Although HIV-infected individuals are living longer as a result of treatment with effective anti-retroviral therapy (ART), these individuals remain at increased risk of morbidity and mortality due to non-AIDS related diseases associated with aging, such as non-AIDS related cancers and osteoporosis. HIV-infected individuals also have an increased risk of cardiovascular disease [1,2]. Research on cardiovascular disease in HIV [3] has focused on direct atherosclerosis measures, such as carotid intima-media thickness (CIMT) as a predictor of clinical cardiovascular events [4] as HIV-infected individuals tend to be relatively young and at low short-term CVD risk. Hsue et al. found that HIV-infected individuals had a greater incidence of plaque in the common and internal carotid artery regions and a higher rate of plaque progression compared to HIV-seronegative controls [5]. HIV drives added CVD risk, although the mechanisms by which this effect is conferred, and effective biomarkers of this process, remain unresolved.
In this manuscript, we examined a panel of candidate biomarkers, both soluble inflammatory mediators and circulating monocyte populations that may mediate inflammation, for their association with CIMT. We conducted a biostatistical modeling analysis based on a cross-sectional study of CVD risk in HIV-infected subjects age >45 years on ART for >6 months. The goal of these models was to identify candidate cellular and plasma biomarkers of CIMT in HIV-1 infected adults. We further sought to determine if these candidate biomarkers were independent of traditional CVD risk factors previously identified in HIV negative adults. We performed this study within the Hawaii Center for AIDS cohort study of older, chronically HIV-1 infected adults with risk factors for CVD on stable virologically suppressive ART. We found evidence for a role for both endothelial cellular adhesion marker expression and altered monocyte phenotypes with higher CIMT in our cohort.
In HIV negative adults, the Framingham risk score (FRS) is employed to estimate 10 year risk for CVD. Over time the FRS system has been extended to encompass several predictive models, each based on distinct outcomes. We wished to determine whether the FRS, and its components, traditional CVD risk factors of age, systolic blood pressure (SBP), gender, current smoking, diabetes and dyslipidemias, associated with CIMT in HIV infected adults. To this end, we selected the FRS Adult Treatment Panel III (ATPIII) [6] that was formulated to predict ‘hard’ CVD outcomes, such as major cardiac events. However we posit that it is likely that HIV drives additional atherosclerosis mechanisms not captured by the FRS system that are involved in the observed elevated risk for CVD in chronic HIV infection.
It has been hypothesized that HIV-infected individuals have increased risk of CVD through several potential mechanisms including chronic immune activation and inflammation secondary to HIV-induced microbial translocation [7] and low-grade endotoxemia, the direct effects of HIV and viral proteins on macrophage cholesterol metabolism [8–10], and dyslipidemia [11,12] related to specific antiretroviral therapies. Prior reports have suggested that higher CD8+ T cell activation level [13] associates with arterial stiffness, and CMV T cell responses [14] associate with higher CIMT in chronically HIV infected adults. Monocytes, an arm of the innate immune response, are believed to play a critical role in HIV disease and atherogenesis [15]. Inflammatory monocytes transmigrate into the arterial wall and are crucial promoters of atherogenesis, a process that may be accelerated by HIV infection. Peripheral blood monocytes have been divided into subpopulations by their CD14 and CD16 expression [16,17]: ‘classical’ CD14+CD16− monocytes, ‘intermediate’ CD14+CD16++ monocytes, and ‘non-classical’ CD14dim/−CD16++ monocytes. Soluble CD14 has been associated with poor HIV disease outcomes [18]. In this report we included a fourth population of cells within the monocyte gate for consideration, a population of CD14dimCD16− monocytes. These are cells commonly observed in studies of monocytes, appear to emerge from the classical monocyte gate, but with reduced CD14 expression. CD14 forms a complex with TLR4 that detects lipopolysaccharide (LPS), a factor that drives immune activation in HIV-1 disease [7]. The dynamics of CD14 expression on monocytes may prove informative in HIV and inflammation studies in CVD research.
Inflammatory mediators in the plasma are markers of systemic inflammation. These soluble inflammatory mediators, found in the plasma, may be biomarkers of CIMT in adults with HIV infection. We characterized plasma soluble inflammatory mediators (such as soluble CD14, C-RP, IL-6, TNF, SAA, MCP-1 and others), alongside the relative proportions of the monocyte subsets discussed above, for their relationships to carotid artery intima media thickness (CIMT), and further determined if these factors associated with CIMT after adjustment for component of the FRS score. The purpose of our study was to identify candidate monocyte or plasma biomarkers that may associate with CIMT measurements independent of traditional CVD risk factors.
