Rhinosinusitis is defined on the basis of the four cardinal symptoms of obstruction, drainage, smell loss, and facial pain or pressure in both acute (ARS) and chronic (CRS) rhinosinusitis, with CRS distinguished by a duration of symptoms of 3 or more months. The determinants of progression from ARS to CRS remain underexplored, but CRS differs from ARS in microbiology, with the most commonly cultured ARS pathogens rarely found in CRS. For the diagnosis of CRS, guidelines further recommend that symptoms be accompanied by objective evidence of sinus inflammation on the basis of sinus computed tomography (CT) scan or nasal endoscopy (1–3). These recommendations were necessary because it remains difficult to accurately differentiate whether a patient presenting with a chief complaint of 4 months of symptoms of facial pain or pressure, bilateral nasal obstruction, and nasal discharge has recurrent ARS, CRS, allergic rhinitis, or migraine without results from diagnostic tests such as nasal endoscopy, a CT scan, or atopy testing. Using symptoms alone, it is also difficult to differentiate important clinical subtypes of CRS such as CRS with nasal polyps (CRSwNP). Indeed, studies from tertiary care institutions report that only about half the patients with characteristic CRS symptoms have objective evidence of sinus inflammation (4, 5). Because diagnosis at the point of encounter has significant impact on treatment, the assumption of a CRS diagnosis on the basis of characteristic symptoms alone likely leads to excessive diagnosis and treatment.
Although directly attributable severe morbidity and mortality from CRS are infrequent, the symptoms and quality-of-life impairment attributed to CRS drive 11.1 million healthcare visits, 250,000 sinus surgeries (CRS being the most common indication), 7.1% of all adult outpatient antibiotic prescriptions, and a conservatively estimated $8.6 billion in direct healthcare costs; placing CRS among the top 10 most costly conditions to U.S. employers (6, 7). Because indirect costs such as workdays missed, increased antibiotic resistance, or antibiotic-related complications have not been fully characterized, the true costs associated with CRS may be significantly higher. Despite these high rates of healthcare use, the epidemiologic study of CRS is in its infancy. Comprehensive multi-institutional research networks have only just begun to apply consensus diagnostic criteria, establish clinical subphenotypes, and use validated disease outcome measures. Thus, there is a paucity of transformative longitudinal studies akin to the National Heart, Lung, and Blood Institute Severe Asthma Research Program or the U-BIOPRED (Unbiased BIOmarkers in PREDiction of respiratory disease outcomes) studies, which have added exciting new information on the phenotyping of asthma. At present, there remains poor information on the incidence, prevalence, natural history, and etiology of CRS and even fewer comprehensive biobanks upon which analysis of biomarkers of CRS severity can be performed. Currently, the World Health Organization has provided no plans for surveillance or prevention and control in its global action plan for chronic respiratory disease (8), and the National Institutes of Health has only recently provided funding for initial studies into the epidemiology of CRS.
In the United States, frequently cited National Health and Nutrition Examination Survey data estimated the prevalence of rhinosinusitis at between 13 and 17% of adults (9). In the National Health and Nutrition Examination Survey, respondents were asked whether they had received a diagnosis of “sinusitis” from a healthcare professional in the previous 12 months. However, the survey did not ascertain duration of symptoms, so ARS and CRS could not be separated; it depended on a healthcare provider diagnosis of sinusitis; not all patients may seek care; and it failed to ascertain and distinguish important variants such as CRSwNP. In 2011, the first population-based epidemiologic study was conducted in Europe using questions about CRS symptoms or prior physician diagnosis and estimated overall adult prevalence at 10.9% (10). In Korea, a population-based survey revealed a prevalence of only 1% in 1991, but this rose to 7% when results were reported again in 2011 (11, 12).
We recently analyzed electronic health records of the primary care patients of the Geisinger Health System and, relying on ICD-9 codes, estimated the incidence for this common condition at 1.1 cases per 100 person-years. Incidence among adults peaked between ages 45 and 54 years. There were sex differences, as more females had CRS without nasal polyps and more males had CRSwNP (13). Our study further demonstrated that CRS was strongly associated with premorbid episodic and chronic conditions of the upper and lower airway with particularly strong associations between CRS and asthma, especially CRSwNP. A number of airway conditions less frequently associated with CRS, including bronchitis, pneumonia, obstructive sleep apnea, and gastroesophageal reflux disease, were also significantly more prevalent in patients who developed CRS, lending strong support for the concept of the unified airway. There is a present need to systematically evaluate the natural history of CRS; the role of developmental, environmental, and occupational risk factors; the long-term effects of CRS on comorbid conditions of the airway; and whether CRS is associated with the development of postmorbid conditions outside the airway.
