Abstract
The role of autoimmune pathology in development and progression of chronic obstructive pulmonary disease (COPD) is becoming increasingly appreciated. In this study, we identified serum autoantibody reactivities associated with chronic bronchitis or emphysema, as well as systemic autoimmunity and associated lung disease. Using autoantigen array analysis, we demonstrated that COPD patients produce autoantibodies reactive to a broad spectrum of self-antigens. Further, the level and reactivities of these antibodies, or autoantibody profile, correlated with disease phenotype. Patients with emphysema produced autoantibodies of higher titer and reactive to an increased number of array antigens. Strikingly, the autoantibody reactivities observed in emphysema were increased over those detected in rheumatoid arthritis patients, and included similar reactivities to those associated with lupus. These findings raise the possibility that autoantibody profiles may be used to determine COPD risk, as well as provide a diagnostic and prognostic tool. They shed light on the heterogeneity of autoantibody reactivities associated with COPD phenotype and could be of use in the personalization of medical treatment, including determining and monitoring therapeutic interventions.
Keywords: COPD, SLE, RA, ILD, Autoantibodies, Antigen array
Introduction
Chronic obstructive pulmonary disease (COPD) is the 4th leading cause of death worldwide, ranking 3rd in the United States [1, 2]. While primarily associated with cigarette smoking, the association is incomplete. Namely, estimates vary with approximately a quarter of all smokers going on to develop COPD [3–5]. Smoking cessation, removal of the causative factor, does not necessarily reverse disease or the associated immune activation [6, 7], and there remains a substantial group of COPD patients who have never smoked [8, 9]. Other contributing factors must therefore exist. One such factor may be autoimmunity. Many studies have shown that hallmarks of autoimmunity are associated with COPD, and conversely, conventional autoimmune disease is often associated with chronic lung disease.
Recent studies have shown that the majority of COPD patients have increased levels of serum antibodies reactive with self-antigens [10–16], and occurrence of antibodies to specific autoantigens correlates with disease severity [12–14, 16]. Others have shown that intentional provocation of the immune system in animal models can cause pulmonary autoimmunity. An example is disease provoked by targeted lung expression of influenza hemagglutinin (HA) peptide in mice transgenic for a HA-specific T cell receptor [17–20]. In another example, immunization of rats with human umbilical vein endothelial cells resulted in an antibody and CD4+ T cell-mediated emphysema [21]. Transfer of T cells from mice exposed to cigarette smoke into Rag2(−/−) hosts has been shown to drive COPD-like disease [22]. Finally, Aire-deficient mice and humans can develop severe lung disease, presumably driven by loss of immune tolerance to antigens found in the lung [23, 24]. Evidence of this type has led to increasing acceptance among the medical community of the concept that COPD has an autoimmune component [25, 26].
Studies of autoimmune etiology in COPD have yielded conflicting results. For example, two studies failed to detect specific autoantibodies in the sera of COPD patients [27, 28]. Also, some accept the evidence supporting the association of autoantibodies, but contend that they arise as bystanders of general inflammation and do not play any significant role in COPD pathology [29]. However, these views are not unlike historical controversy over immuno-pathologic roles in many diseases. Though presently identified autoantibodies may indeed not play a direct role in mediating pulmonary pathology, they are indicative of adaptive immune response to self. We posit that these autoantibodies are produced as a result of an underlying autoimmune process.
Additional circumstantial evidence of a link between autoimmunity and chronic lung disease comes from observations that a significant proportion of systemic lupus erythematous (SLE) [30], rheumatoid arthritis (RA) [31], and systemic scleroderma (Scl) [32] patients present with interstitial lung disease (ILD), which is associated with a worse prognosis. This type of chronic lung disease is characterized by inflammation of the pulmonary interstitium, which can result in fibrosis with ventilatory and gas exchange abnormalities. Due to the high prevalence of ILD in SLE, RA, and Scl, autoimmunity is likely an important contributor to lung pathology.
Interestingly, recent studies have shown a possible link between COPD and ILD [33, 34]. In addition to examining autoantibody profiles of COPD patients, we included sera from RA patients with and without ILD, as well as SLE with and without ILD. Our goal was to determine commonalities associated with chronic lung disease across a spectrum of systemic autoimmunity and lung diseases, in the interest of defining reactivities associated with pulmonary pathology.
We hypothesized that autoimmunity is causally related to development of COPD, likely arising as a result of inflammation triggered by environmental factors such as smoking. A critical step toward understanding the contribution of autoimmunity to COPD is the definition of the specificity of autoantibodies produced in the disease. To this end, we undertook an autoantigen array analysis of the specificity of autoantibodies associated with chronic bronchitis and emphysema. We further hypothesized that during progression to emphysema patients may exhibit differing reactivity to self-antigens than those found in chronic bronchitis. Thus, the set of autoantibody reactivities, or the autoantibody profile, of a patient may correlate with both COPD disease status and phenotype.
Methods
Patient selection
Sera were selected from de-identified banked, frozen samples collected in SST tubes (Becton–Dickinson), originally obtained through the COPDGene® Study. The normal subject group included both healthy smoker and non-smoker sera (n = 5, median age = 44). COPD groups included chronic bronchitis (airway disease, non-emphysematous, n = 7, median age = 61) and emphysema (n = 9, median age = 59). All COPD patients studied were classified as severe by spirometry (GOLD stage III/IV) [5, 35]. A radiologist qualitatively assessed CT scans for all COPD patients to diagnose emphysema or chronic bronchitis (airway disease).
