Abstract
BACKGROUND
It is often a clinical dilemma to determine when to collect autologous peripheral blood progenitor cells (PBPCs) in patients who received prior chemotherapy. It is also challenging to predict if the collected cells will be enough for one or two transplants.
STUDY DESIGN AND METHODS
A total of 103 PBPC donors were followed to evaluate factors that predict poor autologous PBPC collection. The donors were categorized into three groups: plasma cell disorders (PCDs), lymphomas, and normal allogeneic donors.
RESULTS
Our evaluation showed that platelet (PLT) count before growth factor administration significantly correlated with total CD34+ cell yield (Spearman r = 0.38, p < 0.001). Further analysis showed this correlation was only significant in plasma cell disease patients who received prior chemotherapy (Spearman r = 0.5, p = 0.008). Baseline PLT counts did not correlate with PBPC collection yield in untreated PCD, lymphoma, and normal allogeneic donors. In addition, daily PLT count during PBPC harvest correlated with CD34+ cell yield for that day (Spearman r = 0.41, p < 0.001). With a multiple linear regression model (adjusted R2 = 0.31, AIC = 63.1), it has been determined that the baseline PLT count significantly correlates with total CD34+ cell yield in treated PCD patients.
CONCLUSION
Baseline PLT count is a sensitive indicator of autologous PBPC mobilization in PCD patients who received prior chemotherapy. This finding may be considered before growth factor administration to determine the optimal period to mobilize treated PCD patients and to predict if enough cells can be collected for one or two transplants.
Leukapheresis collection of peripheral blood progenitor cells (PBPCs) after granulocyte–colony-stimulating factor (G-CSF; filgrastim) administration has become the preferred method of collecting CD34+ cells for patients with hematologic malignancies receiving high-dose chemotherapy and autologous hematopoietic stem cell transplant (AHSCT). There is no general consensus about adequate number of CD34+ PBPC cell dose needed for successful engraftment after a transplant. In general, 5 million CD34+ cells per kg recipient body weight is considered an adequate cell dose and 2 million CD34+ cell per kg is considered as the minimum acceptable cell dose for an AHSCT.1 The required number of CD34+ stem cells needed for a successful allogeneic stem cell transplant is less well defined.2 In the past 5 years, a handful of studies have reported that infusing higher numbers of allogeneic CD34+ cell per kg is associated with a higher incidence of chronic graft-versus-host disease and higher transplant related mortality.3,4
G-CSF is the most common growth factor used to mobilize patients for PBPC collection.5 When a patient fails to mobilize adequate number of CD34+ cells after G-CSF administration, a combination of two growth factors, usually G-CSF and granulocyte-monocyte–colony-stimulating factor (GM-CSF; sargramostim) or G-CSF and a chemotherapeutic agent, most commonly cyclophosphamide are frequently used. Peripheral CD34+ cell count is performed before collection is begun by apheresis. Most transplant centers in the United States use peripheral CD34+ cell count of 10 per μL as the cutoff to determine when to start collection. Approximately 20 to 30 percent of autologous donors and 10 percent of allogeneic donors fail to mobilize an adequate number of PBPCs for collection. Only about one in four poor mobilizers reaches target CD34+ cell dose despite multiple attempts of remobilization and marrow harvest.6–8
Previous studies have identified several factors that correlate with poor mobilization of PBPCs after G-CSF stimulation. These factors include the effects of prior chemotherapy as well as suppressive effects of the malignant cells on normal hematopoietic progenitors.5 Additional studies have documented the effects of prior chemo-therapy on the ability to harvest sufficient numbers of marrow stem cells or to mobilize CD34+ stem cells for collection by apheresis9,10 Other factors that contribute to poor mobilization include patient age,11 patient diagnosis,12 circulating immature cells,13 immature myeloid cells,14 and white blood cell and mononuclear cell (MNC) counts.15 There is no single established clinical or laboratory test, however, that reliably correlates with marrow reserve and PBPC mobilization.
Several studies have shown a significant correlation between the postmobilization, preapheresis peripheral blood CD34+ cell count (pCD34) with PBPC mobilization and yield.15–17 Predicting the ultimate CD34+ cell yield before mobilization treatment would be of great benefit. Potential risks and complications after mobilization treatment, including the risks associated with central line placement and treatment with high-dose G-CSF, will be avoided.