To date, an incomplete understanding of the etiology and pathogenesis of the disorder, the lack of accepted animal models arising from the uniquely complex paranasal sinus anatomy of humans compared with model organisms, and the paucity of large population-based studies have impeded progress in CRS research (14). Most data on patients with CRS, defined using consensus guidelines requiring evidence of sinus inflammation, are from highly selected patients in tertiary care. Studies of CRS in the general population have generally relied on ICD-9 codes, often from the primary care physician (13), self-reported symptoms without objective determination of sinus inflammation, or self-reported physician diagnosis of CRS. Important unresolved issues include whether etiology, pathophysiology, natural history, and need for treatment differ in the patients described in general population studies and tertiary care populations. Nonetheless, population-representative studies of accurately identified patients with CRS are critical to provide the observations of phenomena and disease history necessary for genetic, epigenetic, immunologic, microbiologic, and other mechanistic investigations.
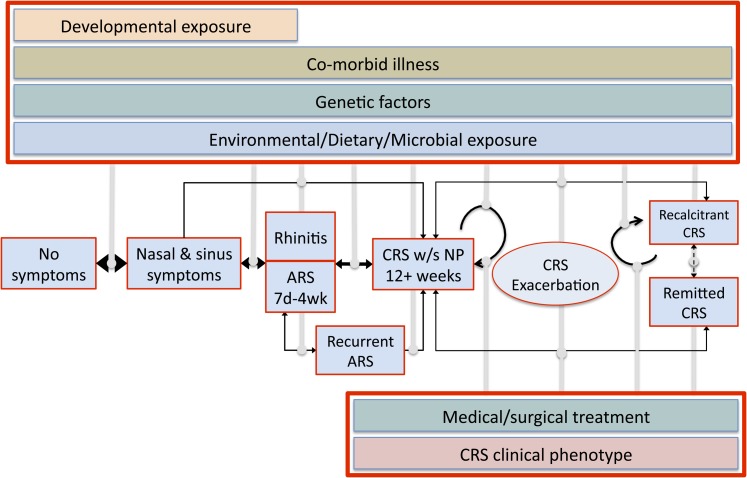
We can make several recommendations for studies that are needed to address the aforementioned limitations in CRS epidemiology. First, to facilitate studies in the general population, a validated questionnaire is needed that can identify patients with sinus inflammation as well as distinguish patients with and without nasal polyps. Second, studies should recruit patients from community and tertiary care populations and biobank samples upon which analyses for biomarkers of severity or phenotype can occur. Third, new natural history models must be investigated because evidence suggests that CRS prevalence plateaus with increasing age, implying that disease remission occurs in a substantial segment of the patient population. CRS should be reevaluated using a conceptual framework applied for the analysis of several other chronic episodic conditions, including asthma, migraine headache, and gastroesophageal reflux disease. In this framework (Figure 1), CRS is hypothesized to begin with a transition from ARS or rhinitis. With continuing insults, including environmental exposures, and influenced by genetic predispositions, disease can progress to a period of durable symptoms meeting the definition of CRS. Once CRS is established, transitions can occur, allowing for extended periods of remission without symptoms; relapse into the symptomatic state after exacerbations, during which healthcare use is likely to occur; and for some, a progressive recalcitrant course with persistent symptoms not durably altered by the effects of treatment. The incidence, prevalence, transition rates, and risk factors for each of the states in the framework are currently unknown, but we believe this model can account for and explain the clinical experience and natural history of the various clinical phenotypes of CRS (e.g., CRS without nasal polyps, CRSwNP, allergic fungal rhinosinusitis, and aspirin-exacerbated respiratory disease) in an epidemiologic framework. In evaluation of this model in the context of published literature, it is apparent that factors such as asthma likely increase rates at which ARS transitions toward CRS and treatment recalcitrance. This explains the high prevalence of individuals with asthma in studies of CRS in tertiary care. Similarly, patients with specific disease phenotypes, such as aspirin-exacerbated respiratory disease, are significantly more likely to remain recalcitrant, receiving repeated surgeries and courses of medication without disease remission. Further studies that carefully elucidate the drivers of these transitions between states are likely to provide novel and critical insights regarding potential mechanisms and thus more effective prevention and management of CRS.
