To the Editor:
Women over the age of 50 who lack antecedent structural lung damage have emerged as a growing population afflicted with pulmonary nontuberculous mycobacteria (PNTM) (1). A common body morphology among these women, who are often tall and lean with scoliosis, pectus excavatum (PE), and mitral valve prolapse (MVP), points to a possible genetic basis for susceptibility to PNTM (2, 3). Exploration of these traits in family members of patients with PNTM provides evidence for the hypothesis that genetic factors modify disease susceptibility. To date, a systematic analysis of a large cohort of patients with PNTM and their relatives has not been performed. We describe here a comprehensive review of families with PNTM designed to identify a familial phenotype of disease. Some of the results of this study have been previously reported in the form of an abstract (4).
Methods
Probands included in our study were adult patients who met diagnostic criteria for PNTM (5) and were enrolled in a natural history of mycobacterial disease protocol at the National Institutes of Health. Probands with other known underlying structural lung diseases or characterized immunodeficiency were excluded. Chart records for each proband were abstracted for demographic data, mycobacterial species identified, and the presence of bronchiectasis, scoliosis, MVP, and PE. Bronchiectasis was identified by chest computed tomography (CT) imaging, scoliosis by spinal X-rays, MVP by echocardiogram, and PE by computing a Haller index greater than 3.5 from chest CT imaging (6). All probands were contacted to construct family pedigrees extending to first- and second-degree relatives. Probands were asked to report the following in all members of the pedigree: current or past diagnosis of PNTM, bronchiectasis, MVP, scoliosis, and PE. When possible, consent was obtained from family members to interview them directly as well. The instrument used to ascertain these characteristics can be found in the online supplement. Summary statistics were performed in Excel (Microsoft Corporation, Redmond, WA).
Results
A total of 383 patients were enrolled in the natural history protocol, 274 of whom were excluded for the following reasons: 87 had an underlying immunodeficiency, 53 were enrolled relatives of patients, 44 had withdrawn from the study, 35 were already deceased, 24 had a known underlying structural lung disorder, 14 were unable to be contacted, 11 had tuberculosis, and 6 were pediatric patients (5 with isolated endobronchial mycobacterial lesions and 1 with pulmonary alveolar proteinosis). The remaining 109 probands included for analysis led to 2,285 first- and second-degree relatives with an average of 21 family members per proband. Seventy-two relatives consented to in-person interviews; the remainder of the relatives’ medical histories were ascertained via interview of the proband.
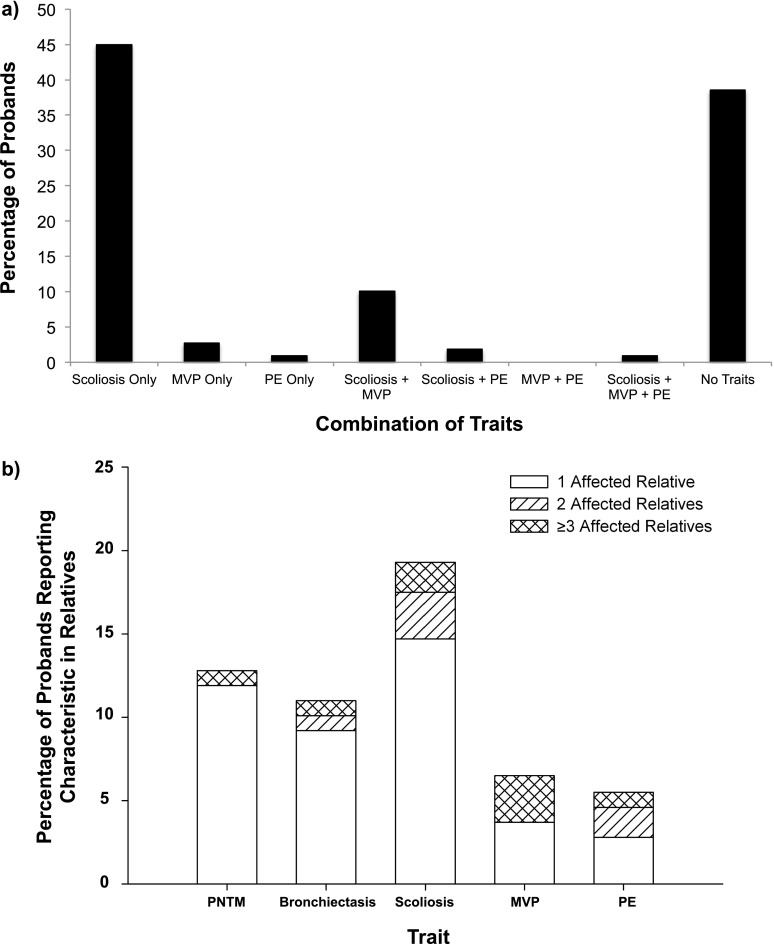
Characteristics of the probands are listed in Table 1. The majority of probands were white females with an average age of 66.7 years. The most common mycobacterial species was Mycobacterium avium complex, followed by M. abscessus and M. fortuitum. Sixty-three (57.8%) patients had scoliosis, 15 (13.8%) had echocardiographic evidence of MVP, and 4 (3.7%) had radiographic evidence of PE by the Haller index. The proportion of probands featuring different combinations of these traits is shown in Figure 1a.