Previous studies have demonstrated that stem cell–megakaryocyte–platelet (PLT) lineage is particularly sensitive to damage of marrow microenvironment.18 It was shown that decrease in stem cell numbers after chemo-and radiotherapy exposures directly affect PLT count. In addition, decrease in maturation from altered marrow environment, accessory cells, and growth factor levels affect megakaryocyte maturation, PLT release, and their migration into circulation.19,20
Peripheral CD34+ cell count is only useful in predicting adequate mobilization after growth factor administration. By that time, patients are already exposed to the risks and side effects of the growth factor and clinicians frequently feel compelled to collect despite the low peripheral CD34+ cell count. We therefore attempted to identify other factors that could be used clinically to predict mobilization before growth factor administration.
We performed this study to assess if premobilization PLT count is a sensitive predictor of adequate CD34+ cell yield in AHSCT candidates. In addition we determined if PLT count on the day of leukapheresis collection is predictive of CD34+ cell yield for the day. We envision that PLT count would serve as a surrogate marker for hematopoietic stem cell mobilization and would correlate with leukapheresis yield. PLT count, unlike peripheral CD34+ count, is simple to perform, inexpensive, and readily available. We have further assessed the confounding factors that could influence the relationship between the PLT count and the CD34+ cell yield. We developed models that predict adequate CD34+ cell collection. We propose that PLT count in addition to patient clinical profile could be used to identify AHSCT candidates at risk for poor mobilization before initiation of mobilization therapy.
MATERIALS AND METHODS
Patient and graft characteristics
We have followed 103 autologous PBPC transplant candidates evaluated at the Mayo Clinic Jacksonville from 2004 to 2006. Patients were followed from the time of initial evaluation to transplant. Factors that may correlate with mobilization potential and poor autologous PBPC collection were evaluated. This is a prospective-retrospective study as it was part of a larger National Institutes of Health funded study entitled “Characterization of Hematopoietic Stem Cells.” Since the main predictor, baseline PLT count, was obtained retrospectively, however, we defined the study design as retrospective. Mayo Clinic Institutional Review Board approval (IRB No. 0242) was obtained before the study was conducted. The median patient age was 51 years (range, 22–76 years). Thirty-eight of the 103 patients were diagnosed with plasma cell disorders (PCDs), primarily multiple myeloma, and 26 patients had lymphoma, while the remaining 39 were normal allogeneic donors (Table 1). The median number of PBPC collections was 3 (range, 1–7).
TABLE 1.
Donor characteristics
| Disease category | PCDs | Lymphoma | Normal | Total |
|---|---|---|---|---|
| Donor | 0 | 0 | 39 | 39 |
| Treated | 27 | 23 | 0 | 50 |
| Untreated | 11 | 3 | 0 | 14 |
| Total | 38 | 26 | 39 | 103 |
PBSC collection procedure
Patients were first mobilized with 10 μg per kg G-CSF for approximately 4 to 5 days. On the day their peripheral CD34+ cell count reached 10 per μL or higher, collection was initiated. Leukapheresis procedures were performed with an apheresis system (COBE Spectra, Gambro BCT, Lakewood, CO). The machine was run with operating software version 5.1. The venous access for all autologous donors was via a trilumen central venous catheter and all allogeneic donors were collected via bilateral puncture of the antecubital or forearm vein. All donors were subjected to 4-blood-volume collection which took approximately 4 to 5 hours to complete. The same operator and machine were used for all collections from each donor. Each donor, however, is randomly assigned to an operator and a machine. All operators were competent and passed their annual competency evaluations. All apheresis machines were validated and received routine biannual preventive maintenance.
Adequate mobilization is defined as the ability of a donor to reach peripheral CD34+ cell count of 10 per μL and/or have a total of 2 million or more CD34+ cells per kg collection yield. In general, the target total CD34+ cells per kg was 5 × 106 for one transplant and 8 × 106 for two transplants. Those who failed to mobilize the first time were mobilized with a combination of G-CSF and either GM-CSF or cyclophosphamide. Occasionally, PBPC products were collected from poor mobilizers with peripheral CD34+ cell counts less than 10 per μL.