SLE and RA patients’ sera were selected from banked, frozen samples obtained through the NJH Interstitial Lung Disease Tissue Bank. SLE patient groups included those with ILD (SLE-ILD, n = 6, median age = 44) and without ILD (n = 6, median age = 51). RA patients with ILD (RA-ILD, n = 13, median age = 57) were compared with RA without ILD (n = 8, median age = 56.5). All patients were diagnosed with their respective autoimmune disease and a positive or negative diagnosis for ILD according to the ATS consensus classification [36].
All patient samples were originally collected under approval by respective Institutional Review Boards. Samples used in this study were banked, de-identified, and results cannot be linked to subjects and are thus exempt from protection of human subjects as defined by 45 CFR 46.
Immunohistochemistry
IgG was isolated from serum samples from representative COPD patients and normal subjects. IgG purification was performed using Protein G (GE Biosciences), and purified IgG was diluted to equivalent concentration for all samples. Lung tissue originating from a lung disease-free organ donor was formalin-fixed, paraffin-embedded, and serially sectioned (4 μm). Purified IgG was diluted and applied to sections using a Dako Autostainer, and binding detected by chromogen IHC kit (Dako). Tissue sections were counterstained with hematoxylin and visualized with an Aberia ScanScope XT digital slide scanner at 20X magnification.
Autoantigen array
An autoantigen array comprised of 70 autoantigens and 8 calibration proteins (hIgG, hIgM, mIgG, mIgM, anti-hIgG, anti-hIgM, anti-mIgG, and anti-mIgM) were printed on FAST-16 slides (Whatman). Autoantigen microarrays were manufactured, hybridizated, and scanned as previously described [37–40]. Briefly, antigens were diluted to printing concentration in printing buffer (Whatman) and transferred to 384-well plates. The antigens were printed in duplicates or triplicates onto nitrocellulose-coated 16-pad FAST™ slides (Whatman) by MicroGrid 610 microarray printer (Genomic Solutions Inc.). After printing, the slides were kept in a chamber with 70 % humidity for 4 h at room temperature (RT), and stored at 4 °C. For hybridization, slides are normalized to RT for 15 min, and blocking buffer (Whatman) was added to each array for 60 min. Serum samples were pretreated with DNAse-I (50 U/ml) for 30 min at RT in buffer containing 50 mM Tris–HCl, 75 mM KCl, 3 mM MgCl2, pH 8.3. The pretreated serum samples were diluted 1:100 in blocking buffer, and diluted serum was added to each array for 1 h. Following hybridization, arrays were washed with washing buffer (Whatman). Cy3-conjugated anti-human IgG and Cy5-conjugated anti-human IgM (Jackson ImmunoResearch) at 1:1000 dilution were applied to each array and incubated at RT for 1 h. Following incubation with secondary antibodies, the arrays were washed and spun dry. Fluorescence was visualized using a Genepix 4000B scanner (Molecular Devices) with 532 nm and 635 nm wavelengths and Genepix Pro6.0 software was used to generate the Gene Pix Result (GPR) files.
Array statistical analyses
From GPR file, the average signal intensity of local background was subtracted from average signal intensity of each spot to generate the background subtracted fluorescent intensity (BSFI) of each antigen spot. The average BSFI of replicate spots is defined as the mean fluorescent intensity (MFI) of replicate assays for each antigen. MFI of each reactivity was normalized to the MFI of the respective calibration proteins spotted on the slide (e.g., human IgG and IgM) using the formula: normalized (nMFI) = antigen MFI/calibration control MFI × 1,000. These nMFI were utilized for all subsequent analyses.
IgM (data not shown) and IgG reactivities were compared between subject groups. Our analyses were focused on IgG as these are indicative of an autoimmune reaction sufficient to drive class switching. Data analyses were performed using GraphPad Prism version 5.0 (GraphPad Software Inc.) software. To calculate statistical significance between groups, the Mann–Whitney test was chosen. For comparison of increased reactivity in disease groups over normal, one-tailed p values of <0.05 were considered significant. In comparisons between disease groups, two-tailed p values were assessed, due to the inability to predict which disease group would have increased reactivity.
Array data visualization
Data are presented as mean ± standard error of the mean (SEM). “Fold increase over baseline” represents the individual sample’s normalized MFI divided by the mean of normal subject group reactivity. Error bars illustrate the SEM in the patient group for a given antigen reactivity. “Galaxy plots” illustrate the relative reactivity of each patient group with each antigen on the array using units of fold increase over baseline. Individual dots represent mean MFI for a given antigen reactivity. X, Y coordinates allow comparison of antigen reactivities between two subject groups, as well as increase over normal (reactivity defined as 1). The error bars represent the SEM of the group (horizontal: X, vertical: Y). Thus, a galaxy plot illustrates variance both within and between groups. The 45-degree line is for illustration of perfect correlation (slope = 1), and for visual reference of skewing between mean group reactivities.
Results
To first determine whether COPD patients express serum autoantibodies reactive to lung tissue, we conducted immunohistochemical (IHC) analysis of antibody binding to healthy lung tissue. The IHC analyses demonstrated that patients with COPD produce serum antibodies reactive with antigens present in healthy lung tissue (Fig. 1). In general, sera from patients with emphysema stained with greater intensity than sera from chronic bronchitis patients. Sera contained antibodies reactive with airway-associated collagen (middle of slides). Chronic bronchitis is associated with reduced reactivity to airway (upper left) and pneumocytes (upper right) as compared with emphysema. Both patient groups showed minimal staining of the arterial endothelium (bottom). Images are representative of multiple lung sections and multiple patients for each group. Together, these findings illustrate both the presence of lung-reactive antibodies in COPD and increased autoreactivity in emphysema.