TABLE 1.
CHARACTERISTICS OF STUDY PROBANDS (N = 109)
| Characteristic | Frequency |
|---|---|
| Female sex, n (%) | 97 (89.0) |
| Mean ± SD age, yr | 66.7 ± 11.6 |
| Mean ± SD BMI, kg/m2 | 22.1 ± 4.0 |
| Mean ± SD FEV1, % predicted | 80.3 ± 21.8 |
| Ethnicity, n (%) | |
| White | 100 (91.7) |
| Asian | 7 (6.4) |
| Hispanic | 2 (1.8) |
| Mycobacterium species, n (%) | |
| M. avium complex | 82 (75.2) |
| M. abscessus | 39 (35.8) |
| M. fortuitum | 8 (7.3) |
| M. kansasii | 3 (2.8) |
| M. mucogenicum | 4 (3.7) |
| Other | 7 (6.4) |
| Radiographic presentation, n (%) | |
| Nodular bronchiectasis | 106 (97.2) |
| Cavitary | 23 (21.1) |
| Scoliosis, n (%) | 63 (57.8) |
| Mitral valve prolapse, n (%) | 15 (13.8) |
| Pectus excavatum, n (%) | 4 (3.7) |
Figure 1.
(a) The percentage of probands exhibiting different combinations of scoliosis, mitral valve prolapse (MVP), and pectus excavatum (PE) is shown. The largest proportions of probands exhibited either only scoliosis (45%) or none of the pertinent traits (39%). Combinations of traits included 11 patients (10%) with both scoliosis and MVP, 2 patients (1.8%) with both scoliosis and PE, and 1 patient (0.92%) with all three traits. (b) The percentage of probands reporting pertinent characteristics in one or more of their relatives is shown, with the distribution of the number of affected relatives per family shown in white (1 affected relative), diagonal stripes (2 affected relatives), and cross-hatch (≥3 affected relatives). Of 109 probands, 14 (12.8%) reported at least 1 relative with PNTM, with 1 individual reporting having 3 affected relatives. Twelve (11.0%) reported at least 1 relative with bronchiectasis, with 1 individual reporting having 4 affected relatives. Twenty-one (19.2%) reported at least 1 relative with scoliosis, with 1 individual reporting having 10 affected relatives. Seven (6.4%) reported at least 1 relative with MVP, with 3 individuals reporting having 3 affected relatives each. Six (5.5%) reported at least 1 relative with PE, with 1 individual reporting having 5 affected relatives.
The proportion of probands reporting pertinent characteristics in at least one family member is displayed in Figure 1b. Of 109 probands, 14 (12.8%) reported having at least one other first- or second-degree relative diagnosed with PNTM. In addition, 13 (11.9%) probands reported bronchiectasis and 21 (19.2%) probands reported scoliosis in at least one relative. MVP was found in 7 (6.4%) families and PE in 6 (5.5%) families. Twenty-three relatives exhibited just scoliosis, 14 exhibited just MVP, and 12 exhibited just PE. Two relatives exhibited scoliosis and MVP together, and one relative exhibited MVP and PE together. Of the 14 families with more than one member with PNTM, 8 were sibling pairs, 4 were parent–child pairs, and 2 were aunt–niece pairs.