PLT counts
PLT counts were part of complete blood count (CBC). CBC was performed with a cell and particle counter (Coulter Counter, Beckman Coulter, Fullerton, CA). Baseline PLT count was the latest count before growth factor administration. PLT count on the day of collection was measured before each collection. Therefore, PLT count on the first day of collection was not affected by the collection procedure. Subsequent daily PLT counts might be affected by the previous day collection procedure but so also the CD34+ cell yield. None of the patients received PLT transfusion during collection.
CD34+ cell enumeration
The stem cell product was assayed for the total number of MNCs and CD34+ cells. Total CD34+ cell yield is defined as cumulative total number of CD34+ cells from all collections. MNCs were stained with anti-CD45-FITC (Phar-Mingen, San Diego, CA) and anti-CD34-PE (Becton Dickinson Immunocytometry Systems, San Jose, CA) and analyzed with a flow cytometer (FACSCalibur, BD Biosciences, Waltham, MA). The number of CD34+ cells for autologous marrow products was determined before cryopreservation. The data analysis was performed with computer software (CellQuest, BD Biosciences) after acquisition layouts (Procount, BD Biosciences). The percentage of CD34+ cells in each sample was determined and the total number of CD34+ cells infused was calculated. The median peripheral CD34+ count on the first day of collection was 16.9 per μL (range, 1.9–135.9 μL). The median CD34+ cell per kg count on the first day of collection was 1.78 × 106 per kg (range, 0.06–14.53/kg) and the median total CD34+ cell yield per patient was 5.78 × 106 per kg (range, 0.06–14.53/kg; Table 2).
TABLE 2.
Donor collection profile
| Variable | Median | Minimum | Maximum |
|---|---|---|---|
| Age (years) | 51 | 22 | 76 |
| Weight (kg) | 78.3 | 49 | 143 |
| Number of collections | 3 | 1 | 7 |
| Baseline PLT count (×109/L) | 253 | 6 | 593 |
| Peripheral blood CD34 count (/μL) | 16.9 | 1.9 | 135.9 |
| Day 1 CD34+ cell count (×106/kg) | 1.78 | 0.06 | 14.53 |
| Total CD34+ cell yield (×106/kg) | 5.98 | 0.06 | 14.53 |
Statistical analysis
The primary endpoint was CD34+ cells per kg yield and the primary predictor was PLT count at baseline. We used regression models to ascertain if the PLT count predicted CD34+ cell yield, unadjusted and adjusted for other factors. Factors included in the regression analyses were patient weight, previous treatment status, and disease. Our objective was to determine if baseline PLT count can be used to predict total CD34+ cell yield.
Associations between continuous and categorical variables were assessed by the two-sample t test or the Wilcoxon rank-sum test. The linear correlation between two continuous factors was assessed by Spearman's rank correlation. Receiver operating curve (ROC) was used to evaluate the best cutoff for baseline PLT count that predict collection of 5 × 106 CD34+ cells per kg from treated PCD patients. No statistical adjustment was made for performing multiple tests. All probability values are two-sided.
RESULTS
Baseline PLT count before growth factor mobilization correlates with total CD34+ cell yield
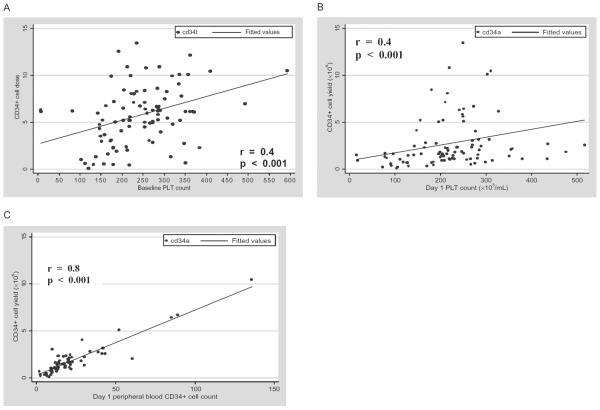
The median baseline PLT count (PLT count before growth factor administration began) was 253 × 109 per L (range 6 × 109–593 × 109/L). Our evaluation showed baseline PLT count before growth factor administration significantly correlated with total CD34+ cell yield (Spearman r = 0.38, p = 0.001; Fig. 1A). In addition, baseline PLT count significantly correlated with peripheral blood CD34+ cell count (Spearman r = 0.36, p = 0.002).
Fig. 1.