Fig. 1.
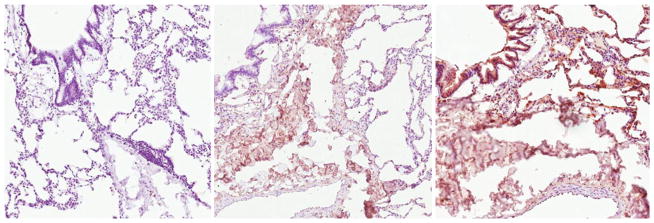
COPD patients have serum autoantibodies reactive to healthy lung tissue. Purified IgG fractions from serum of representative healthy smokers (left), and chronic bronchitis (middle) and emphysema (right) patients were used to stain serial sections of non-smoker lung tissue. Brown coloration indicates immunoreactivity. Hematoxylin counterstain
Previous studies have utilized an autoantigen array to test sera for reactivity to antigens associated with SLE, RA, and other systemic autoimmune disease [37–40]. This array consists of 70 intact human, mammalian, and bacterial antigens. These antigens include either recombinant or native preparations as previously described [40]. The antigens to which significantly elevated reactivities were detected in patient sera as compared with controls are listed in Table 1.
Table 1.
COPD disease phenotypes are associated with partially distinct serum autoantibody profiles
| Chronic bronchitis versus normal | Emphysema versus normal | COPD versus normal | Emphysema versus chronic bronchitis |
|---|---|---|---|
| Cardiolipin | Aggrecan** | B2-microglobulin | Aggrecan** |
| H1 | Amyloid** | Cardiolipin** | Collagen II |
| La/SS-B | B2-glycoprotein 1 | CENP-B | Intaktin |
| LC1 | B2-microglobulin | Collagen II | TPO |
| TPO | Cardiolipin** | Collagen IV | U1-snRNP-BB′ |
| U1-snRNP-BB′ | CENP-B** | Cytochrome C | U1-snRNP-C |
| Collagen I | DGPS** | ||
| Collagen II** | Elastin | ||
| Collagen IV | H1** | ||
| Cytochrome C*** | H2A | ||
| DGPS** | H2B | ||
| Elastin | Histone | ||
| H1** | JO-1 | ||
| H2A** | La/SS-B** | ||
| H2B** | LC1 | ||
| Histone | PL-12 | ||
| Intaktin EDTA | PL-7 | ||
| JO-1** | Ro-52 (SSA) | ||
| La/SS-B** | Thyroglobulin | ||
| LC1 | Topoisomerase | ||
| PCNA | TPO | ||
| PL-12** | U1-snRNP-68 | ||
| PL-7 | U1-snRNP-A | ||
| Ro-52 (SSA)** | U1-snRNP-BB′** | ||
| Scl-70** | |||
| Thyroglobulin** | |||
| Topoisomerase** | |||
| U1-snRNP-68 | |||
| U1-snRNP-BB′** | |||
| U1-snRNP-C |
Listed (below/above) are the specific antigen reactivities that are increased above baseline and differ significantly between groups compared. Statistical significance was calculated using Mann–Whitney test for comparison of populations with unequal variance (all listed are p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001)
To begin to characterize the specificity of autoantibodies produced in COPD, we compared autoantigen reactivities of all patients diagnosed with severe COPD (GOLD stage III/IV) to normal subjects. The array analyses (Figs. 2; Table 1) confirmed the presence of IgG autoantibodies in COPD patients. Statistically significant increases in levels of antibodies to 24 antigens, including ubiquitous nuclear proteins and those of tissue-specific origin were detected in sera of COPD patients relative to healthy control subjects. These findings are consistent with previous reports documenting autoantibodies in sera of COPD patients [8, 14, 15], and extend earlier findings to include previously unidentified self-reactivities.
Fig. 2.