2. Methods
This is a cross-sectional examination of baseline data from the Hawaii Aging with HIV Cardiovascular Study cohort, a 5-year longitudinal natural history cohort study designed to investigate the role of oxidative stress and inflammation in the pathogenesis of CVD in HIV-infected individuals. Details on enrollment and clinical characteristics are published elsewhere [19]. Briefly, 158 HIV-infected individuals ≥40 years of age were on stable combination antiretroviral therapy (cART) for ≥6 months. Routine HIV and CVD medical histories were obtained. A consensus panel of two study physicians (DCC, CMS) identified prevalent CVD through a process of adjudication of study participants’ case report forms. Prevalent CVD was defined as a history of myocardial infarction, angina related to coronary heart disease, coronary artery bypass graft, coronary angioplasty or stent to treat coronary heart disease, ischemic stroke or peripheral vascular disease. Medical assessments obtained included vital signs, plasma HIV RNA, CD4+ T cell count, and lipid profile and glucose after a 12-hour fast, and a 2-hour oral glucose tolerance test (OGTT). Blood pressure was obtained in triplicate after 5 min of resting in triplicate and averaged. Fasting lipid profile included total cholesterol, high-density lipoprotein (HDL-C), directly measured low-density lipoprotein (LDL-C) cholesterol and total triglycerides by enzymatic, colorimetric assay. Hypertension was defined as self-reported diagnosis, use of an antihypertensive medication or measured systolic blood pressure (SBP) equal to or greater than 140 mm Hg or diastolic blood pressure (DBP) equal to or greater than 90 mm Hg [20] or if the subject reported the diagnosis of hypertension. Diabetes mellitus was defined as the use of diabetes medication, or a fasting plasma glucose greater than 126 mg/dL or 2-hour glucose greater than 200 mg/dL on OGTT [21], or if the subject reported the diagnosis of diabetes. Direct vascular measures including CIMT were also obtained. The Committee on Human Studies at the University of Hawaii approved the protocol. Written informed consent was obtained from participants. Participants agreed to have their blood and urine specimens banked and utilized for future research related to HIV and/or cardiovascular health. Subjects were eligible for this analysis if they had completed a CIMT measurement, had calculable Framingham risk scores, and adequate volumes of plasma and viably preserved peripheral blood mononuclear cells (PBMCs).
Framingham risk score (FRS Adult Treatment Panel III (ATPIII)) was calculated based on a model comprised of age, gender, total cholesterol, HDL cholesterol, SBP, treatment of hypertension, and any cigarette smoking in the past month as previously described [6]. FRS was used to categorize subjects into a Framingham Risk Class (FRC) defined as “low” (<10% 10-year risk of CVD), “intermediate” (10–19% risk of CVD), and “high” risk (>20% risk of CVD). Participants with diagnosis of diabetes or adjudicated to have evidence of CVD were automatically classified into the “high risk” (>20% risk of CVD) group.
Immuno-assays
Plasma samples were assayed for soluble inflammatory mediators using antibody coated beads in a high-sensitivity Milliplex assay (Human CVD panels A, B and C, EMD Millipore, Billerica, MA). Standard curves and samples were tested in duplicate. Samples were acquired on a Labscan 200 analyzer (Luminex, Austin, TX) using Bio-Plex manager software (Bio-Rad, Hercules, CA).
Cellular Phenotyping
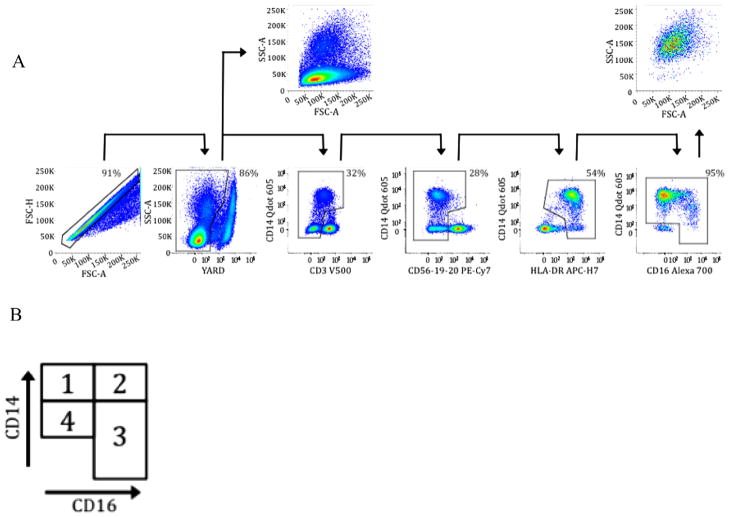
Using multi-parametric flow cytometry, monocyte subsets were defined by CD14 and CD16 expression as classical (CD14+CD16−) Mono 1, and monocyte subsets associated with inflammation: intermediate (CD14+CD16+) Mono 2 or non-classical (CD14dim/−CD16+) Mono 3, and a population of CD14dimCD16− monocytes (Mono 4). CD8+ T cell activation levels were assessed as described elsewhere [22]. Data were acquired on an LSRFortessa, and all data were gated and analyzed in FlowJo as described elsewhere [23]. Fig. 1 shows the gating strategy employed to identify monocyte subsets.
Fig. 1.
Panel A. Identification of monocytes from peripheral blood mononuclear cells as CD3−, CD56−, CD19−, CD20−, HLA-DR+ and then categorized into subsets by expression of CD14 and CD16, excluding double negative cells. Panel B. Diagram of assignment of monocyte subset categories. Mono 1 is CD14+CD16− or classical monocytes, Mono 2 is CD14+CD16++ or ‘intermediate’ monocytes, Mono 3 is CD14dim/−CD16+ or non-classical monocytes, and Mono 4 is CD14dimCD16− monocytes.