(A) Correlation of baseline PLT count and total CD34+ cell yield for all donors. (B) Correlation of first day PLT count and first day CD34+ cell harvest for all donors. (C) Correlation of peripheral blood CD34+ cell count and first day CD34+ cell harvest for all donors.
PLT count on day of harvest correlates with CD34+ cell yield
We examined whether PLT count on the first day of harvest correlated with CD34+ cell yield for that day. Figure 1B shows a significant linear correlation between PLT count on the first day of collection and the CD34+ cell yield for that day (r = 0.4, p < 0.001). Similarly, a significant relationship was observed when the peripheral CD34+ cell count on first day of collection was compared with CD34+ cell yield for the day (r = 0.78, p < 0.001; Fig. 1C). In addition, daily PLT count during PBPC harvest correlated with CD34+ cell yield for the day (data not shown).
Influence of disease and prior treatment on PBPC mobilization and CD34+ cell yield
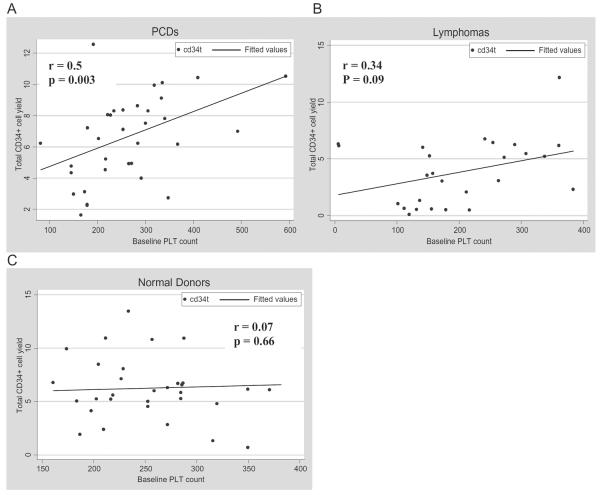
We also examined the influence of disease characteristics on PBPC mobilization and collection. Donors were stratified based on their diseases: lymphoma and PCD with normal allogeneic donors as control group. The median baseline PLT count was 253 × 109, 176 × 109, and 257 × 109 per L for PCD, lymphoma, and normal donors, respectively. The median total CD34+ cell yields per kilogram of body weight were 7.04 × 106, 3.6 × 106, and 5.98 × 106 for PCD, lymphoma, and normal allogeneic donors, respectively. The median number of collections was 4, 3, and 2 for PCD, lymphoma, and normal donors, respectively. Only baseline PLT counts from donors with PCD have significant correlation with total CD34+ cell yield (Spearman r = 0.46, p = 0.003; Fig. 2A). Patients with lymphoma and normal donors showed no significant correlation between baseline PLT count and CD34+ cell yield (Figs. 2B and 2C).
Fig. 2.
Correlation of baseline PLT count and total CD34+ cell yield.
Forty-nine percent of our donor cohort received prior therapies. Among the treated donors, 54 percent were PCD donors and 46 percent were lymphoma donors. Among the donors without history of prior therapies, 74 percent were normal allogeneic donors, 6 percent lymphoma patients, and 20 percent PCD patients. When the donors were stratified according to their disease and prior therapies, only treated PCD patients showed significant correlation between baseline PLT counts and total CD34+ cell yield (Spearman r = 0.5, p = 0.008). PCD donors who received prior therapies significantly yield higher total CD34+ cells than previously treated lymphoma donors (p < 0.001). There was no significant difference, however, in total CD34+ cell yield between the untreated PCD and untreated lymphoma donors (Fig. 3A). Similarly, median baseline PLT count for previously treated PCD donors was significantly higher than median PLT count for previously treated lymphoma donors (p = 0.05; Fig. 3B).
Fig. 3.
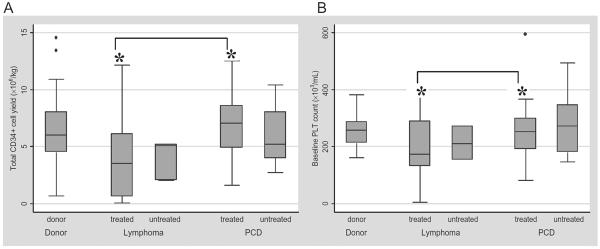
(A) Distribution of total CD34+ cell yield by disease and prior treatment status. *Significant difference between the treated PCD and treated lymphoma groups (p < 0.001). (B) Distribution of baseline PLT count by disease and prior treatment status. *Significant difference between the treated PCD and treated lymphoma groups (p = 0.05).