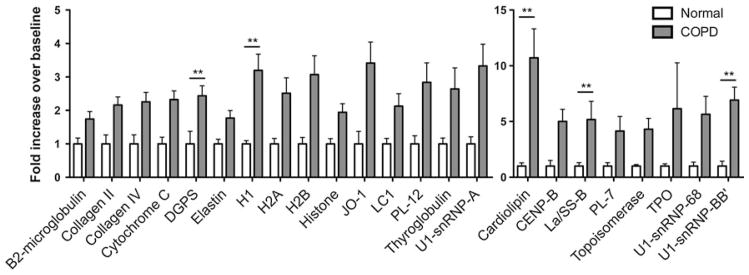
Severe COPD is associated with multiple autoantibody reactivities. Autoantigen reactivities of serum antibodies from patients diagnosed with GOLD stage III/IV COPD (n = 16) were compared with normal subjects. Shown are reactivities from array of 70 antigens that were significantly increased in the disease group. Statistical significance calculated using Mann–Whitney test for populations with unequal variance (one-tailed, all listed are p ≤ 0.05, **p ≤ 0.01)
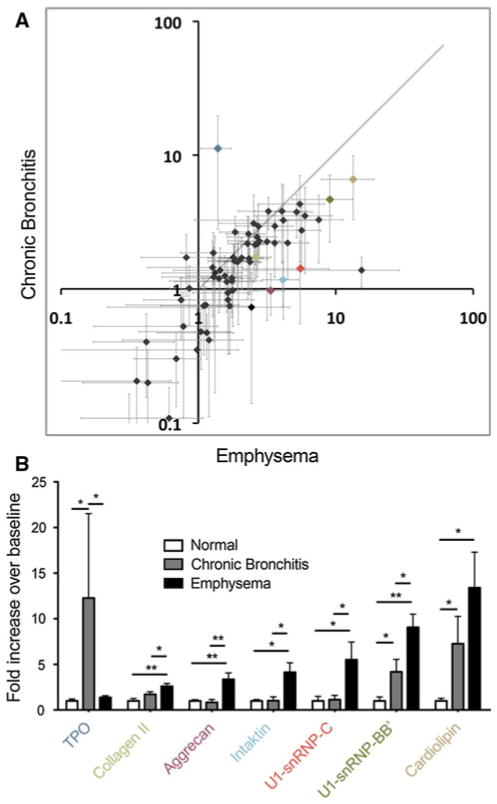
In an attempt to determine whether autoantibody profiles differ with disease phenotype, we compared reactivities of COPD patients diagnosed with chronic bronchitis (airways disease) to those with emphysema (Fig. 3; Table 1). This comparison demonstrated that although while chronic bronchitis is associated with autoreactivity, emphysema is characterized by higher levels of reactivity to many of the same antigens, as well as reactivity with additional auto-antigens. In total, emphysema is associated with significant sera reactivity to 30 of the 70 autoantigens represented on the array. Notably, a significantly increased production of antibodies reactive to aggrecan, collagen, and molecules of the spliceosome was observed in emphysema sera. These data reveal an increased level of autoimmunity in emphysema compared with chronic bronchitis.
Fig. 3.
Autoantigen reactivity of serum antibodies are increased in emphysema relative to subjects with chronic bronchitis. Represented are the mean serum antibody reactivities to individual antigens (each dot denotes an antigen, crosshairs = SEM) of the chronic bronchitis versus emphysema groups as fold increase over normal (a). The diagonal line represents a slope of 1, and is illustrative of relative correlation. Dots falling along the diagonal reflect reactivities that are increased in both subject groups, while those removed from the diagonal are more likely to reflect variation. Relatively more autoantigen reactivity is observed in sera from emphysematous patients. Large colored dots (a) are re-illustrated below (b) as examples of variation in chronic bronchitis and emphysema antibody reactivities (*p ≤ 0.05, **p ≤ 0.01)
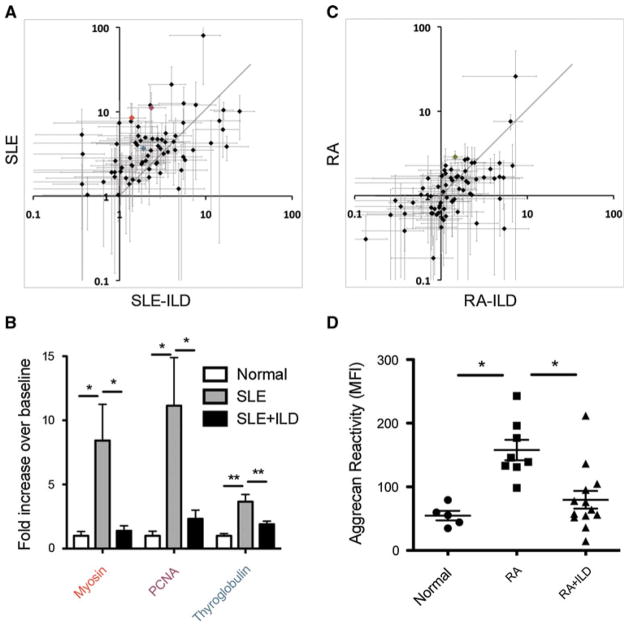
RA and SLE are often associated with ILD, suggesting a spread of immunoreactivity to lung antigens. For comparison with COPD—associated reactivities, as well as to identify common autoantibody profiles associated with lung disease—we analyzed profiles of patients with diagnosed autoimmune disorders with or without lung involvement (Fig. 4). Surprisingly, the analysis revealed that SLE-ILD patients exhibit significantly decreased reactivity to myosin, proliferating cell nuclear antigen (PCNA), and thyroglobulin than autoimmune patients without diagnosed lung disease. Additionally, RA-ILD patients showed a single significantly decreased reactivity to aggrecan, compared with RA without lung disease (RA = 2.881: RA-ILD = 1.454 mean fold increase over normal). Together, these data show that lung disease was associated with significant decreases in specific antigen reactivities in two separate autoimmune disorders.
Fig. 4.