CIMT
High-resolution B-mode ultrasound images of the right common carotid common artery (CCA) were obtained. A single reader measured the intima-media thickness of the far wall of the distal common carotid artery along a 1-cm length just proximal to the carotid artery bulb with automated edge detection [24]. Standardized carotid artery images were acquired at Queens Medical Center in Honolulu and analyzed at the USC Atherosclerosis Research Unit Core Imaging and Reading Center.
Statistical analysis
Univariate analysis with Spearman correlations was used to explore associations between log cIMT as the dependent variable and leukocyte activation markers, plasma cytokines and chemokines, gender, prevalent CVD, diabetes, and FRC as the independent variables (Table 2). The natural log transformation of CIMT was used as the outcome in modeling exercises. Stepwise multivariable linear regression was subsequently performed, including independent variables with a p = 0.15 on univariate analysis. Partial r-squared values were calculated for each independent variable included to estimate the proportion of variation in CIMT that is accounted for by each term.
Table 2.
Summary of cIMT candidate predictors.
| Median | IQR | N | |
|---|---|---|---|
| Leukocyte activation markers | |||
| CD8+ T cell activation (% CD38+/HLA-DR+) | 10.5 | 7.6, 17.1 | 108 |
| CD14+CD16−% or Mono 1 | 71.1 | 65.6, 81.1 | 108 |
| CD14+CD16++% or Mono 2 | 1.62 | 0.54, 4.07 | 108 |
| CD14dim/−CD16++% or Mono 3 | 5.07 | 3.4, 7.5 | 108 |
| CD14dimCD16−% or Mono 4 | 11.9 | 8.82, 14.5 | 108 |
| Plasma cytokines and chemokines | |||
| C-RP | 12,022 | 4355, 53,564 | 121 |
| IL-6 | 1.44 | 0.85, 2.43 | 121 |
| IL-8 | 3.55 | 2.74, 4.46 | 121 |
| IL-10 | 1.78 | 0.68, 4.69 | 121 |
| IL-1β | 0.31 | 0.27, 0.35 | 121 |
| TNF | 2.97 | 1.75, 4.45 | 121 |
| Myeloperoxidase (MPO) | 16.3 | 12.0, 23.3 | 121 |
| Matrix Metalloproteinase 9 (MMP-9) | 55.4 | 36.8, 86.7 | 121 |
| tPAI-1 | 8.9 | 70.4, 120.3 | 121 |
| NT ProBNP | 8.64 | 7.75,8.8 | 121 |
| sICAM-1 | 140.9 | 110,171.9 | 121 |
| sVCAM-1 | 1198 | 1011, 1342 | 121 |
| sE-Selectin | 34.5 | 23.0, 50.0 | 121 |
| MCP-1 | 143.1 | 114.8,176.5 | 121 |
| VEGF | 23.68 | 13.78, 51.28 | 121 |
| Soluble CD14 | 1,817,878 | 1,579,162, 2,119,672 | 121 |
| SAA | 18,906 | 4070, 48,020 | 121 |
| SAP | 96,090 | 57,052, 190,993 | 121 |
Abbreviations: FRS ATPIII, Framingham risk score ATPIII, C-RP = C-reactive protein, IL-6 = Interleukin-6, IL-8 = Interleukin-8, IL-10 = Interleukin-10, IL-1b = Interleukin-1 beta, TNF = tumor necrosis factor, MPO = myeloperoxidase, MMP-9 = matrix metalloproteinase 9, tPAI-1 = tissue plasminogen activator inhibitor-1, NT ProBNP = N-terminal fragment of brain natriuretic peptide, sICAM-1 = soluble intercellular cell adhesion molecule 1, sVCAM-1 = soluble vascular adhesion molecule 1, sE-Selectin = soluble E (CD62E), endothelial-leukocyte adhesion molecule 1, MCP-1 = monocyte chemoattractant protein 1, soluble CD14 = free plasma CD14, SAA = serum amyloid alpha, SAP = serum amyloid P.
3. Results
Of the 158 subjects in the parent cohort, 125 subjects were eligible for this analysis and their demographics and clinical characteristics are summarized in Table 1. In brief, study subjects were a median of 49.5 years old (interquartile range, IQR: 45–57), were 87% male, had a CIMT median value of 0.73 mm (IQR: 0.67–0.83 mm), and had a median FRS of 6% (IQR: 3–13), with 35% of participants having an intermediate or high risk FRC. Eight percent of subjects (10/125) had diabetes and prevalent cardiovascular disease. Ninety-six percent of patients had undetectable plasma HIV viral loads <48 copies/mL, and a median CD4+ T cell count of 479 cells/μL (IQR: 333–612 cells/uL). These 125 subjects did not differ from the balance of the cohort with respect to age, FRS, current plasma HIV-1 RNA level, current CD4+ T cell count, gender or ethnicity. Median untransformed values of CIMT are shown in Table 1 for the study cohort.
Table 1.
