Abstract
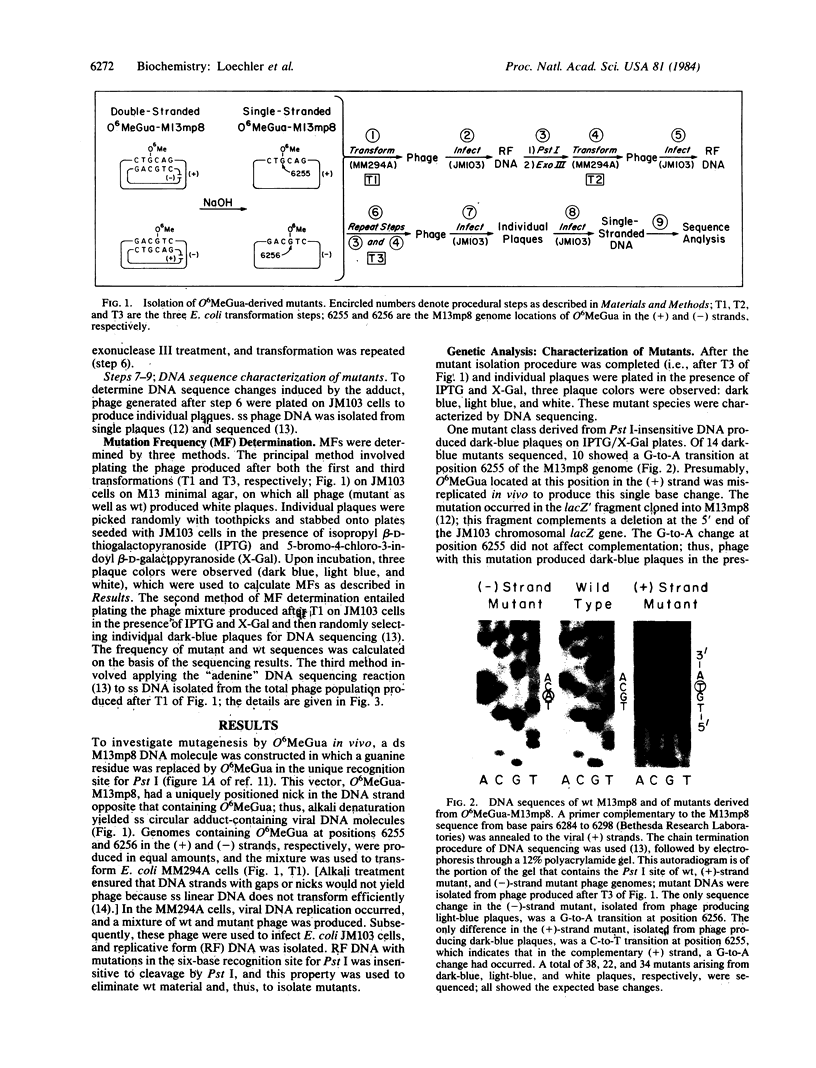
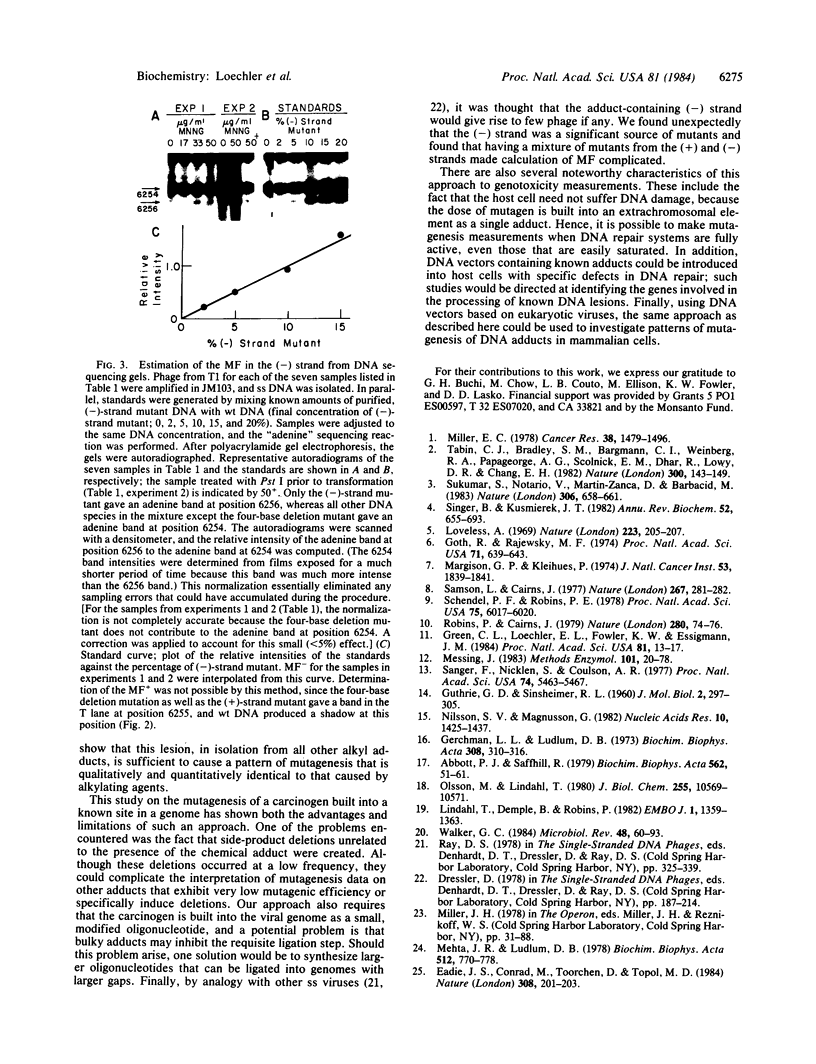
The mutagenicity of O6-methylguanine (O6MeGua), a chemical carcinogen-DNA adduct, has been studied in vivo by using a single-stranded M13mp8 genome in which a single O6MeGua residue was positioned in the unique recognition site for the restriction endonuclease Pst I. Transformation of Escherichia coli MM294A cells with this vector gave progeny phage, of which 0.4% were mutated in their Pst I site. In a separate experiment, cellular levels of O6MeGua-DNA methyltransferase (an O6MeGua-repair protein) were depleted by treatment with N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) prior to viral DNA uptake. In these cells, the mutation frequency due to O6MeGua increased with increasing MNNG dose (the highest mutation frequency observed was 20%). DNA sequence analysis of 60 mutant genomes revealed that O6MeGua induced exclusively G-to-A transitions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott P. J., Saffhill R. DNA synthesis with methylated poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O(6)-methylguanine. Biochim Biophys Acta. 1979 Mar 28;562(1):51–61. doi: 10.1016/0005-2787(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Eadie J. S., Conrad M., Toorchen D., Topal M. D. Mechanism of mutagenesis by O6-methylguanine. Nature. 