Autoimmunity-associated ILD shows autoantibody profile variability, including reduced reactivity to array antigens. Mean reactivities to individual antigens compared from patient groups: SLE and SLE-ILD (a), RA and RA-ILD (c). The colored antigens found to be significantly different with autoimmunity-associated ILD are reillustrated for SLE (b). Aggrecan (c, green dot) reactivity is illustrated as individual subjects’ normalized MFI (d)
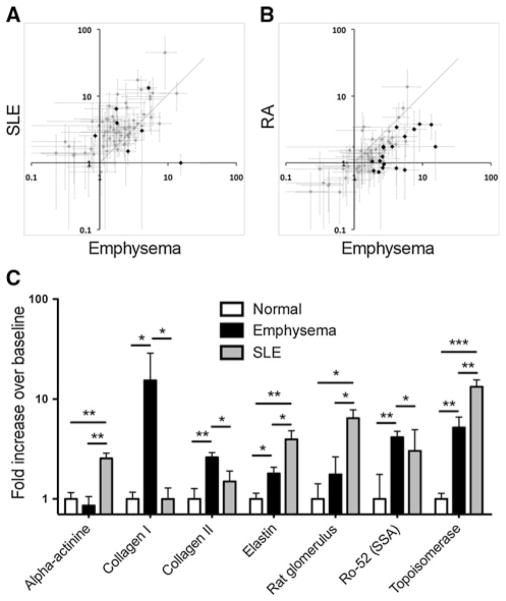
To evaluate differences in autoantibody reactivity profile in patients with emphysema to those with diagnosed autoimmunity, we compared emphysema with RA or SLE subject groups (Fig. 5). Emphysema subject sera showed both similar and reduced IgG reactivities as compared with SLE subject sera. Of the 7 reactivities that significantly differed between disease groups, 4 were increased in SLE and 3 were increased in emphysema. However, a general increase in autoantibody reactivities was observed in emphysema over RA, 18 of which were significant (p = <0.05). No autoreactivities were significantly increased in RA over emphysema. Together, these data show that the autoantibody profile associated with emphysema lies between those observed in SLE and RA.
Fig. 5.
Emphysema is characterized by less antibody autoreactivity than SLE, and greater than RA. Mean reactivities to individual antigens compared from patient groups: all SLE and emphysema (a), all RA and emphysema (b). Large black dots represent antigens that had statistically significant difference (p ≤ 0.05) between patient groups. The 18 antigens that varied between RA and emphysema were all increased in emphysema (b). The 7 that differed between SLE and emphysema included 4 increased in SLE, and 3 increased in emphysema (c) (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001)
Discussion
This study confirms and extends previous analyses of occurrence of autoantibodies in COPD patients, identifying self-antigen reactivities and their relative titers in subject sera using an array 70 of antigens previously associated with RA, SLE, Scl, and other autoimmune disorders [40]. Additionally, we included in the study sera from patients with RA or SLE, with and without lung disease, in an effort to define reactivities both common and unique to lung disease. The results of the study demonstrate that COPD patients produce autoantibodies against multiple self-antigens, including those expressed in healthy lung (Figs. 1, 2; Table 1). Reactivities of these antibodies include antigens previously identified in COPD [8, 14, 15], as well as antigens associated with other autoimmune diseases [40]. These findings support the hypothesis that autoimmunity is present in COPD and may be a driving factor in both disease pathology and exacerbation. The fact that the reactivities observed are not restricted to lung autoantigens raises the possibility that autoimmunity in COPD may also drive extrapulmonary disease comorbidities. Further, the breadth of the observed reactivities, including ubiquitous antigens, is not uncommon in autoimmune disorders and does not exclude a tissue-restricted pathology [41].
Along with ubiquitous antigen reactivities, we found an increase in autoantibodies to thyroid-specific antigens, thyroid peroxidase (TPO), and thyroglobulin (Fig. 2). These specificities are normally associated with autoimmune thyroid disease; however, the COPD patients studied here were not diagnosed with any clinical thyroid disease. Interestingly, in an earlier study by Birring et al. [8], patients with non-smoking-related COPD had a high incidence of thyroid disease and/or anti-thyroid antigen antibodies. This raises the possibility that a link exists between thyroid autoimmunity and lung disease. Autoimmune individuals may simply be prone to develop a second, unrelated autoimmunity due to defects in the maintenance of tolerance, or lung and thyroid autoimmunity may have a direct link, in which development of one autoimmune response drives the second.
Of particular interest, we found that among COPD groups, patients diagnosed with emphysema had a broader spectrum of autoantibody reactivities, and a trend toward increased titer for most of these antibodies (Fig. 3; Table 1). This finding confirms our hypothesis that autoantibody profiles differ somewhat between these disease phenotypes. The implications of this finding are that autoantibody profile may correlate with risk for development of emphysema, and may therefore be a biomarker for this disease phenotype. Additionally, the correlation of autoreactivity profiles with differential disease phenotype is further circumstantial evidence for autoimmune pathology in COPD.
Our findings of decreased autoantibodies in the subset of patients with SLE-ILD or RA-ILD, relative to autoimmune patients without lung disease (Fig. 4) are very surprising. We suggest that this may be due to two possible mechanisms. The first is that in autoimmune patients with ILD there may be some level of immune suppression. However, this would seem inconsistent with previous findings demonstrating that decreased ANA titers are associated with a better prognosis in ILD [42]. An alternative explanation lies in the possibility that autoimmune ILD patients may have skewed antibody reactivity to other autoantigens that are immunodominant over those represented on the array. These could include antigens that are important in driving the added lung disease. Immunodominance of lung antigens would explain both the decrease in sera antibodies to the primarily ubiquitous self-antigens represented on the array and could drive the development of ILD in association with autoimmunity.
The answer to how such immunodominance of lung antigens would arise may lie in the etiology of the auto-immunity. It is thought that in both RA and SLE, tissue-specific inflammation or injury is an important instigator of immune responses that spread to eventually cause systemic autoimmunity. Perhaps in patients with ILD associated with autoimmunity, the lung is the organ in which inflammation first arises, skewing the adaptive immune response. Smoking is a risk factor for development of both RA [43] and SLE [44], so an association with lung inflammation and initiation of autoimmunity exists. Additionally, recent studies in animal models of arthritis have implicated a role for cigarette smoke in exacerbation of arthritis, though notably; cigarette smoke was insufficient to initiate arthritis or ILD in the SKG mouse model. In any case, the investigation of a role for lung-specific immunity in autoimmune initiation is difficult to test in human subjects. Assuming autoantibody reactivities are identified for pulmonary antigens as proposed above, future studies could examine the procession of immunoreactivity. In patients who develop a lung-initiated autoimmunity, one could expect to see early, perhaps preclinical onset, autoantibodies against a set of lung-specific antigens, with a spreading to ubiquitous antigens as disease progresses.