Demographics and clinical characteristics.
| N = 125 | |
|---|---|
| Age, median years (interquartile range, IQR) | 49.5 (45, 57) |
| Male (%) | 109 (87%) |
| Ethnicity, N (%) | |
| White | 69 (55%) |
| Right carotid artery intima medial thickness (mm) | 0.73 (0.67, 0.83) |
| Plasma HIV-1 RNA, median copies/mL (IQR) | 48 (48, 48) |
| CD4+ T cell count, median cells/mL (IQR) | 479 (333, 612) |
| On combination anti-retroviral treatment | 100% |
| Framingham risk score (ATPIII) | 6 (3, 13) |
| Framingham risk class | |
| Low risk classa | 81 (65%) |
| Intermediate risk classb | 22 (18%) |
| High risk classc | 21 (17%) |
| Prevalent cardiovascular disease, N (%) | 10 (8%) |
| Systolic blood pressure, median mm Hg (IQR) | 120 (111, 130) |
| Total cholesterol, median mg/dL (IQR) | 179.5 (156, 209) |
| HDL cholesterol, median mg/dL (IQR) | 45 (33, 55) |
| LDL cholesterol, median mg/dL (IQR) | 110 (90, 135) |
| Diabetes (%) | 10 (8%) |
| Current smoker (%) | 28 (22%) |
ATPIII = adult treatment panel III; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Low Framingham risk class is defined as <10% 10-year risk of cardiovascular disease.
Intermediate Framingham risk class is defined as <10–19% 10-year risk of cardiovascular disease.
High Framingham risk class is defined as ≥20% 10-year risk of cardiovascular disease.
Median values of levels of plasma inflammatory markers found in the plasma, CD8+ T cell activation (% of CD8+ T cells expressing CD38 and HLA-DR) levels, and distribution of monocyte subsets for the study subjects are found in Table 2.
Univariate associations (Spearman Rank correlations) of candidate predictor variables with cIMT are shown in Table 3. In brief, a panel of demographic, clinical and immunologic predictors were screened for univariate association with CIMT. The following immunologic predictors, sVCAM-1, C-RP, VEGF, SAA, IL-6, IL-8 and Mono 4 were each associated with CIMT at a p-value of 0.15 or less, and were forwarded for further model building of predictors of CIMT.
Table 3.
Univariate association of candidate predictors with CIMT.
| Wilcoxon | p-value | N | Modeled? | |
|---|---|---|---|---|
| Male gender | 0.03 | 125 | Yes | |
| Prevalent cardiovascular disease | 0.10 | 125 | No | |
| Hypertension treatment | 0.19 | 125 | Yes | |
| Diabetes mellitus | 0.41 | 125 | Yes | |
| Framingam risk class | 0.007 | 125 | No |
| Rho | p-value | N | Modeled? | |
|---|---|---|---|---|
| FRS ATPIII | 0.31 | 0.0004 | 125 | Yes |
| Age | 0.31 | 0.0003 | 125 | Yes |
| Plasma HIV RNA | 0.06 | 0.51 | 125 | No |
| CD4+ T cell count | −0.04 | 0.66 | 125 | No |
| CD8+ T cell activation (% CD38+/HLA-DR+) | −0.08 | 0.38 | 108 | No |
| CD14+CD16−% or Mono 1 | −0.11 | 0.22 | 108 | No |
| CD14+CD16++ % or Mono 2 | 0.03 | 0.71 | 108 | No |
| CD14dim/−CD16++% or Mono 3 | −0.04 | 0.62 | 108 | No |
| CD14dimCD16−% or Mono 4 | 0.15 | 0.11 | 108 | Yes |
| C-RP | 0.22 | 0.01 | 121 | Yes |
| IL-6 | 0.17 | 0.06 | 121 | Yes |
| IL-8 | 0.23 | 0.02 | 121 | Yes |
| IL-10 | 0.09 | 0.31 | 121 | No |
| IL-1β | 0.07 | 0.41 | 121 | No |
| TNF | 0.13 | 0.16 | 121 | No |
| MPO | 0.04 | 0.66 | 121 | No |
| MMP-9 | −0.04 | 0.68 | 121 | No |
| tPAI-1 | 0.02 | 0.76 | 121 | No |
| NT ProBNP | 0.01 | 0.86 | 121 | No |
| sICAM-1 | 0.01 | 0.90 | 121 | No |
| sVCAM-1 | 0.25 | 0.006 | 121 | Yes |
| sE-Selectin | 0.12 | 0.19 | 121 | No |
| MCP-1 | 0.12 | 0.19 | 121 | No |
| VEGF | 0.15 | 0.10 | 121 | Yes |
| Soluble CD14 | −0.06 | 0.52 | 121 | No |
| SAA | 0.21 | 0.02 | 121 | Yes |
| SAP | 0.10 | 0.25 | 121 | No |
Abbreviations: FRS ATPIII, Framingham risk score ATPIII, C-RP = C-reactive protein, IL-6 = Interleukin-6, IL-8 = Interleukin 8, IL-10 = Interleukin-10, IL-1b = Interleukin-1 beta, TNF = tumor necrosis factor, MPO = myeloperoxidase, MMP-9 = matrix metalloproteinase 9, tPAI-1 = tissue plasminogen activator inhibitor-1, NT ProBNP = N-terminal fragment of brain natriuretic peptide, sICAM-1 = soluble intercellular cell adhesion molecule 1, sVCAM-1 = soluble vascular adhesion molecule 1, sE-Selectin = soluble E (CD62E), endothelial-leukocyte adhesion molecule 1, MCP-1 = monocyte chemoattractant protein 1, soluble CD14 = free plasma CD14, SAA = serum amyloid alpha, SAP = serum amyloid P.