1984 Mar 8;308(5955):201–203. doi: 10.1038/308201a0. [DOI] [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. L., Loechler E. L., Fowler K. W., Essigmann J. M. Construction and characterization of extrachromosomal probes for mutagenesis by carcinogens: site-specific incorporation of O6-methylguanine into viral and plasmid genomes. Proc Natl Acad Sci U S A. 1984 Jan;81(1):13–17. doi: 10.1073/pnas.81.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Carcinogenicity of N-methyl-N-nitrosourea: possible role of excision repair of O6-methylguanine from DNA. J Natl Cancer Inst. 1974 Dec;53(6):1839–1841. [PubMed] [Google Scholar]
- Lindahl T., Demple B., Robins P. Suicide inactivation of the E. coli O6-methylguanine-DNA methyltransferase. EMBO J. 1982;1(11):1359–1363. doi: 10.1002/j.1460-2075.1982.tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B. Synthesis and properties of O6-methyldeoxyguanylic acid and its copolymers with deoxycytidylic acid. Biochim Biophys Acta. 1978 Dec 21;521(2):770–778. doi: 10.1016/0005-2787(78)90316-7. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nilsson S. V., Magnusson G. Sealing of gaps in duplex DNA by T4 DNA ligase. Nucleic Acids Res. 1982 Mar 11;10(5):1425–1437. doi: 10.1093/nar/10.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Robins P., Cairns J. Quantitation of the adaptive response to alkylating agents. Nature. 1979 Jul 5;280(5717):74–76. doi: 10.1038/280074a0. [DOI] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel P. F., Robins P. E. Repair of O6-methylguanine in adapted Escherichia coli. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6017–6020. doi: 10.1073/pnas.75.12.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]




