Abstract
Rationale: The prognostic significance of delirium symptoms in intensive care unit (ICU) patients with focal neurologic injury is unclear.
Objectives: To determine the relationship between delirium symptoms and subsequent functional outcomes and quality of life (QOL) after intracerebral hemorrhage.
Methods: We prospectively enrolled 114 patients. Delirium symptoms were routinely assessed twice daily using the Confusion Assessment Method for the ICU by trained nurses. Functional outcomes were recorded with modified Rankin Scale (scored from 0 [no symptoms] to 6 [dead]), and QOL outcomes with Neuro-QOL at 28 days, 3 months, and 12 months.
Measurements and Main Results: Thirty-one (27%) patients had delirium symptoms (“ever delirious”), 67 (59%) were never delirious, and the remainder (14%) had persistent coma. Delirium symptoms were nearly always hypoactive, were detected mean 6 days after intracerebral hemorrhage presentation, and were associated with longer ICU length of stay (mean 3.5 d longer in ever vs. never delirious patients; 95% confidence interval, 1.5–8.3; P = 0.004) after correction for age, admit National Institutes of Health (NIH) Stroke Scale, and any benzodiazepine exposure. Delirium symptoms were associated with increased odds of poor outcome at 28 days (odds ratio, 8.7; 95% confidence interval, 1.4–52.5; P = 0.018) after correction for admission NIH Stroke Scale and age, and with worse QOL in the domains of applied cognition–executive function and fatigue after correcting for the NIH Stroke Scale, age, benzodiazepine exposure, and time of follow-up.
Conclusions: After focal neurologic injury, delirium symptoms were common despite low rates of infection and sedation exposure, and were predictive of subsequent worse functional outcomes and lower QOL.
Keywords: delirium, outcomes, quality of life
At a Glance Commentary
Scientific Knowledge on the Subject
Delirium symptoms are common in the intensive care unit (ICU), generally related to sedation and infection, and predictive of subsequent worse outcomes.
What This Study Adds to the Field
This paper describes a cohort of ICU patients with intracerebral hemorrhage in whom delirium symptoms were common in the relative absence of sedative use or infection. Delirium symptoms were not predictable at admission but were independently associated with functional outcomes and quality of life at follow-up. The prognostic significance of the clinical findings of delirium symptoms in neuro-ICU patients may offer new insights into predictors of poor outcomes for these patients.
The symptoms of delirium, a potential consequence of multiple clinical disease states and physiologic aberrations, include a shift in baseline mental status, inattention, and disorganized thinking or altered level of consciousness. Although nonspecific, this syndrome is an independent predictor of higher mortality (1), longer length of stay (LOS), higher cost of care, and worse long-term cognitive outcomes in medical, surgical, burn, and trauma intensive care unit (ICU) patients (2). There are few such data, however, in ICU patients with focal neurologic injury without systemic illness.
Risk factors for delirium symptoms are typically global (infection [3] and intravenous sedation, particularly benzodiazepines [BZDs]) (4, 5) as opposed to focal lesions (e.g., hematoma). Most mechanically ventilated patients are delirious during hospitalization (1), potentially because of the sedation regimen (e.g., BZD infusion), but sedation is typically minimized in neurologically injured patients to permit repeated neurologic assessment that may lead to an acute intervention (6).
Screening tests for delirium have been recently validated in neurologically ill patients (7–9), including ischemic stroke (7, 10) and intracerebral hemorrhage (ICH) (9). The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) is a commonly used and well-validated instrument (11–14) that could be used to track the presence of absence or such a symptom complex in the neuro-ICU patient population (7, 10, 15). Some distinguish between hyperactive (agitated) delirium and hypoactive delirium (“encephalopathy”) depending on the level of arousal. We use “delirium” here for consistency with the literature that is specifically not mechanistic, but highlights the presence of delirium symptoms and later outcomes.
Among patient populations with focal neurologic injury, acute ischemic stroke is best studied, with documented rates of delirium between 11% (8) and 50% (7). Although these patients were typically not critically ill, mortality was higher in patients with delirium. ICH is an attractive disease model because it is typically defined by the acute hematoma and is not complicated by cerebral vasospasm (as opposed to aneurysmal subarachnoid hemorrhage [SAH]). Patients are routinely admitted to an ICU for management of severe hypertension and because there is a high risk of clinical decompensation.
In this acutely neurologically ill cohort who routinely receive ICU care, we sought to understand the prognostic significance of delirium symptoms assessed with a standard assessment and associations with LOS and follow-up functional outcomes and quality of life (QOL). Specifically, we tested the null hypothesis that delirium symptoms would have no association with LOS, functional outcomes, or selected domains of QOL.
Some of the results of these studies have been previously reported in the form of an abstract (9).
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Institutional Review Board. Written informed consent to collect data and outcomes was obtained from the patient or a legally authorized representative with the exception for patients who died while in the hospital (generally those with persistent coma), for whom the requirement for consent was waived.