We selected 7 immunologic predictors (Table 3), as well as the individual components of the FRS ATPIII score (age, gender, SBP, medication treated hypertension, diabetes, current smoker, total cholesterol and HDL). We elected to test for an association of cIMT with inclusion of separate CVD risk factors (from the FRS ATPIII) rather than utilize prevalent CVD or the composite Framingham risk score. We adopted this approach to understand the FRS components that associated with CIMT in HIV infected persons, and to determine which FRS components dropped out of significance when inflammatory markers were included in the same model. Prevalent cardiovascular disease, a two level variable, was marginally associated with cIMT (p = 0.10 Wilcoxon), and the three level Framingham Risk Class variable was strongly associated with cIMT (p = 0.007 Kruskal Wallis). However neither term was included in the modeling process, due to high information content overlap with FRS itself.
Regression modeling to assess the presence of independent predictors of CIMT is shown in Tables 4 and 5. In Table 4 we present the results of a modeling exercise intended to determine whether candidate immunologic predictors (Table 3) are associated with CIMT independent of traditional CVD risk factors (the component values of the FRS ATPIII) are presented. In columns 2 and 3, Table 4 shows the association of the FRS ATPIII composite score with CIMT, and the association of the FRS ATPIII individual components run in an adjusted multivariate model with cIMT. FRS ATPIII composite score was significantly associated with CIMT (column 2). Of the FRS ATPIII components (column 3) only age, gender, SBP and total cholesterol were significantly or marginally significantly associated with CIMT. In columns 3–9 are models showing the association of CIMT with each of the 7 candidate inflammatory markers, adjusted for each of the FRS ATPIII components, versus CIMT. In brief, higher sVCAM-1, C-RP, VEGF and Mono 4 were significantly or marginally significantly associated with CIMT after adjustment for FRS ATPIII components.
Table 4.
Relationship of candidate immunologic predictors of carotid artery intima media thickness (CIMT) upon adjustment for Framingham risk score ATPIII components.
| FRS
|
FRS components | sVCAM1
|
C-RP
|
VEGF
|
SAA
|
IL-6
|
IL-8
|
Mono 4
|
|
|---|---|---|---|---|---|---|---|---|---|
| β | β | β | β | β | β | β | β | ||
| p-value | p-value | p-value | p-value | p-value | p-value | p-value | p-value | ||
| 0.0081 | 8.7E-5 | 2.6E-7 | 7.9E-4 | −1.6E-8 | −4.6E-4 | 7.7E-4 | 3.0E-3 | ||
| <0.001 | 0.04 | 0.13 | 0.01 | 0.69 | 0.9 | 0.88 | 0.12 | ||
| Age in years | 6.0E-3 | 7.0E-3 | 6.0E-3 | 7.0E-3 | 7.0E-3 | 7.0E-3 | 7.0E-3 | 6.0E-3 | |
| 0.002 | <0.001 | 0.001 | 0.003 | <0.001 | <0.001 | <0.001 | 0.001 | ||
| Male gender | 0.09 | 0.08 | 0.07 | 0.09 | 0.1 | 0.1 | 0.1 | 0.06 | |
| 0.06 | 0.08 | 0.04 | 0.04 | 0.04 | 0.03 | 0.04 | 0.18 | ||
| Systolic blood pressure | 0.24 | 0.27 | 0.26 | 0.22 | 0.25 | 0.25 | 0.25 | 0.21 | |
| 0.05 | 0.03 | 0.09 | 0.07 | 0.04 | 0.04 | 0.04 | 0.09 | ||
| On antihypertensives | 0.02 | 9.0E-3 | −2.0E-3 | −6.2E-4 | 0.01 | 9.0E-3 | 7.0E-3 | 2.0E-3 | |
| 0.57 | 0.8 | 0.96 | 0.98 | 0.77 | 0.81 | 0.85 | 0.95 | ||
| Current smoker | −1.4E-2 | 3.5E-3 | −1.8E-2 | 8.0E-4 | 5.0E-3 | 6.0E-3 | −7.6E-3 | −4.0E-3 | |
| 0.71 | 0.92 | 0.63 | 0.98 | 0.9 | 0.88 | 0.84 | 0.93 | ||
| Total cholesterol | 0.12 | 0.12 | 0.11 | 0.12 | 0.1 | 0.1 | 0.11 | 6.3E-3 | |
| 0.09 | 0.11 | 0.12 | 0.09 | 0.16 | 0.14 | 0.13 | 0.36 | ||
| HDL | 4.0E-3 | 6.0E-3 | 0.01 | 5.0E-3 | 0.01 | 0.01 | 0.01 | 0.02 | |
| 0.94 | 0.9 | 0.83 | 0.92 | 0.77 | 0.88 | 0.78 | 0.7 | ||
| Diabetes mellitus | 0.05 | 0.08 | 0.09 | 0.08 | 0.09 | 0.09 | 0.09 | 0.05 | |
| 0.36 | 0.16 | 0.11 | 0.18 | 0.9 | 0.14 | 0.13 | 0.34 |
Far left column lists the component variables of the Framingham risk score (ATPIII). Starting with column two, each column represents a distinct regression model. The second column, FRS ATPIII describes the univariate association of the Framingham risk score ATPIII to CIMT levels. The third column, FRS components, describes adjusted associations of all FRS ATPIII components regressed against CIMT in the same model. The fourth to seventh columns show the values of each candidate immunologic predictors of CIMT modeled singly alongside all FRS ATPIII components. For example, column four shows the association of soluble VCAM1 (sVCAM1) to CIMT value, after adjustment for FRS ATPIII components (Age, Gender, Systolic Blood Pressure, Hypertension Treatment, Current Smoking, Total Cholesterol, HDL and Diabetes). For the adjusted sVCAM1/FRS ATPIII model in column four, the immunologic predictor sVCAM1 remained significantly associated with CIMT (p = 0.04).