Figure 1.
Application of a dynamic chronic episodic disease model to the natural history of chronic rhinosinusitis (CRS). Ordinary nasal and sinus symptoms progress through a transition through a state of rhinitis or acute rhinosinusitis (ARS) into CRS. In the model, CRS can be worsened by exacerbations of disease, durably remit at various points, or transition to recalcitrant CRS after exacerbations. Environmental, genetic, dietary, and developmental factors, the presence of comorbid diseases such as asthma, and specific microbial exposures, CRS phenotype, and surgical or medical management all likely affect transition rates to the various disease states.
In the changing environment of a healthcare system that places increasingly heavy emphasis on preventing costly escalations in care and demands metrics of efficacy to justify resource use, CRS appears to be an inviting disease for careful evaluation of current clinical practice and reconceptualization of our current disease paradigm. It is clear that critical new tools are needed to understand the natural history of the disease and identify factors leading to disease onset, symptomatic exacerbation, and remission in CRS. Epidemiologic studies are urgently needed to fill the immense gaps in knowledge and a fundamental reconsideration of CRS as a chronic episodic disease may provide a critical framework with which future studies can conceptualize this epidemic that silently claims a heavy individual and societal toll.
Footnotes
Supported by the Triological Society/American College of Surgeons and NIH grant K23DC012067 (B.K.T.); NIH grants R01 HL068546, R01 HL078860, and R01 AI072570 and the Ernest S. Bazley Trust (R.P.S.); and NIH grant P01 AI106683 (all authors).
Author Contributions: Article conception, drafting, editing, and approval: B.K.T., R.C.K., R.P.S., and B.S.S.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Bachert C, Baraniuk J, Baroody FM, Benninger MS, et al. American Academy of Allergy, Asthma and Immunology (AAAAI); American Academy of Otolaryngic Allergy (AAOA); American Academy of Otolaryngology--Head and Neck Surgery (AAO-HNS); American College of Allergy, Asthma and Immunology (ACAAI); American Rhinologic Society (ARS) Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(6 Suppl):155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfeld RM, Andes D, Bhattacharyya N, Cheung D, Eisenberg S, Ganiats TG, Gelzer A, Hamilos D, Haydon RC, III, Hudgins PA, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 Suppl):S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 4.Hsueh WD, Conley DB, Kim H, Shintani-Smith S, Chandra RK, Kern RC, Tan BK. Identifying clinical symptoms for improving the symptomatic diagnosis of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3:307–314. doi: 10.1002/alr.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson BJ, Narita M, Yu VL, Wagener MM, Gwaltney JM., Jr Prospective observational study of chronic rhinosinusitis: environmental triggers and antibiotic implications. Clin Infect Dis. 2012;54:62–68. doi: 10.1093/cid/cir747. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya N. Contemporary assessment of the disease burden of sinusitis. Am J Rhinol Allergy. 2009;23:392–395. doi: 10.2500/ajra.2009.23.3355. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: demographics and perioperative outcomes. Laryngoscope. 2010;120:635–638. doi: 10.1002/lary.20777. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Khaltaev N World Health Organization. Geneva: World Health Organization; 2007. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. [Google Scholar]
- 9.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat 10. 2009;(242):1–157. [PubMed] [Google Scholar]
- 10.Jarvis D, Newson R, Lotvall J, Hastan D, Tomassen P, Keil T, Gjomarkaj M, Forsberg B, Gunnbjornsdottir M, Minov J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67:91–98. doi: 10.1111/j.1398-9995.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011;25:117–121. doi: 10.2500/ajra.2011.25.3630. [DOI] [PubMed] [Google Scholar]
- 12.Min YG, Jung HW, Kim HS, Park SK, Yoo KY. Prevalence and risk factors of chronic sinusitis in Korea: results of a nationwide survey. Eur Arch Otorhinolaryngol. 1996;253:435–439. doi: 10.1007/BF00168498. [DOI] [PubMed] [Google Scholar]
- 13.Tan BK, Chandra RK, Pollak J, Kato A, Conley DB, Peters AT, Grammer LC, Avila PC, Kern RC, Stewart WF, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–1360. doi: 10.1016/j.jaci.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, Schleimer RP, Ledford D. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–1490. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]