Discussion
To date, no systematic review of a large cohort has evaluated whether conditions previously ascribed to patients with PNTM, such as scoliosis, MVP, and PE, cluster among family members. The infrequency of these traits in large population-based epidemiologic studies suggests that they are much more prevalent among relatives of patients with PNTM than in the general population. Whereas studies of general pediatric populations demonstrate rates of scoliosis of between 0.5 and 3.2% (7–10), we observe here 21 (19.2%) families with at least one member with scoliosis. Comparisons with populations similar to patients with PNTM also suggest a higher rate. For example, an estimate of scoliosis in a population of patients with cystic fibrosis, who also carry higher risk for developing PNTM, only revealed a prevalence of 2.2% (11). Similarly, our observations exceed those found in a comparable cohort of postmenopausal women, where lumbar scoliosis was found in 12.9% (12). Estimates of MVP in the general population range from 2.4 to 2.7% (13, 14) whereas we observed 7 (6.4%) families with at least one affected relative. Even rarer conditions such as PE, affecting 0.12% in one autopsy series (15) and 0.49% in one pediatric screening study (16), were found in 6 (5.5%) families. Patients with primary ciliary dyskinesia, another structural lung disease associated with PNTM, may feature similar rates of PE compared with our population, with one study finding a 9% prevalence on CT scans (17). Although inherited syndromes of these traits certainly exist, how frequently these traits are expressed in first- and second-degree relatives of comparable populations remains unknown.
The genetic underpinnings of this familial syndrome remain undetermined. The triad of scoliosis, MVP, and PE points strongly to a Marfan-like syndrome, although it should be noted that none of our probands met diagnostic criteria for Marfan syndrome. As a corollary to our findings, Marfan syndrome is known to be associated with bronchiectasis (18). Other connective tissue diseases, such as congenital contractural arachnodactyly, have been associated with PNTM infection (19). Involvement of the transforming growth factor-β pathway, alterations in which account for many connective tissue diseases, could potentially explain the marked similarities in body morphotype between the families in our cohort and the connective tissue disease population (20).
Our study has several limitations, most notably that traits identified by probands and their family members need in-person verification. These findings, based on family member report, are subject to ascertainment bias; however, this bias is likely to underestimate the prevalence, particularly for mild forms of conditions such as scoliosis and MVP, which may not be apparent to individuals without full medical examinations. Second, the fact that PNTM is a disease largely affecting postmenopausal women makes the estimation of disease difficult for younger relatives who have not yet reached the “at-risk” age for disease onset. Longitudinal follow-up over decades will be necessary to determine the true degree of risk for family members. Moreover, the identification of PNTM, bronchiectasis, and associated traits is challenging in the oldest generations of pedigrees, many of whom are already deceased or may not have undergone diagnostic testing during their lifetimes. Despite the limitations intrinsic to using generations who are presymptomatic and those who may not have had adequate testing, at least some of these families show parent–child transmission, most consistent with dominant disease. It is also possible that there are recessive families in this cohort as well. Finally, the relative rarity of exhibiting all associated traits together in both the probands and the relatives raises the possibility that although they are clustered, these traits may not necessarily be inherited together. Further explorations of larger populations of patients with PNTM in addition to clarifying the inheritance pattern of these traits would better demonstrate whether a true syndrome of traits exists, rather than mere clustering.
This study is the first to show a familial clustering of traits related to PNTM infections in otherwise unaffected relatives. The higher-than-expected prevalence of PNTM, bronchiectasis, scoliosis, MVP, and PE strongly supports a genetic basis for at least some cases of PNTM.