Significant associations with CIMT (p < 0.05), or marginally significant associations (0.05 < p < 0.15). Abbreviations: FRS = Framingham risk score (ATPIII), sVCAM1 = soluble vascular cell adhesion molecule 1, C-RP = C reactive protein, VEGF = vasoendothelial growth factor, SAA = serum amyloid alpha, IL-6 = interleukin-6, IL-8 = interleukin-8, Mono 4 = monocyte population 4 (CD14dimCD16− monocytes). HDL = high density lipoprotein.
Table 5.
Stepwise selected model of candidate FRS components and immunologic predictors of CIMT.
| Variable | β | p-value |
|---|---|---|
| Age | 7.1E-3 | <0.0001 |
| Blood pressure | 0.25 | 0.02 |
| sVCAM-1 | 8.4E-5 | 0.02 |
| C-RP | 2.4E-7 | 0.10 |
| Mono 4 | 5.0E-3 | 0.01 |
All FRS components (Age, Gender, Systolic Blood Pressure, Hypertension Treatment, Current Smoking, Total Cholesterol, HDL and Diabetes), and all candidate immunologic predictors (sVCAM1, C-RP, VEGF, SAA, IL-6, IL-8 and Mono 4) were entered in a stepwise regression model. To be entered into the model as a candidate predictor the variable must associate with cIMT below a p-value threshold of 0.25, and to be retained in the model must associate with cIMT below a p-value threshold of 0.15. Abbreviations: sVCAM1 = soluble vascular cell adhesion molecule 1, C-RP = C reactive protein, Mono 4 = monocyte population 4 (CD14dimCD16− monocytes).
A stepwise selection model to determine a concise set of predictors of CIMT was performed (Table 5) entering all FRS ATPIII components and all 7 candidate immunologic predictors from Table 3 (CRP, IL-6, IL-8, sVCAM-1, VEGF, SAA and Mono 4). Terms were allowed into the model if they associated with CIMT at a p-value of less than 0.25 and were retained if they associated with CIMT at a p-value of 0.15 or less. Higher age, SBP, sVCAM-1 and Mono 4 were significantly associated with higher CIMT.
We then sought to determine the fraction of variation in CIMT that was accounted for by our final predictor set in Table 5. We calculated model r-squared values that allow an estimate of the fraction of variation in CIMT accounted for by the final predictor set. From the concise model of significant predictors of CIMT we observed that the model containing age, SBP, sVCAM-1 and Mono 4 accounted for 27% of the variation in CIMT.
4. Discussion
We conducted a multi-stage biostatistical analysis to identify cellular and plasma biomarkers of CIMT that were independent of traditional CVD risk factors, in HIV-1 infected adults on stable anti-retroviral therapy. After adjustment for two persistent, robust risk factors for HIV CVD, age and systolic blood pressure, our findings suggest two possible biomarkers of cIMT, one cellular (CD14dimCD16−) and one soluble plasma factor (sVCAM-1) that independently associate with higher CIMT in treated chronic HIV infection. These two factors represent distinct components of atherogenesis: 1) vascular adhesion molecule expression that promotes tethering of circulating cells to the arterial wall; and, 2) innate immunity through the monocytes that enter into the arterial wall and promote fatty streak formation and plaque.