Multiple regression models for prediction of PBPC mobilization and total CD34+ collection for PCD patients
Univariate and multivariate linear regression was used to predict the total CD34+ cell yield for PCD patients. Four potential predicting covariates were considered: baseline PLT count, donor weight, peripheral CD34+ cell count, and the treatment status (treated, 1; untreated, 0). In univariate analysis, baseline PLT count (p = 0.006), donor weight (p = 0.021), and peripheral CD34+ cell count (p = 0.023) were identified as the significant predicting factors for the total CD34+ cell yield. In a multivariate analysis, baseline PLT count (p = 0.034), donor weight (p = 0.024), and peripheral CD34+ cell count (p = 0.038) remain significant. The regression model with best goodness of fit is the model that includes baseline PLT count, peripheral CD34+ cell count, and patient weight in kilograms (total CD34+ cell × 106/kg = 0.01 × baseline PLT count [in 103] + 0.03 × peripheral CD34+ cell count – 0.06 × weight [kg]; adjusted R2 = 0.31, AIC = 63.1).
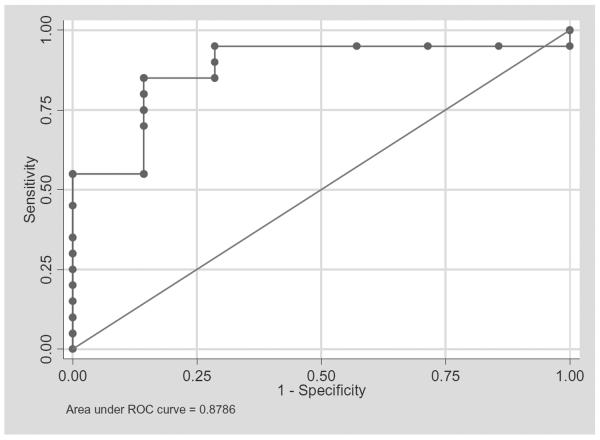
ROC analysis to determine the best baseline PLT count cutoff that predicts 5 × 106 CD34+ cells per kg yield from treated PCD patients
Total CD34+ cell yields from treated PCD patients were categorized into less than 5 and at least 5. We then performed ROC analysis to determine the baseline PLT count cutoff that has the best sensitivity and specificity to predict 5 × 106 CD34+ cell yield from these patients. We have determined PLT count of 192 × 109 per L will predict at least 5 × 106 CD34+ cell yield with 95 percent sensitivity and 71 percent specificity and estimated correct classification of 88 percent. Increasing the cutoff to 220 × 109 per L will decrease the sensitivity to 85 percent but increase the specificity to 86 percent The ROC analysis result was summarized in Table 3 and Fig. 4.
TABLE 3.
ROC analysis for determining baseline PLT count with best sensitivity and specificity to predict 5 × 106 CD34+ cells per kg yield from treated PCD patients
| Baseline PLT count cut point | Sensitivity (%) | Specificity (%) | Correctly classified (%) |
|---|---|---|---|
| ≥151 | 95 | 14 | 74 |
| ≥166 | 95 | 28 | 77 |
| ≥179 | 95 | 42 | 81 |
| ≥192 | 95 | 71 | 89 |
| ≥203 | 90 | 71 | 85 |
| ≥220 | 85 | 86 | 85 |
| ≥235 | 75 | 86 | 78 |
| ≥266 | 55 | 86 | 63 |
Fig. 4.
ROC analysis of baseline PLT count as a predictor of at least 5 × 106 CD34+ cells per kg yield from treated PCD patients. The best cutoff PLT count was 192 × 109 per L (sensitivity, 95%; specificity, 71%; correct classification, 89%).