In light of these considerations, it is likely that COPD patients produce antibodies to self-antigens not represented on the array. These could include antigens that are important in lung disease pathology and are common to those produced in autoimmune ILD patients. Two separate studies have identified specific self-antigens relevant to ILD pathology driven by Aire deficiency. These antigens are vomeromodulin in mice, or a related protein, LPLUNC1 in humans [24], and KCNRG [23, 45], both of which are absent from the autoantigen array employed in this study. Additionally, cytokeratin 18 antibodies have been shown to be present and correlate with disease progression in COPD and were not included in this array [13]. Finally, post-translational modifications have been proposed as an origin of neo-self antigens, and a recent study has demonstrated the presence of antibodies reactive to modified self-proteins in COPD [12]. Thus, we propose that both ILD and COPD patients have additional serum antibodies reactive to self-antigens not represented on the array.
To address this possibility, future studies should utilize approaches that assess autoreactivity to an expanded set of lung antigens. Ideally, this would include the majority of the lung proteome, as well as post-translational modifications that could result in neo-self antigens, such as those listed above. We predict that autoimmune patients with ILD will produce an increased level of autoantibodies reactive to lung-derived antigens, likely critical to ILD pathology and correlated to the decrease in reactivity to some of the ubiquitous antigens assayed in this study. Additionally, we predict that COPD patients will have both common and unique autoantibody profiles, the common self-antigens indicative of chronic lung disease, and unique characteristic of disease phenotype.
Nonetheless, it is reassuring that the array analysis was successful in confirming reactivities with both of the previously identified COPD self-antigens which were represented. These included elastin, previously shown to correlate with disease severity, and CENP-B [14, 15]. Together, these findings strengthen the positive association of autoantibodies with COPD. Limitations of this study include the number of patients studied and the advanced disease stage of patients in the COPD groups. It is possible that a study of a patient group with GOLD stage 1–2 would not yield the same findings. We plan to extend this study to investigate autoantibody profiles earlier in disease progression and attempt to replicate findings using other serum antibody detection methods, such as ELISA.
Strikingly, by the method employed, the reactivity of emphysema patient sera is remarkably similar to that observed in patients with SLE, and significantly increased over RA (Fig. 5). The comparison of the autoantibody profiles of emphysema patients to all RA or SLE groups shows a hierarchy of autoreactivity, in which emphysema falls below that observed in SLE and above RA. This is surprising in that RA is well accepted as an autoimmune disorder, while immunopathologies in emphysema, and COPD as a whole, remain controversial.
In conclusion, we have demonstrated that COPD is associated with production of autoantibodies against a broad spectrum of self-antigens, and immunoreactivity to lung. Further, we suggest that among COPD patient groups, the autoantibody profile associated with emphysema is broader and titers increased relative to chronic bronchitis. This finding is important in defining a correlation of autoreactivity to a differential disease phenotype. We propose that future studies should attempt to identify autoantibody profiles useful as risk, diagnostic, and prognostic indicators. In fact, such findings could be utilized in predicting candidates for specific therapy, and in monitoring the success of these therapeutic interventions.
We propose that COPD is indeed associated with a substantial level of autoimmunity, as defined by an adaptive immune response to self-antigen. The spectrum of this reactivity, or autoantibody profile, is correlated to both disease status and phenotype. The grand total of autoreactivities is yet to be defined, and these findings merit a redoubling of efforts to identify the remaining unknown antigens. Further, pathologic roles for candidate autoantibodies should be investigated in transgenic or passively immunized animal models. Finally, given the association of B cell infiltrates in COPD [46], and the findings of autoantibodies as an indicator of activation, B cell targeted therapy should be considered for trials in therapeutic intervention in COPD.
Acknowledgments
The authors would like to thank Dr. Steve Groshong for his contribution to histological analyses, and the patients of National Jewish Health for their blood donations. This work was funded by the NJC COPD Institutional Program and the generous donation by Joel Farkas to National Jewish Health.