Footnotes
Supported entirely by the Divisions of Intramural Research of the National Institute of Allergy and Infectious Diseases and the Clinical Center at the National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, Brown MR, Chernick M, Steagall WK, Glasgow CG, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914–916. doi: 10.1164/ajrccm/144.4.914. [DOI] [PubMed] [Google Scholar]
- 4.Leung JM, Adjemian J, Dastmalchi N, Davis J, Claypool RJ, Holland SM, Prevots DR, Olivier KN. A familial syndrome of pulmonary nontuberculous mycobacteria infections [abstract] Am J Respir Crit Care Med. 2012;185:A4020. doi: 10.1164/rccm.201306-1059LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 6.Haller JA, Jr, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg. 1987;22:904–906. doi: 10.1016/s0022-3468(87)80585-7. [DOI] [PubMed] [Google Scholar]
- 7.Suh SW, Modi HN, Yang JH, Hong JY. Idiopathic scoliosis in Korean schoolchildren: a prospective screening study of over 1 million children. Eur Spine J. 2011;20:1087–1094. doi: 10.1007/s00586-011-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stirling AJ, Howel D, Millner PA, Sadiq S, Sharples D, Dickson RA. Late-onset idiopathic scoliosis in children six to fourteen years old. A cross-sectional prevalence study. J Bone Joint Surg Am. 1996;78:1330–1336. doi: 10.2106/00004623-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Gore DR, Passehl R, Sepic S, Dalton A. Scoliosis screening: results of a community project. Pediatrics. 1981;67:196–200. [PubMed] [Google Scholar]
- 10.Wong HK, Hui JH, Rajan U, Chia HP. Idiopathic scoliosis in Singapore schoolchildren: a prevalence study 15 years into the screening program. Spine (Phila Pa 1976) 2005;30:1188–1196. doi: 10.1097/01.brs.0000162280.95076.bb. [DOI] [PubMed] [Google Scholar]
- 11.Fainardi V, Koo SD, Padley SP, Lam SH, Bush A. Prevalence of scoliosis in cystic fibrosis. Pediatr Pulmonol. 2013;48:553–555. doi: 10.1002/ppul.22624. [DOI] [PubMed] [Google Scholar]
- 12.Urrutia J, Diaz-Ledezma C, Espinosa J, Berven SH. Lumbar scoliosis in postmenopausal women: prevalence and relationship with bone density, age, and body mass index. Spine (Phila Pa 1976) 2011;36:737–740. doi: 10.1097/BRS.0b013e3181db7456. [DOI] [PubMed] [Google Scholar]
- 13.Theal M, Sleik K, Anand S, Yi Q, Yusuf S, Lonn E. Prevalence of mitral valve prolapse in ethnic groups. Can J Cardiol. 2004;20:511–515. [PubMed] [Google Scholar]
- 14.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 15.Kelly RE, Jr, Lawson ML, Paidas CN, Hruban RH. Pectus excavatum in a 112-year autopsy series: anatomic findings and the effect on survival. J Pediatr Surg. 2005;40:1275–1278. doi: 10.1016/j.jpedsurg.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Rajabi-Mashhadi MT, Ebrahimi M, Mobarhan MG, Moohebati M, Boskabady MH, Kazemi-Bajestani SM. Prevalence of chest wall deformities in a large sample of Iranian children aged 7-14 years. Iran J Pediatr. 2010;20:221–224. [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy MP, Noone PG, Leigh MW, Zariwala MA, Minnix SL, Knowles MR, Molina PL. High-resolution CT of patients with primary ciliary dyskinesia. AJR Am J Roentgenol. 2007;188:1232–1238. doi: 10.2214/AJR.06.0965. [DOI] [PubMed] [Google Scholar]
- 18.Foster ME, Foster DR. Bronchiectasis and Marfan’s syndrome. Postgrad Med J. 1980;56:718–719. doi: 10.1136/pgmj.56.660.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulson ML, Olivier KN, Holland SM. Pulmonary non-tuberculous mycobacterial infection in congenital contractural arachnodactyly. Int J Tuberc Lung Dis. 2012;16:561–563. doi: 10.5588/ijtld.11.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ovrutsky AR, Merkel PA, Schonteich E, Bai X, Kinney W, Iseman MD, Kartalija M, Knight V, Chan ED. Patients with non-tuberculous mycobacterial lung disease have elevated transforming growth factor-beta following ex vivo stimulation of blood with live Mycobacterium intracellulare. Scand J Infect Dis. 2013;45:711–714. doi: 10.3109/00365548.2013.800947. [DOI] [PubMed] [Google Scholar]