DISCUSSION
Our analysis shows PLT count is a significant predictor of mobilization potential. In addition, PLT count correlates with daily yield of CD34+ cell yield. This correlation was observed in treated PCD patients, because when the data set was stratified by donor disease and prior treatment only treated PCD patients showed significant correlation. Therefore, treated PCD patients were influencing the results of our initial analysis (presented in Fig. 1) in such a way to make the correlation of PLT count and CD34+ count significant for all donors. This suggests that the patient's disease and treatment status significantly influences the correlation between PLT count and CD34+ cell collection yield. The importance of a subtle interaction between the underlying disease, in this case PCD, and prior treatment was suggested by our observation. Patients with non-Hodgkin's lymphoma tend to be heavily treated,7 whereas patients with multiple myeloma commonly undergo PBSCT without prior exposure to alkylating agent chemotherapy. Exposure to alkylating agents may influence CD34+ cell yield; nevertheless, in our study this did not appear to impact the relation between PLT count and CD34+ cell yield since we did not observe a significant relationship between baseline PLT and CD34+ cell yield in treated lymphoma donors who commonly receive prior alkylating agents. None of the treated PCD patients in our study received prior melphalan treatment, a common alkylating agent for treatment of multiple myeloma that is known to be myelosuppressive. Therefore, it is the combination of biologic nature of the disease and prior treatments that influences the quantity and quality of marrow reserve and mobilization potential.
PLT count is an important factor to consider among many other factors that influence successful mobilization and collection of treated PCD patients. We provided a multivariate regression model that could be used to predict total CD34+ cell yield. Our institution considers all the significant factors when determining who to mobilize. To further enhance the utility of our study for those who prefer a simple algorithm based on baseline PLT count, we performed ROC analysis to examine the sensitivity and specificity of potential baseline PLT count cutoffs that has the highest correct classification. We determined that PLT count of at least 192 × 109 per L was likely to result in at least 5 × 106 per kg CD34+ cell yield in treated PCD patients. We point out that prognostic models, like our PLT cut point under ROC, however, are very data dependent; that is, what we obtained here is based on our data only, which may not be applicable to other institutions. Further prospective confirmation is needed to validate our data.
We did not consider number of collections as a good correlate measure because some patients were mobilized and collected with intention to collect cells enough for two transplants. Naturally, those that would be having two transplants would have higher target cell numbers and hence would have more collections than those who would be receiving a single transplant.
Multiple direct and indirect factors interact together to maintain the quality and quantity of marrow stem cells in their niche. Among the direct factors are the accessory cells that constitute the marrow microenvironment. The marrow microenvironment also contains many extracellular membrane proteins such as fibronectin, collagen, laminin, and osteopontin. The physical interaction of multiple myeloma cells with these proteins and the accessory cells has a significant role in the disease pathogenesis.21 The association of high levels of interleukin-6, a potential mobilizing agent, with most plasma cell diseases22 may contribute to the increased CD34+ cell mobilization observed in our PCD patients.
Seventy-one percent of the PCD patients studied have their PBPC collected after they received some type of therapies for their disease. The majority of those who received prior therapies were treated with dexamethasone alone or in combination with other agents such as prednisone, thalidomide, lenalidomide, vincristine, and doxorubicin. We suspect that dexamethasone, the most common steroid treatment for PCD, may be responsible for the observed correlation between PLT count and PBPC yield. This is unsubstantiated, however, and is currently part of an ongoing study in our lab. More studies will be needed to validate this perception.
PLT count is a simple inexpensive test that is routinely performed as part of CBC during patient evaluation. It therefore does not increase cost of patient care. Unlike peripheral CD34+ cell count, it does not require special flow cytometry equipment and technicians. Very recently, Fu and colleagues23 reported a retrospective study that shows premobilization therapy blood CD34+ cell count predicts the likelihood of successful HSC mobilization. The study proposed a complicated algorithm for predicting postmobilization CD34+ cell yield. The overall correlation coefficient between the premobilization CD34+ cell count and CD34+ cell yield was 0.37, which is comparable with the correlation coefficient between baseline PLT count and total CD34+ cell yield.
PLT production is closely associated with overall hematopoiesis. Thrombopoietin, the only growth factor known to stimulate thrombopoiesis, is also a powerful inducer of hematopoiesis.24 Thrombopoietin has been used successfully to mobilize PBPCs for leukapheresis collection.25 In addition, CD34+ cell dose correlates with time to PLT engraftment after transplant and time to PLT engraftment was shown to be the best indicator of short-term engraftment.5 A study was conducted in 2005 by Lysak and colleagues11 from Czechoslovakia to examine the factors affecting PBSC mobilization and collection in healthy donors. The study seemed to suggest in a univariate regression model prestimulation PLT counts correlated with CD34+ cell yield in healthy donors. The R2 for the model was only 14 percent. This observation was not true in a multivariate model, however. It was unclear from the report when the prestimulation PLT counts were performed and whether the PLT counts in the paper were equivalent to our baseline PLT counts, which were obtained before growth factor stimulation. Therefore, it is challenging to make a comparison between the two studies. If the conclusion from the study was prestimulation PLT counts in healthy donors have no significant correlation with total CD34+ cell yield, then our findings agree with their report as we did not observe significant correlation in normal donors. The mechanism of how PLT counts predict marrow reserve and mobilization potential warrant further studies to assess the molecular basis of marrow damage that impedes PLT production and survival.