We prospectively enrolled consecutive patients from December 2009 through April 2013. All patients were prospectively diagnosed by a board-certified neurologist using computed tomography. We measured change in hematoma volume as previously described (16). Patients were excluded if their ICH was attributable to trauma, hemorrhagic conversion of ischemic stroke, or structural lesions (aneurysm, tumor, arteriovenous malformation, vessel dissection, and so forth).
All patients with ICH are admitted to the Neuro/Spine ICU with a standardized order set. We prospectively recorded baseline demographic, past medical history, clinical data, and follow-up. Pneumonia was prospectively defined per criteria from the U.S. Centers for Disease Control and Prevention (17). EEG monitoring was routinely performed for at least 24 hours in unresponsive patients and interpreted by a board-certified epileptologist. Severity was prospectively assessed on admission with the National Institutes of Health (NIH) Stroke Scale (NIHSS) (18), a validated neurologic examination from 0 (best) to 42 (worst theoretically possible score), with a score of 8 or more indicating a moderately severe deficit.
Assessment of Delirium Symptoms
The CAM-ICU (11, 12), a dichotomous clinical bedside rating, was routinely used to track the presence or absence of delirium symptoms and assessed by trained ICU nurses twice daily in the ICU and the stroke unit (ICU step-down). Delirium symptom screening and training on the CAM-ICU was implemented in all hospital ICUs in early to mid-2009, several months before we began data collection. The CAM-ICU was electronically documented and later all assessments were retrieved by automated query. A positive CAM-ICU indicated the presence of a change from the patient’s new baseline mental status (established on admission after ICH symptom onset) plus inattention and either an altered level of consciousness or disorganized thinking, as previously shown in a validation and reliability study in 129 patients with ischemic stroke or ICH (7). In the 1,003 paired assessments against a neurologist Diagnostic and Statistical Manual-IV reference rater (7), the CAM-ICU demonstrated a sensitivity of 76% and specificity of 98% with an accuracy of 94% and an interrater reliability (kappa) of 0.94.
The level of arousal was routinely assessed twice daily with the Richmond Agitation- Sedation Scale (RASS) as previously described (19). Briefly, the RASS is scored from −5 (unresponsive) to +4 (combative, violent); the protocolized goal was 0 (alert and calm) to −2 (briefly awakens with eye contact to voice).
Medication administration (e.g., BZD) and the timing of administration in relationship to the CAM-ICU ratings were electronically retrieved as previously described (20). Patients with at least one positive CAM-ICU (delirium symptoms) were classified as ever delirious, patients with all negative assessable CAM-ICU scores were classified at never delirious, and patients who could never be assessed as positive or negative within 28 days of symptom onset because of coma were classified as persistent coma (all of these patients were dead at 28 d). Duration of delirium symptoms was calculated as described elsewhere (2).
Outcome Assessment
The usual outcome measure after ischemic stroke and ICH is the modified Rankin Scale (mRS) (21), a validated scale from 0 (no symptoms) to 6 (dead), assessed at 1 (22) and 3 months after onset. Scores of 0–2 indicate mild disability or better (good outcome, as defined by the National Institute of Neurological Disorders and Stroke for the High Dose Desferoxamine in ICH trial). The mRS was prospectively recorded at 14 days or discharge, 28 days, and 3 months after ICH symptom onset with a validated questionnaire (23, 24). The mRS is valid when assessed by telephone or by proxy (25). Interviewers were unaware of delirium status. We began to attempt mRS assessments at 12 months alongside Neuro-QOL assessments (discussed next).
Neuro-QOL and the Patient Reported Outcomes Measurement Information System (PROMIS), developed by the NIH, provide valid and reliable tools for QOL assessment in patients with stroke, cancer, and other disorders (26). Neuro-QOL became available for limited (research) use in January 2011 (www.assessmentcenter.net). For the subset of patients with follow-up scheduled after early 2011 (see Table E1 in the online supplement), we attempted to obtain QOL assessments at 28 days, 3 months, and 12 months. Patients or proxies were sent a scripted, deidentified email to complete QOL assessments online, the typical method for assessing QOL. Neuro-QOL banks were validated for entry by proxy as part of their development, an advantage in patients with neurologic injury, where outcomes (including the mRS, especially for devastated or uncommunicative patients) are often reported by proxy (e.g., a family member). Patients without Internet access had the option of completing the assessment over the telephone as a form of proxy entry. We administered computer adaptive banks (where the patient’s response determines subsequent questions) in the following instruments at each assessment: upper extremity function (fine motor activity, such as picking up coins or opening bottles), lower extremity function (mobility, such as walking on uneven surfaces or stairs), applied cognition executive function (such as the ability to manage finances), and fatigue (difficulty starting or completing tasks because of tiredness). Results are expressed in T scores, centered on demographics of the general U.S. population at 50 ± 10. Further information is available at www.nihpromis.org and www.neuroqol.org.