Contributor Information
Thomas A. Packard, Department of Immunology, University of Colorado School of Medicine, National Jewish Health, 1400 Jackson St, Denver, CO 80206, USA
Quan Z. Li, Department of Immunology, University of Texas Southwestern, Medical Center, Dallas, TX, USA
Gregory P. Cosgrove, Department of Medicine, National Jewish Health, Denver, CO, USA
Russell P. Bowler, Department of Medicine, National Jewish Health, Denver, CO, USA
John C. Cambier, Email: cambierj@njhealth.org, Department of Immunology, University of Colorado School of Medicine, National Jewish Health, 1400 Jackson St, Denver, CO 80206, USA
References
- 1.Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. National Vital Statistics Reports: US Department of Health and Human Services. Centers for Disease Control and Prevention. National Center for Health Statistics; 2011. [Accessed 14 Jun 2012]. Deaths: preliminary data for 2009; p. 51. http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_04.pdf. [Google Scholar]
- 2.WHO Media Centre: Fact Sheet N∘310. World Health Organization; 2011. [Accessed 14 Jun 2012]. The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en/ [Google Scholar]
- 3.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–8. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61:935–9. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, Committee GS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 6.Gamble E, Grootendorst DC, Hattotuwa K, O’Shaughnessy T, Ram FS, Qiu Y, Zhu J, Vignola AM, Kroegel C, Morell F, Pavord ID, Rabe KF, Jeffery PK, Barnes NC. Airway mucosal inflammation in COPD is similar in smokers and ex-smokers: a pooled analysis. Eur Respir J. 2007;30:467–71. doi: 10.1183/09031936.00013006. [DOI] [PubMed] [Google Scholar]
- 7.Motz GT, Eppert BL, Sun G, Wesselkamper SC, Linke MJ, Deka R, Borchers MT. Persistence of lung CD8 T cell oligoclonal expansions upon smoking cessation in a mouse model of cigarette smoke-induced emphysema. J Immunol. 2008;181:8036–43. doi: 10.4049/jimmunol.181.11.8036. [DOI] [PubMed] [Google Scholar]
- 8.Birring SS, Brightling CE, Bradding P, Entwisle JJ, Vara DD, Grigg J, Wardlaw AJ, Pavord ID. Clinical, radiologic, and induced sputum features of chronic obstructive pulmonary disease in nonsmokers: a descriptive study. Am J Respir Crit Care Med. 2002;166:1078–83. doi: 10.1164/rccm.200203-245oc. [DOI] [PubMed] [Google Scholar]
- 9.Hagstad S, Ekerljung L, Lindberg A, Backman H, Rönmark E, Lundbäck B. COPD among non-smokers—Report from the Obstructive Lung Disease in Northern Sweden (OLIN) studies. Respir Med. 2012;106(7):980–8. doi: 10.1016/j.rmed.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Feghali-Bostwick CA, Gadgil AS, Otterbein LE, Pilewski JM, Stoner MW, Csizmadia E, Zhang Y, Sciurba FC, Duncan SR. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:156–63. doi: 10.1164/rccm.200701-014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karayama M, Inui N, Suda T, Nakamura Y, Nakamura H, Chida K. Antiendothelial cell antibodies in patients with COPD. Chest. 2010;138:1303–8. doi: 10.1378/chest.10-0863. [DOI] [PubMed] [Google Scholar]
- 12.Kirkham PA, Caramori G, Casolari P, Papi AA, Edwards M, Shamji B, Triantaphyllopoulos K, Hussain F, Pinart M, Khan Y, Heinemann L, Stevens L, Yeadon M, Barnes PJ, Chung KF, Adcock IM. Oxidative stress-induced antibodies to carbonyl-modified protein correlate with severity of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:796–802. doi: 10.1164/rccm.201010-1605OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo YB, Chang CA, Wu YK, Hsieh MJ, Tsai CH, Chen KT, Chen CY, Chan EC. Identification and clinical association of anti-cytokeratin 18 autoantibody in COPD. Immunol Lett. 2010;128:131–6. doi: 10.1016/j.imlet.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Cogswell S, Storness-Bliss C, Corry DB, Kheradmand F. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–9. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 15.Leidinger P, Keller A, Heisel S, Ludwig N, Rheinheimer S, Klein V, Andres C, Hamacher J, Huwer H, Stephan B, Stehle I, Lenhof HP, Meese E. Novel autoantigens immunogenic in COPD patients. Respir Res. 2009;10:20. doi: 10.1186/1465-9921-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Núñez B, Sauleda J, Antó JM, Julià MR, Orozco M, Monsó E, Noguera A, Gómez FP, Garcia-Aymerich J, Agustí A, Investigators P-C. Anti-tissue antibodies are related to lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1025–31. doi: 10.1164/rccm.201001-0029OC. [DOI] [PubMed] [Google Scholar]
- 17.Bruder D, Westendorf AM, Geffers R, Gruber AD, Gereke M, Enelow RI, Buer J. CD4 T Lymphocyte-mediated lung disease: steady state between pathological and tolerogenic immune reactions. Am J Respir Crit Care Med. 2004;170:1145–52. doi: 10.1164/rccm.200404-464OC. [DOI] [PubMed] [Google Scholar]
- 18.Gereke M, Gröbe L, Prettin S, Kasper M, Deppenmeier S, Gruber AD, Enelow RI, Buer J, Bruder D. Phenotypic alterations in type II alveolar epithelial cells in CD4 + T cell mediated lung inflammation. Respir Res. 2007;8:47. doi: 10.1186/1465-9921-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp3(+) regulatory T cells. Am J Respir Crit Care Med. 2009;179:344–55. doi: 10.1164/rccm.200804-592OC. [DOI] [PubMed] [Google Scholar]
- 20.Tosiek MJ, Gruber AD, Bader SR, Mauel S, Hoymann HG, Prettin S, Tschernig T, Buer J, Gereke M, Bruder D. CD4 + CD25 + Foxp3 + regulatory T cells are dispensable for controlling CD8 + T cell-mediated lung inflammation. J Immunol. 2011;186:6106–18. doi: 10.4049/jimmunol.1000632. [DOI] [PubMed] [Google Scholar]