We believe that taking PLT count into consideration when evaluating treated PCD patients for bone marrow transplantation will have a significant clinical impact by minimizing patient exposure to unnecessary and potentially risky mobilization therapies. This will improve quality of patient care and potentially save money.
Acknowledgments
Supported by NIH Grant CA102824.
ABBREVIATIONS
- AHSCT
autologous hematopoietic stem cell transplant
- CBC
complete blood count
- PCD(s)
plasma cell disorder(s)
- ROC
receiver operating curve
REFERENCES
- 1.Gianni AM. Where do we stand with respect to the use of peripheral blood progenitor cells? Ann Oncol. 1994;5:781–4. doi: 10.1093/oxfordjournals.annonc.a059003. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Bacigalupo A, Hasenclever D, Nagler A, Gluckman E, Clark P, Bourquelot P, Greinix H, Frickhofen N, Ringdén O, Zander A, Apperley JF, Gorin C, Borkett K, Schwab G, Goebel M, Russell NH, Gratwohl A. Allogeneic bone marrow transplantation vs. filgrastim-mobilised peripheral blood progenitor cell transplantation in patients with early leukaemia: first results of a randomised multi-centre trial of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1998;21:995–1003. doi: 10.1038/sj.bmt.1701234. [DOI] [PubMed] [Google Scholar]
- 3.Zaucha JM, Gooley T, Bensinger WI, Heimfeld S, Chauncey TR, Zaucha R, Martin PJ, Flowers ME, Storek J, Georges G, Storb R, Torok-Storb B. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood. 2001;98:3221–7. doi: 10.1182/blood.v98.12.3221. [DOI] [PubMed] [Google Scholar]
- 4.Urbano-Ispizua A, Carreras E, Marin P, Rovira M, Martinez C, Fernandez-Aviles F, Xicoy B, Hernandez-Boluda J-C, Montserrat E. Allogeneic transplantation of CD34(+) selected cells from peripheral blood from human leukocyte antigen-identical siblings: detrimental effect of a high number of donor CD34(+) cells? Blood. 2001;98:2352–7. doi: 10.1182/blood.v98.8.2352. [DOI] [PubMed] [Google Scholar]
- 5.To L, Haylock D, Simmons P, Juttner C. The biology and clinical uses of blood stem cells. Blood. 1997;89:2233–58. [PubMed] [Google Scholar]
- 6.Watts MJ, Ings SJ, Flynn M, Dodds D, Goldstone AH, Linch DC. Remobilization of patients who fail to achieve minimal progenitor thresholds at the first attempt is clinically worthwhile. Br J Haematol. 2000;111:287–91. doi: 10.1046/j.1365-2141.2000.02346.x. [DOI] [PubMed] [Google Scholar]
- 7.Sugrue MW, Williams K, Pollock BH, Khan S, Peracha S, Wingard JR, Moreb JS. Characterization and outcome of “hard to mobilize” lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma. 2000;39:509–19. doi: 10.3109/10428190009113381. [DOI] [PubMed] [Google Scholar]
- 8.Stockerl-Goldstein KE, Reddy SA, Horning SF, Blume KG, Chao NF, Hu WW, Johnston LF, Long GD, Strober S, Wong RM, Feiner RH, Kobler S, Negrin RS. Favorable treatment outcome in non-Hodgkin's lymphoma patients with “poor” mobilization of peripheral blood progenitor cells. Biol Blood Marrow Transplant. 2000;6:506–12. doi: 10.1016/s1083-8791(00)70021-8. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan A, Bhatia S, Slovak ML, Arber DA, Niland JC, Nademanee A, Fung H, Bhatia R, Kashyap A, Molina A, O'Donnell MR, Parker PA, Sniecinski I, Snyder DS, Spielberger R, Stein A, Forman SJ. Predictors of therapy-related leukemia and myelodysplasia following autologous trans plantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588–93. [PubMed] [Google Scholar]