Statistical Analysis
Data are expressed as n (%), mean ± SD, or median (Q1–Q3) as appropriate. Categorical data (e.g., seizures or not) were tested for an association with delirium status with chi-square statistics, using Fisher exact test when there were less than five observations per cell. Normally distributed numbers (age) were compared with analysis of variance (for three groups) or t tests (for two groups). Nonnormally distributed numbers (as detected by examination of a histogram and testing with the Kolmogorov-Smirnov distribution) were compared with the Kruskal-Wallis H (three groups) or Mann-Whitney U (two groups). We analyzed LOS in the ICU and hospital as a time-to-event variable in a Cox proportional hazards model to evaluate for the immortal-time bias as previously described (27). We performed logistic regression for predictors of good outcome. We were unable to analyze the impact of delirium on the mRS with ordinal regression because the proportional odds assumption did not hold. QOL scores were analyzed with mixed effects models because the repeated observations in a given patient are likely to be correlated from one assessment to the next (i.e., QOL at 28 d and 3 mo are likely to be correlated in a given patient); each instrument (fatigue, fine motor, and so forth) was considered separately. When QOL was reported by a proxy, we repeated the model with QOL scores corrected for proxy report with data from Neuro-QOL validation studies. We adjusted models for age and NIHSS, a previously accepted model for outcome prediction (20, 28). Calculations were made with standard statistical software (SPSS v. 21; IBM, Armonk, NY). A statistician from Neuro-QOL and the PROMIS Statistical Center who was not involved in the acquisition of data (J.L.B.) directed and reviewed the statistical analysis.
Results
Demographics of the sample are shown in Table 1. The population was typical of those with ICH, and most had a history of hypertension. Of 114 patients, 16 (14%) could never be assessed for delirium because of persistent coma. These patients had a greater severity of injury on admission (Table 2) and all were dead by 28 days after ICH symptom onset; this left 98 patients who could be assessed as ever or never delirious with the CAM-ICU, of whom 31 (27% overall) were ever delirious.
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS AT ADMISSION
| Variable | N (%) or Mean ± SD |
|---|---|
| N | 114 |
| Age, yr | 63.0 ± 13.8 |
| Ethnicity | |
| White | 58 (51) |
| African American | 54 (47) |
| Asian | 2 (2) |
| Women | 52 (46) |
| History of diabetes | 22 (19) |
| History of hypertension | 86 (75) |
| History of atrial fibrillation | 10 (9) |
| Coronary artery disease | 14 (12) |
| Dementia | 2 (2) |
TABLE 2.
POTENTIAL CLINICAL RISK FACTORS FOR DELIRIUM*
| Variable | Persistent Coma | No Delirium Symptoms | Delirium Symptoms | P Value for Three-Way Comparison | P Value for Ever vs. Never Delirious |
|---|---|---|---|---|---|
| N | 16 (14) | 67 (59) | 31 (27) | ||
| NIH Stroke Scale on admission | 29 (25 to 35) | 6 (2 to 16) | 8 (3 to 15) | <0.001 | 0.3 |
| Age, yr | 66.4 ± 13.5 | 61.7 ± 14.6 | 63.9 ± 12.1 | 0.4 | 0.5 |
| Length of stay in the intensive care unit | 2.2 (0.6 to 6.3) | 2.3 (1.1 to 6.7) | 7.0 (3.4 to 10.2) | <0.002 | 0.001 |
| Length of stay in the hospital | 2.4 (0.6 to 6.3) | 6.4 (4.0 to 13.2) | 13.2 (7.9 to 24.2) | <0.001 | 0.001 |
| Location | |||||
| Subcortical | 5 (31) | 37 (55) | 17 (55) | 0.6 | 0.8 |
| Lobar | 5 (31) | 20 (29) | 9 (29) | ||
| Infratentorial | 5 (31) | 9 (13) | 4 (13) | ||
| Other | 1 (1) | 1 (1) | 1 (3) | ||
| Hematoma volume, ml | 35 (15 to 72) | 6 (1.4 to 15.8) | 10 (3 to 29) | <0.001 | 0.1 |
| Change in hematoma volume | 1.5 (−3.5 to 11.3) | 0.1 (−0.3 to 1.4) | −0.4 (−1 to 0.5) | 0.3 | 0.1 |
| Intraventricular hemorrhage | 15 (94) | 18 (27) | 14 (45) | <0.001 | 0.07 |
| Pneumonia | 2 (13) | 6 (9) | 4 (13) | 0.8 | 0.5 |
| Ventilator-free days (maximum 14) | >0 (0 to 0) | 14 (6 to 14) | 12 (6 to 14) | <0.001 | 0.3 |
| Levetiracetam, total mg | 1,500 (0 to 3,500) | 0 (0 to 5,250) | 0 (0 to 7,000) | 0.01 | 0.9 |
| Levetiracetam for prophylaxis | 9 (56) | 22 (33) | 10 (33) | 0.2 | 0.9 |
| Phenytoin for prophylaxis | 1 (6) | 5 (8) | 2 (7) | 0.9 | 0.9 |
| Seizure | 1 (6) | 4 (6) | 0 | 0.4 | 0.2 |
| Lorazepam, total mg | 0 (0 to 2) | 0 (0 to 1) | 0 (0 to 1.5) | 0.3 | 0.4 |
| Midazolam, total mg | 0 | 0 (0 to 0.5) | 0 (0 to 3) | 0.2 | 0.04 |
| Any benzodiazepine use | 6 (37) | 31 (46) | 19 (61) | 0.2 | 0.2 |
Definition of abbreviation: NIH = National Institutes of Health.