Notably, we did not find an association of the ‘intermediate’ monocyte subset, CD14++CD16+ (Mono 2, sometimes referred to as ‘activated’) [25], or the classical monocyte subset (Mono 1, CD14+CD16−) [26] with variation in cIMT. Sub-populations of CD16− monocytes with reduced CD14 expression may be seen in the primary flow plots from multiple prior reports on CVD and HIV in humans [27,28], but have not been previously considered for study. We have included this population, which we call Mono 4, for consideration in this report, as this population is commonly encountered in monocytes, appears to emerge from the classical monocyte population, and differs with respect to CD14 expression. The reduction of CD14 expression on the monocyte cell surface is intriguing, soluble CD14 has been associated with all-cause mortality [18] in HIV disease. Moreover, CD14 is part of the TLR4 signaling complex that detects LPS, a known trigger of immune activation in HIV disease. Increased levels of soluble CD14 (sCD14) in the plasma has been suggested to arise from the release of surface CD14 from monocytes [29,30]. We feel the results are intriguing, and while the CD14dimCD16negative monocyte bears further study that this population may represent a novel biomarker for CIMT in HIV infected adults.
sVCAM-1 withstood adjustment for FRS ATPIII components, and was observed to have a significant effect upon variation in CIMT across the statistical models in Tables 4 and 5. VEGF and sVCAM-1 may capture overlapping relationships with CIMT. The presence of sVCAM-1 in the blood is a marker of endothelial activation [31]. Soluble vascular cell adhesion molecule 1, or sVCAM-1, promotes the physical adhesion of multiple cell types, including monocytes and lymphocytes, to vascular endothelium via integrins. sVCAM-1 may be induced by inflammatory cytokines, such as IL-1β and TNF produced by monocytes and macrophages, and in this way act to promote and amplify inflammatory processes that lead to migration of activated monocytes and other cell types through the vascular endothelium, and accumulation of macrophages and other inflammatory cells in the arterial wall. Taken together, the blood inflammatory markers that associated with CIMT in this study, sVCAM-1 and CD14dimCD16− monocytes, delineate distinct components of a process of drawing activated monocyte populations to the vascular endothelial lining of the artery and transmigration of circulating monocytes into the arterial wall.
We found a number of candidate clinical, immunologic or inflammatory factors were not associated with CIMT at the univariate level. We observed that CIMT was not linked to CD8+ T cell activation in contrast to prior studies [13,32]. Our cohort may differ from other reports, as our HIV cohort is older, more male, more likely to be virologically suppressed, to have lower CD8+ T cell activation levels and to have higher CD4+ T cell counts.
Several FRS ATPIII traditional CVD risk factors such as male gender and HDL-C did not associate with CIMT, while age and SBP associated with outcome. This suggests the features of the FRS ATPIII that predict ‘hard outcomes’ in non-HIV populations may differ from those factors that drive CIMT, a direct measure of atherosclerosis. In univariate associations higher CIMT was associated with three candidate inflammatory plasma markers that did not survive adjustment for FRS components for an association with CIMT (SAA, IL-6, IL-8). SAA is serum-amyloid A, an apoliprotein produced predominantly in the liver, is involved in acute inflammatory responses, and plays multiple physiologic roles, such as cholesterol transport, and immune cell migration. SAA may be induced by inflammatory cytokines such as IL-1β, IL-6 and IL-8. Plasma IL-1β was not associated with CIMT, and neither SAA, IL-6 or IL-8 withstood adjustment for FRS ATPIII components. This suggests that the relationships of these acute inflammatory response markers to variation in CIMT are captured by components of the FRS ATPIII, such as age or systolic blood pressure. In fact we observed that higher SAA and IL-6 were correlated with higher age (data not shown) in our cohort. Across models, the FRS ATPIII components of age and SBP were consistently associated with CIMT, and may capture the effect of higher plasma SAA, IL-6 and IL-8 on thickening of the carotid intima-media. SAA, IL-6 and Il-8 may rise with age, and the measurement of age included in FRS ATPIII may effectively capture the rise in certain inflammatory agents in the blood.
We assessed the degree to which our final statistical model accounts for variation in the CIMT measurement. Put another way, we calculated the fraction of the observed variation in CIMT that our model could explain. The model we identified (Mono 4, sVCAM-1, age and SBP) accounted for 27% of the variation in CIMT level, suggesting other unmeasured factors, not captured by the FRS or our panel of inflammatory cellular and plasma factors, play a role in determining CIMT in this population. Additional factors or pathways that associate with CIMT may include unmeasured or unknown genetic or environmental determinants of CIMT, or novel biomarkers not yet considered or assessed here.
Our study was subject to some limitations. This evaluation of candidate cellular and plasma biomarkers for CIMT was based on a small cross-sectional study of 125 subjects. Our cohort population may be distinct from other clinical populations, as our study subjects had a low rate of prevalent CVD, a median CD4+ T cell count of nearly 500 cells/ul and very well controlled viremia. That said, our study subjects had high residual immune inflammation as marked by elevated CD8+ T cell activation, abnormal HDL-C levels, and was older (half over the age of 50, and a quarter over the age of 57) with very long-standing HIV infection (more than 10 years). Comprehensive clinical information was collected, and paired to measures of plasma inflammatory markers, leukocyte phenotypes (CD8+ T cell activation, monocyte phenotype) and CIMT values. We conducted a careful screen process, testing each candidate factor against our outcome, choosing only significant and marginally significant factors for entry into multi-variate models. These terms were then further test for association with CIMT after adjustment by individual FRS components, known CVD risk factors in HIV-1 negative persons. In this way our analysis set a high bar, and only those factors showing robust association with the outcome, that is those factors showing significance across several alternative formulations of the models, are being proposed here as candidate biomarkers. And we would emphasize that these candidate biomarkers will still require independent verification in distinct populations of adults at risk for HIV CVD.