- 21.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, Nicolls MR, Fontenot AP, Tuder RM, Voelkel NF. An animal model of autoimmune emphysema. Am J Respir Crit Care Med. 2005;171:734–42. doi: 10.1164/rccm.200409-1275OC. [DOI] [PubMed] [Google Scholar]
- 22.Motz GT, Eppert BL, Wesselkamper SC, Flury JL, Borchers MT. Chronic cigarette smoke exposure generates pathogenic T cells capable of driving COPD-like disease in Rag2−/− mice. Am J Respir Crit Care Med. 2010;181:1223–33. doi: 10.1164/rccm.200910-1485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alimohammadi M, Dubois N, Sköldberg F, Hallgren A, Tardivel I, Hedstrand H, Haavik J, Husebye ES, Gustafsson J, Rorsman F, Meloni A, Janson C, Vialettes B, Kajosaari M, Egner W, Sargur R, Pontén F, Amoura Z, Grimfeld A, De Luca F, Betterle C, Perheentupa J, Kämpe O, Carel JC. Pulmonary autoimmunity as a feature of autoimmune polyendocrine syndrome type 1 and identification of KCNRG as a bronchial autoantigen. Proc Natl Acad Sci USA. 2009;106:4396–401. doi: 10.1073/pnas.0809986106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shum AK, DeVoss J, Tan CL, Hou Y, Johannes K, O’Gorman CS, Jones KD, Sochett EB, Fong L, Anderson MS. Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance. Sci Transl Med. 2009;1:9ra20. doi: 10.1126/scitranslmed.3000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–26. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- 26.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–54. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 27.Brandsma CA, Kerstjens HA, Geerlings M, Kerkhof M, Hylkema MN, Postma DS, Timens W. The search for autoantibodies against elastin, collagen and decorin in COPD. Eur Respir J. 2011;37:1289–92. doi: 10.1183/09031936.00116710. [DOI] [PubMed] [Google Scholar]
- 28.Greene CM, Low TB, O’Neill SJ, McElvaney NG. Anti-proline-glycine-proline or antielastin autoantibodies are not evident in chronic inflammatory lung disease. Am J Respir Crit Care Med. 2010;181:31–5. doi: 10.1164/rccm.200904-0545OC. [DOI] [PubMed] [Google Scholar]
- 29.Toyoshima M, Chida K, Suda T, Sato M. Is autoimmunity really related to the pathogenesis of COPD? Am J Respir Crit Care Med. 2011;184:1212–3. doi: 10.1164/ajrccm.184.10.1212a. author reply 3. [DOI] [PubMed] [Google Scholar]
- 30.Sant SM, Doran M, Fenelon HM, Breatnach ES. Pleuropulmonary abnormalities in patients with systemic lupus erythematosus: assessment with high resolution computed tomography, chest radiography and pulmonary function tests. Clin Exp Rheumatol. 1997;15:507–13. [PubMed] [Google Scholar]
- 31.Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, MacDonald SD, Travis WD, Stylianou MP, Rosas IO. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168:159–66. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 32.Steen VD, Medsger TA. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–44. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 33.Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, Israel-Biet D, Court-Fortune I, Valeyre D, Cordier JF. Combined pulmonary fibrosis and emphysema: a distinct under-recognised entity. Eur Respir J. 2005;26:586–93. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 34.Samara KD, Margaritopoulos G, Wells AU, Siafakas NM, Antoniou KM. Smoking and pulmonary fibrosis: novel insights. Pulm Med. 2011;2011:461439. doi: 10.1155/2011/461439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease, Inc; 2011. p. 80. [Google Scholar]
- 36.Society AT, Society ER. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 37.Li QZ, Karp DR, Quan J, Branch VK, Zhou J, Lian Y, Chong BF, Wakeland EK, Olsen NJ. Risk factors for ANA positivity in healthy persons. Arthritis Res Ther. 2011;13:R38. doi: 10.1186/ar3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li QZ, Zhen QL, Xie C, Wu T, Mackay M, Aranow C, Putterman C, Mohan C. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115:3428–39. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li QZ, Zhou J, Lian Y, Zhang B, Branch VK, Carr-Johnson F, Karp DR, Mohan C, Wakeland EK, Olsen NJ. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol. 2010;159:281–91. doi: 10.1111/j.1365-2249.2009.04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, Mohan C, Wakeland EK, Olsen NJ. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol. 2007;147:60–70. doi: 10.1111/j.1365-2249.2006.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marrack P, Kappler J, Kotzin BL. Autoimmune disease: why and where it occurs. Nat Med. 2001;7:899–905. doi: 10.1038/90935. [DOI] [PubMed] [Google Scholar]
- 42.Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct ntity. Chest. 2011;140:1292–9. doi: 10.1378/chest.10-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Källberg H, Ding B, Padyukov L, Bengtsson C, Rönnelid J, Klareskog L, Alfredsson L, Group ES. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70:508–11. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costenbader KH, Karlson EW. Cigarette smoking and systemic lupus erythematosus: a smoking gun? Autoimmunity. 2005;38:541–7. doi: 10.1080/08916930500285758. [DOI] [PubMed] [Google Scholar]
- 45.Popler J, Alimohammadi M, Kämpe O, Dalin F, Dishop MK, Barker JM, Moriarty-Kelsey M, Soep JB, Deterding RR. Auto-immune polyendocrine syndrome type 1: utility of KCNRG autoantibodies as a marker of active pulmonary disease and successful treatment with rituximab. Pediatr Pulmonol. 2012;47:84–7. doi: 10.1002/ppul.21520. [DOI] [PubMed] [Google Scholar]
- 46.Drolet JP, Frangie H, Guay J, Hajoui O, Hamid Q, Mazer BD. B lymphocytes in inflammatory airway diseases. Clin Exp Allergy. 2010;40:841–9. doi: 10.1111/j.1365-2222.2010.03512.x. [DOI] [PubMed] [Google Scholar]