Data are N (%), mean ± SD, or median (Q1 to Q3) as appropriate. The NIH Stroke Scale is a validated neurologic examination from 0 (no abnormality) to 42 (worst possible score), with scores of 8 indicating a moderately severe deficit.
Patients with persistent coma had large hematomas, severe neurologic injury, and a dismal prognosis. Among assessable patients the severity of neurologic injury, age, hematoma volume, and so forth were similar.
Delirium symptoms were nearly always hypoactive. Only two (2%) patients were ever very agitated (RASS 3, both of whom were ever delirious) and another nine (8%) were ever agitated (RASS 2, of whom five were ever delirious).
Timing and Duration of Delirium
Delirium symptoms were first detected 5.9 ± 6.1 days after ICH symptom onset, and after discharge from the ICU in five (16%). Delirium symptoms were usually short lived, with most patients delirious for 1 (n = 16), 2 (n = 3), or 3 (n = 3) days.
Inpatient Medication Use
The doses of BZD used during the hospital stay are shown in Table 2, and overall the use of any BZD was not associated with delirium symptoms. Midazolam was usually given once (in 32 [70%] of 46 patients), typically for a bedside procedure (e.g., external ventricular drain placement). Most doses of midazolam were not temporally related to delirium. Of the 14 patients who received midazolam and were ever delirious, the first dose of midazolam was given more than 2 days before the first delirium symptoms in six, within 2 days of the first delirium symptoms in six, and more than 2 days after the first delirium symptoms in two.
Other psychotropic medications were seldom used in assessable patients: quetiapine in five patients, risperidone and haloperidol in four patients, olanzapine in three patients, and diazepam in two patients.
Length of Stay
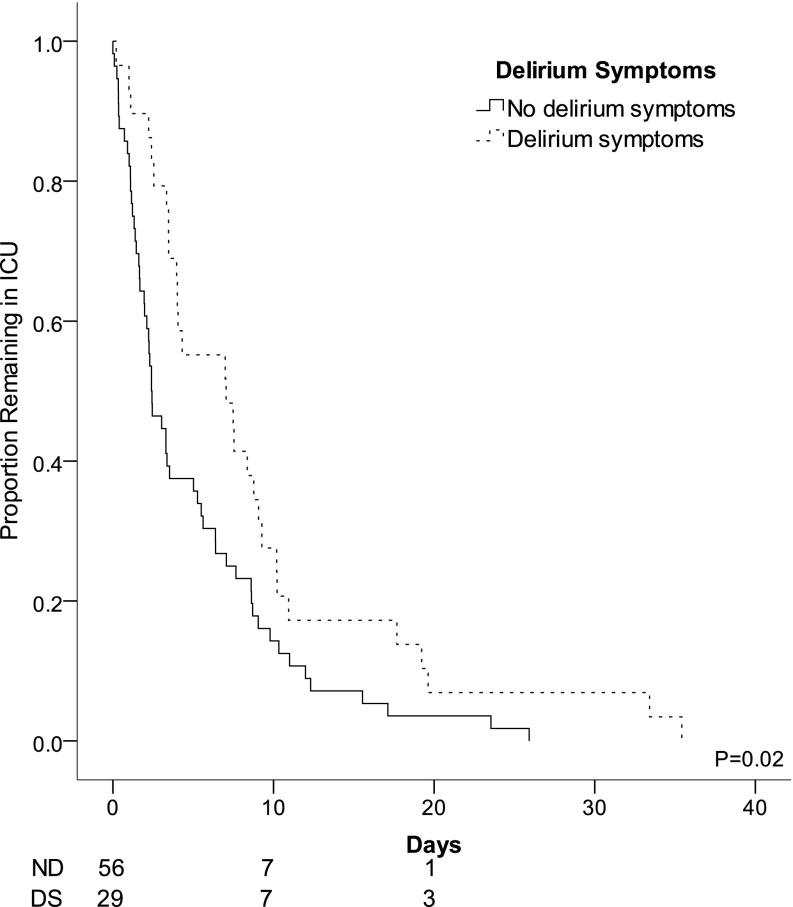
LOS in the ICU and hospital were associated with delirium status. In univariate analysis, patients who were ever delirious had longer LOS in the ICU and the hospital overall compared with patients who were never delirious (Figure 1, Table 2). When hospital and ICU LOS were considered as time-to-event endpoints, patients who were ever delirious had longer LOS after correction for the NIHSS, age, and any BZD use (P ≤ 0.03 for both). When considered as a time-dependent variable, delirium symptoms in assessable patients was predictive of longer ICU LOS (median, 2.1 d longer in ever vs. never delirious patients; 95% confidence interval [CI], 1.1–4.5; P = 0.03) after correction for age, admit NIHSS, and any BZD exposure. Delirium symptoms in assessable patients were also predictive of longer hospital LOS (median 3.5 d longer in ever vs. never delirious patients; 95% CI, 1.5–8.3; P = 0.004) after correction for age, admit NIHSS, and any BZD exposure.