Our study suggests increased CIMT among HIV-infected adults on long-term ART is associated with expansion of a population of monocytes with reduced CD14 expression (CD14dimCD16−) and increased levels of a vascular adhesion molecule, sVCAM-1, in addition to FRS components of age and SBP. The two new factors identified from our inflammatory marker screen each represent different components of the inflammatory process that drives atherogenesis. These components bear further evaluation as potential biomarkers for CIMT and merit further study for a role in atherogenesis in chronic, treated HIV infection.
Acknowledgments
We thank our study participants and community physicians for their roles in this study.
This work was supported by NIH grants U54RR026136, U54MD007584, R01HL095135 (CMS), K23 HL088981 (DC), and N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-RR-024156 and UL1-RR-025005 from NCRR.
Footnotes
Presented in part at the 20th Conference on Retroviruses and Opportunistic Infections, R-149. March 3–6th, 2013, Atlanta, Georgia, USA.
The authors have declared no conflict of interests.
References
- 1.Esser S, Gelbrich G, Brockmeyer N, et al. Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study. Clin Res Cardiol. 102:203–13. doi: 10.1007/s00392-012-0519-0. [DOI] [PubMed] [Google Scholar]
- 2.Hsue PY, Deeks SG, Hunt PW. Immunologic Basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 205(Suppl 3):S375–82. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 120:4599–608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hsue PY, Scherzer R, Hunt PW, et al. Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc. 1 doi: 10.1161/JAHA.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 7.Brenchley JM. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Retrovirology. 2006;3(Suppl 1):S98. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 8.Feeney ER, McAuley N, O’Halloran JA, et al. The expression of cholesterol metabolism genes in monocytes from HIV-infected subjects suggests intra-cellular cholesterol accumulation. J Infect Dis. 207:628–37. doi: 10.1093/infdis/jis723. [DOI] [PubMed] [Google Scholar]
- 9.Bukrinsky M, Sviridov D. Human immunodeficiency virus infection and macrophage cholesterol metabolism. J Leukoc Biol. 2006;80:1044–51. doi: 10.1189/jlb.0206113. [DOI] [PubMed] [Google Scholar]
- 10.Dube MP, Cadden JJ. Lipid metabolism in treated HIV Infection. Best Pract Res Clin Endocrinol Metab. 25:429–42. doi: 10.1016/j.beem.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Feeney ER, Mallon PW. Impact of mitochondrial toxicity of HIV-1 antiretroviral drugs on lipodystrophy and metabolic dysregulation. Curr Pharm Des. 16:3339–51. doi: 10.2174/138161210793563482. [DOI] [PubMed] [Google Scholar]
- 12.Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–27. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 217:207–13. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–83. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 308:379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zawada AM, Rogacev KS, Rotter B, et al. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 118:e50–61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 17.Rogacev KS, Seiler S, Zawada AM, et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 32: 84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 18.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shikuma CM, Seto T, Liang CY, et al. Vitamin D levels and markers of arterial dysfunction in HIV. AIDS Res Hum Retroviruses. 28:793–97. doi: 10.1089/aid.2011.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Screening for high blood pressure: U. S Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2007;147:783–6. doi: 10.7326/0003-4819-147-11-200712040-00009. [DOI] [PubMed] [Google Scholar]
- 21.Standards of medical care in diabetes–2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford TQ, Ndhlovu LC, Tan A, et al. HIV-1 infection Abrogates CD8+ T cell MAPK signaling responses. J Virol. 2011;85(23):12343–50. doi: 10.1128/JVI.05682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalbert E, Shikuma CM, Ndhlovu LC, Barbour JD. Sequential staining improves detection of CCR2 and CX3CR1 on monocytes when simultaneously evaluating CCR5 by multicolor flow cytometry. Cytometry A. 83:280–6. doi: 10.1002/cyto.a.22257. [DOI] [PubMed] [Google Scholar]
- 24.Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–53. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 25.Hearps AC, Maisa A, Cheng WJ, et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 26:843–53. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- 26.Berg KE, Ljungcrantz I, Andersson L, et al. Elevated CD14++CD16− monocytes predict cardiovascular events. Circ Cardiovasc Genet. 5:122–31. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- 27.Poitou C, Dalmas E, Renovato M, et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 31:2322–30. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 28.Baker J, Hullsiek Huppler K, Singh A, et al. for CDC SUN Study Investigators. Monocyte activation, but not T cell activation, predicts progression of coronary arterial calcium in a contemporary HIV cohort. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA, USA. 2013. [Google Scholar]
- 29.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 206: 1558–67. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longenecker C, Funderburg N, Jiang Y, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andresen TK, Svennevig JL, Videm V. Soluble VCAM-1 is a very early marker of endothelial cell activation in cardiopulmonary bypass. Perfusion. 2002;17:15–21. doi: 10.1191/0267659102pf531oa. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 203: 452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]