Figure 1.
Unadjusted Kaplan-Meier plot of length of stay in the intensive care unit (ICU). Lines are stratified by patients who had no delirium symptoms (ND, solid line) or who had delirium symptoms (DS, stippled line). In Kaplan-Meier analysis, delirium symptoms were associated with median ICU length of stay of 7.0 (1.5–12.6) versus 2.4 (1.4–3.4) days (P = 0.02 by log-rank test).
Functional Outcomes among Assessable Patients
Among assessable patients at 14 days, those who were ever delirious had a higher (worse) median NIHSS (11 [6–19] vs. 3 [1–14]; P = 0.002) and a higher (worse) median mRS (5 [4–5] vs. 4 [2–5]; P = 0.003). In multivariate models, being ever delirious was associated with increased odds of poor outcome (mRS ≥3 vs. mRS ≤2) at 28 days (odds ratio [OR], 8.7; 95% CI, 1.4–52.5; P = 0.018) after correction for admission NIHSS (1.5 per point; 95% CI, 1.1–1.9; P = 0.003) and age (OR, 1.06 per year; 95% CI, 1.006–1.1; P = 0.03). At 3 and 12 months, however, only NIHSS was associated with good outcomes in multivariate models.
QOL Outcomes
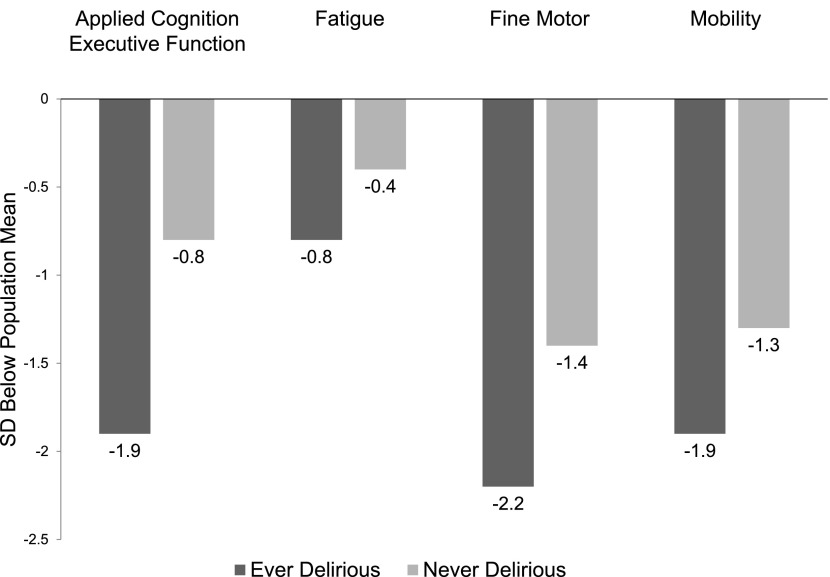
Of 78 patients known to be alive at 28 days, QOL data were available in 52 patients (66%). In univariate analysis, delirium was associated with worse QOL (lower score) in all domains of QOL (Figure 2). After correction for age, NIHSS on admit, and any BZD use, being ever delirious remained associated with worse QOL in the domains of applied cognition – executive function and fatigue (Table 3).
Figure 2.
Domain-specific quality of life (QOL) scores at 28 days, stratified by delirium. Patients with delirium had poorer mean QOL in all domains. T scores from Neuro-QOL are presented as SD below the mean compared with the U.S. general population for ease of viewing. In mixed models accounting all time-points of follow-up (Table 3), delirium symptoms were associated with lower QOL in the domains of applied cognition–executive function and fatigue.
TABLE 3.
MIXED MODELS FOR QUALITY OF LIFE ASSESSMENT*
| Neuro-QOL Instrument | Domain | Estimate | 95% Confidence Interval | P Value |
|---|---|---|---|---|
| Applied cognition executive function | Ability to manage tasks involving finances, taking medications, and so forth | 5.8 | 0.15 to 11.5 | 0.045 |
| Fatigue | Difficulty starting or completing activities | 7.4 | 1.7 to 13 | 0.01 |
| Fine motor | Fine motor function with the upper extremities | 0.9 | −3.7 to 5.6 | 0.7 |
| Mobility | Mobility in a variety of settings | 0.8 | −4.5 to 6.2 | 0.7 |
Definition of abbreviation: QOL = quality of life.
The estimate is how much poorer T scores were for patients who were ever versus never delirious (i.e., patients who were never delirious had applied cognition–executive function scores 5.8 points worse than those ever delirious; SD for all assessments is 10 points, so 5.8 points is 0.58 SD. We analyzed Neuro-QOL data at every point of follow-up. Mixed models were constructed for each instrument individually controlling for National Institutes of Health Stroke Scale on admission, age, any benzodiazepine use, and point of follow-up (28 d, 3 mo, and 12 mo). Results were similar when data were adjusted for proxy report.
Delirium was independently associated with worse QOL in the domains of applied cognition–executive function, and fatigue at 1-, 3-, and 12-month follow-up.
Discussion
Delirium symptoms were common in this cohort of patients with life-threatening brain injury despite the scarcity of infection and minimal levels of sedation, delirium’s usual risk factors. The detection of delirium symptoms in patients with acute ICH mean 6 days after ICH onset (not at the time of presentation, as one might have expected) was independently predictive of mean 3.5-day longer ICU LOS, nearly nine times the odds of poor functional outcomes at 1 month, and nearly a SD worse lower QOL in applied cognition–executive function and fatigue. This independent effect on functional outcomes (good vs. poor outcome) at 1 month, a reliable time for outcome assessment (22), is important given that it was present after correction for NIHSS and age, strong predictors of outcome in this population (28). These data underscore that delirium symptoms, which may be overlooked because of ICH, had implications for outcomes independent of the severity of the underlying neurologic injury.
BZDs have been associated with delirium (12, 29) in general ICUs with subsequent worse functional (8) and cognitive outcomes (2, 30), but in these investigations BZDs were typically infused and at much higher doses (5) than described here. Several patients were treated several days after delirium was detected, making causality questionable.
We did not confirm that older age and greater severity of injury are risk factors (31). How to predict delirium symptoms after ICH is not clear from these data because severity of injury was not associated with delirium symptoms. There may be unmeasured characteristics of ICH that predict the later manifestation of delirium; if detected, such characteristics might be appropriately considered in ICH severity scores.
Partial or subclinical seizures are a potential underlying cause of delirium because seizures after ICH (28) may alter consciousness. Although prophylactic phenytoin use has been shown to be associated with worse functional outcomes after ICH (20) and worse cognitive function after SAH (32), we routinely minimized its use. Pneumonia was uncommon and not associated with delirium in this cohort. Cerebral ischemia is common after ICH (33, 34), might be an underlying risk factor for delirium, and deserves future study.
Antipsychotic medication use may decrease the occurrence and duration of hyperactive delirium (35, 36), but might depress mental status. The brief duration of delirium in this population, median 1 day, argues against standing orders for antipsychotic medications, as recently recommended (15), and they were seldom used in this cohort. Our findings are likely to apply most to other patient populations who have hypoactive delirium.
Domain-specific QOL assessments at follow-up may be more meaningful than functional outcomes. Patients who had delirium symptoms in the hospital had worse QOL in particularly applied cognition–executive function and fatigue. Delirium’s limited association with mobility is probably why delirium was not associated with good functional outcome at 3 months because the mRS is heavily linked with mobility. Although there are data on cognitive dysfunction after delirium in general (2) and SAH (37), these data demonstrate that Neuro-QOL (and PROMIS, because they are similarly assessed) measures are feasible to obtain in survivors of critical care, and this may be a helpful standard for future outcomes research. QOL data require somewhat more effort from a patient or proxy to report than the validated questionnaire for the mRS. Proxy reporting is a potential source of bias in dependent patients, although other validated tests obtain QOL data by proxy in some cases (38), as we did here. Importantly, QOL results were similar when data were corrected for proxy reporting.
There are limitations to these data. Temporary alterations in consciousness might be severe enough to preclude assessment of CAM-ICU, but might resolve before delirium symptoms are detected. Added to the relatively low sensitivity of the CAM-ICU for delirium (39), this might artificially lower the apparent incidence of delirium. If anything, this would mean that our already striking findings could be an underestimate of the strength of the relationships. Certain aspects of the CAM-ICU, including test-retest reliability, have not been verified specifically in ICH patients, but they have been tested and found very strong in a large cohort of stroke patients. Taken together, the strong and reproducible data on the validity and reliability of the CAM-ICU in critically ill, mechanically ventilated, and now stroke patients (7, 10) lend support to the practice of screening for delirium symptoms in neurologically injured patients. These data were collected in a specialty neurologic ICU, and whether there would be different results for patients in a medical ICU with ICH is not known. We did not assess the implications of failure of other organ systems or physiologic derangement on delirium symptoms. We did not quantify cerebral edema or midline shift, which might also alter mental status. Severity of delirium symptoms cannot be assessed with the dichotomous CAM-ICU; whether another cognitive assessment is valid and reliable for this is not clear. Thus, this area of severity of symptoms is for now relegated to an area of future research. Dementia is common in patients with ICH and is associated with worse recovery, but was uncommon in this cohort. We did not record if the validated interview for the mRS was with the patient or a caregiver, nor the setting in which it was assessed. We took care to choose covariates previously associated with outcomes, although further studies with larger sample sizes and broader variation in ICH severity and baseline health are needed to confirm these results.
The terminology used to describe delirium in the literature is a recurring limitation. We used the term “delirium symptoms” for consistency, although delirium likely represents a spectrum of conditions that impair consciousness and attention. Our chosen terminology is a deliberate effort to be less committal about the presence of the actual entity of delirium, much like some papers in the field of post-traumatic stress disorder sometimes adjudicate that a population had “post-traumatic stress disorder symptoms” rather than a confirmed diagnosis of post-traumatic stress disorder (40). Nearly all of the delirium symptoms could also be referred to as “hypoactive.” Neither of these terms, however, allows for valid and reliable quantification and so would not likely enhance our study objectives.
Several strengths to our methods warrant further comment. Trained ICU nurses assessed delirium symptoms in our cohort after several months of use, which has been shown to be highly correlated with the reference standard in patients with ischemic stroke (7), neurosurgical patients (41), and ICH (9). Research nurses may have greater sensitivity than routine bedside assessment but specificity remains excellent (39), so our reported rates of delirium are, if in error, an underestimate. Delirium symptoms were usually 1 day in duration, so frequent screening is needed to detect it in most cases.
We found that symptoms of delirium were common after acute ICH, usually hypoactive and brief in duration, and associated with longer LOS, subsequent worse functional outcomes, and domain-specific QOL. The impact of delirium symptoms on longer LOS and worse outcomes indicates that further research on the importance and mechanism of delirium symptoms in this population (and other neurologically ill patients in the ICU) should be routinely pursued. Only through such work can we advance the knowledge of potentially modifiable aspects of neurologic injury in these high-risk patients.
Footnotes
Supported in part by National Institutes of Health grants 1 R01 AG 027472-01A1 and 1R01-AG035117-01A1 and VA Project ID 1123773 (Delirium and Dementia in Veterans Surviving ICU Care Project No. 0005) (E.W.E.) and NINDS contract HHSN271201200036C (D.C.). The infrastructure for automated data retrieval was funded in part by National Institutes of Health through a grant to Northwestern University’s Clinical and Translational Sciences (UL1RR025741).
Author Contributions: A.M.N. performed the statistical analysis and wrote the paper. J.L.B. reviewed and directed the statistical analysis. A.R.K. identified patients, obtained outcomes data, and revised the manuscript. N.F.R. identified patients, ensured patients met entry criteria, and critically revised the manuscript. M.B.M. and M.L.A. critically revised the manuscript. D.C. specified the domains of quality of life to be measured, critically reviewed the analysis of quality of life data, and critically revised the manuscript. E.W.E. specified the interpretation of delirium symptom data and critically revised the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201307-1256OC on October 8, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 2.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin SM, Huang CD, Liu CY, Lin HC, Wang CH, Huang PY, Fang YF, Shieh MH, Kuo HP. Risk factors for the development of early-onset delirium and the subsequent clinical outcome in mechanically ventilated patients. J Crit Care. 2008;23:372–379. doi: 10.1016/j.jcrc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 5.Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Maas MB, Rosenberg NF, Kosteva AR, Bauer RM, Guth JC, Liotta EM, Prabhakaran S, Naidech AM. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology. 2013;81:107–112. doi: 10.1212/WNL.0b013e31829a33e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitasova A, Kostalova M, Bednarik J, Michalcakova R, Kasparek T, Balabanova P, Dusek L, Vohanka S, Ely EW. Poststroke delirium incidence and outcomes: validation of the confusion assessment method for the intensive care unit (CAM-ICU) Crit Care Med. 2012;40:484–490. doi: 10.1097/CCM.0b013e318232da12. [DOI] [PubMed] [Google Scholar]
- 8.Oldenbeuving AW, de Kort PLM, Jansen BPW, Algra A, Kappelle LJ, Roks G. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology. 2011;76:993–999. doi: 10.1212/WNL.0b013e318210411f. [DOI] [PubMed] [Google Scholar]
- 9.Klein K. Detection of delirium in the neurological intensive care unit: validity of 2 tools and neurointensive examination for delirium screening [abstract] Crit Care Med. 2012;40:1–328. [Google Scholar]
- 10.Gusmao-Flores D, Salluh JI, Chalhub RA, Quarantini LC. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012;16:R115. doi: 10.1186/cc11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 13.Plaschke K, von Haken R, Scholz M, Engelhardt R, Brobeil A, Martin E, Weigand MA. Comparison of the confusion assessment method for the intensive care unit (CAM-ICU) with the Intensive Care Delirium Screening Checklist (ICDSC) for delirium in critical care patients gives high agreement rate(s) Intensive Care Med. 2008;34:431–436. doi: 10.1007/s00134-007-0920-8. [DOI] [PubMed] [Google Scholar]
- 14.Van Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Elseviers M, Bossaert L. A comparison of the CAM-ICU and the NEECHAM Confusion Scale in intensive care delirium assessment: an observational study in non-intubated patients. Crit Care. 2008;12:R16. doi: 10.1186/cc6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 16.Naidech AM, Jovanovic B, Liebling S, Garg RK, Bassin SL, Bendok BR, Bernstein RA, Alberts MJ, Batjer HH. Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke. 2009;40:2398–2401. doi: 10.1161/STROKEAHA.109.550939. [DOI] [PubMed] [Google Scholar]
- 17.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 20.Naidech AM, Garg RK, Liebling S, Levasseur K, Macken MP, Schuele SU, Batjer HH. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke. 2009;40:3810–3815. doi: 10.1161/STROKEAHA.109.559948. [DOI] [PubMed] [Google Scholar]
- 21.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 22.Ovbiagele B, Lyden PD, Saver JL VISTA Collaborators. Disability status at 1 month is a reliable proxy for final ischemic stroke outcome. Neurology. 2010;75:688–692. doi: 10.1212/WNL.0b013e3181eee426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson JTL, Hareendran A, Grant M, Baird T, Schulz UGR, Muir KW, Bone I. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33:2243–2246. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 24.Saver JL, Filip B, Hamilton S, Yanes A, Craig S, Cho M, Conwit R, Starkman S FAST-MAG Investigators and Coordinators. Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA) Stroke. 2010;41:992–995. doi: 10.1161/STROKEAHA.109.571364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruno A, Akinwuntan AE, Lin C, Close B, Davis K, Baute V, Aryal T, Brooks D, Hess DC, Switzer JA, et al. Simplified modified Rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke. 2011;42:2276–2279. doi: 10.1161/STROKEAHA.111.613273. [DOI] [PubMed] [Google Scholar]
- 26.Cella D, Lai JS, Nowinski CJ, Victorson D, Peterman A, Miller D, Bethoux F, Heinemann A, Rubin S, Cavazos JE, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78:1860–1867. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shintani AK, Girard TD, Eden SK, Arbogast PG, Moons KG, Ely EW. Immortal time bias in critical care research: application of time-varying Cox regression for observational cohort studies. Crit Care Med. 2009;37:2939–2945. doi: 10.1097/CCM.0b013e3181b7fbbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, Saver J, Nuwer MR, Frazee JG, McArthur DA, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441–1446. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]
- 29.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Boogaard M, Schoonhoven L, Evers AW, van der Hoeven JG, van Achterberg T, Pickkers P. Delirium in critically ill patients: impact on long-term health-related quality of life and cognitive functioning. Crit Care Med. 2012;40:112–118. doi: 10.1097/CCM.0b013e31822e9fc9. [DOI] [PubMed] [Google Scholar]
- 31.Oldenbeuving AW, de Kort PLM, van Eck van der Sluijs JF, Kappelle LJ, Roks G. An early prediction of delirium in the acute phase after stroke. J Neurol Neurosurg Psychiatry. doi: 10.1136/jnnp-2013-304920. (In press) [DOI] [PubMed] [Google Scholar]
- 32.Naidech AM, Kreiter KT, Janjua N, Ostapkovich N, Parra A, Commichau C, Connolly ES, Mayer SA, Fitzsimmons BF. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke. 2005;36:583–587. doi: 10.1161/01.STR.0000141936.36596.1e. [DOI] [PubMed] [Google Scholar]
- 33.Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on MRI, and outcomes after intracerebral hemorrhage. Stroke. 2012;43:67–71. doi: 10.1161/STROKEAHA.111.629493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menon RS, Burgess RE, Wing JJ, Gibbons MC, Shara NM, Fernandez S, Jayam-Trouth A, German L, Sobotka I, Edwards D, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71:199–205. doi: 10.1002/ana.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, Robbins T, Garpestad E. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419–427. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Li HL, Wang DX, Zhu X, Li SL, Yao GQ, Chen KS, Gu XE, Zhu SN. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial. Crit Care Med. 2012;40:731–739. doi: 10.1097/CCM.0b013e3182376e4f. [DOI] [PubMed] [Google Scholar]
- 37.Mayer SA, Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, Claassen J, Du YE, Connolly ES., Jr Global and domain-specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology. 2002;59:1750–1758. doi: 10.1212/01.wnl.0000035748.91128.c2. [DOI] [PubMed] [Google Scholar]
- 38.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Eijk MM, van den Boogaard M, van Marum RJ, Benner P, Eikelenboom P, Honing ML, van der Hoven B, Horn J, Izaks GJ, Kalf A, et al. Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med. 2011;184:340–344. doi: 10.1164/rccm.201101-0065OC. [DOI] [PubMed] [Google Scholar]
- 40.Girard TD, Shintani AK, Jackson JC, Gordon SM, Pun BT, Henderson MS, Dittus RS, Bernard GR, Ely EW. Risk factors for post-traumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care. 2007;11:R28. doi: 10.1186/cc5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luetz A, Heymann A, Radtke FM, Chenitir C, Neuhaus U, Nachtigall I, von Dossow V, Marz S, Eggers V, Heinz A, et al. Different assessment tools for intensive care unit delirium: which score to use? Crit Care Med. 2010;38:409–418. doi: 10.1097/CCM.0b013e3181cabb42. [DOI] [PubMed] [Google Scholar]