- 10.Vantelon JM, Koscielny S, Brault P, Bourhis JH, Ribrag V, Pico J, Fenaux P, Munck JN. Scoring system for the prediction of successful peripheral blood stem cell (PBSC) collection in non-Hodgkin's lymphoma (NHL): application in clinical practice. Bone Marrow Transplant. 2000;25:495–9. doi: 10.1038/sj.bmt.1702201. [DOI] [PubMed] [Google Scholar]
- 11.Lysak D, Koza V, Jindra P. Factors affecting PBSC mobilization and collection in healthy donors. Transfus Apher Sci. 2005;33:275–83. doi: 10.1016/j.transci.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Koenigsmann M, Jentsch-Ullrich K, Mohren M, Becker E, Heim M, Franke A. The role of diagnosis in patients failing peripheral blood progenitor cell mobilization. Transfusion. 2004;44:777–84. doi: 10.1111/j.0041-1132.2004.03321.x. [DOI] [PubMed] [Google Scholar]
- 13.Kozuka T, Ikeda K, Teshima T, Kojima K, Matsuo K, Bessho A, Sunami K, Hiramatsu Y, Maeda Y, Noguchi T, Yamamoto K, Fujii N, Imai T, Takenaka K, Shinagawa K, Ishimaru F, Niiya K, Koide N, Tanimoto M, Harada M. Predictive value of circulating immature cell counts in peripheral blood for timing of peripheral blood progenitor cell collection after G-CSF plus chemotherapy-induced mobilization. Transfusion. 2002;42:1514–22. doi: 10.1046/j.1537-2995.2002.00218.x. [DOI] [PubMed] [Google Scholar]
- 14.Park KU, Kim SH, Suh C, Kim S, Lee SJ, Park JS, Cho HJ, Kim KW, Lee K, Kim HJ, Park J, Joo Min Y, Kim JG, Kim T, Lee JH, Kim SB, Kim SW, Lee KH, Lee JS, Kim WK, Park CJ, Chi HS. Correlation of hematopoietic progenitor cell count determined by the SE-automated hematology analyzer with CD34(+) cell count by flow cytometry in leukapheresis products. Am J Hematol. 2001;67:42–7. doi: 10.1002/ajh.1074. [DOI] [PubMed] [Google Scholar]
- 15.Moncada V, Bolan C, Yau YY, Leitman SF. Analysis of PBPC cell yields during large-volume leukapheresis of subjects with a poor mobilization response to filgrastim. Transfusion. 2003;43:495–501. doi: 10.1046/j.1537-2995.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 16.Elliott C, Samson DM, Armitage S, Lyttelton MP, McGuigan D, Hargreaves R, Giles C, Abrahamson G, Abboudi Z, Brennan M, Kanfer EJ. When to harvest peripheral-blood stem cells after mobilization therapy: prediction of CD34-positive cell yield by preceding day CD34-positive concentration in peripheral blood. J Clin Oncol. 1996;14:970–3. doi: 10.1200/JCO.1996.14.3.970. [DOI] [PubMed] [Google Scholar]
- 17.Lane TA, Bashey A, Carrier E, Holman P, Castro J, Mullen M, Ward DM, Ada O, Ball ED. Improving the efficiency of PBPC collection by pre-apheresis peripheral blood and mid-apheresis product measurements of CD34 cells. Cytotherapy. 2004;6:318–27. doi: 10.1080/14653240410004880. [DOI] [PubMed] [Google Scholar]
- 18.Messner HA. Hemopoietic precursors in human bone marrow transplantation. Int J Cell Cloning. 1986;4(Suppl 1):11–8. doi: 10.1002/stem.5530040706. [DOI] [PubMed] [Google Scholar]
- 19.Warren MK, Zujewski J, Rose WL, Szabo JM, O'Shaughnessy JA, Halverson DC, Cowan KH, Gress RE, Schwartz GN. Early suppressive effects of chemotherapy on recovery of bone marrow megakaryocyte precursors: possible relationship to platelet recovery. Stem Cells. 1996;14(Suppl 1):31–7. doi: 10.1002/stem.5530140704. [DOI] [PubMed] [Google Scholar]
- 20.Tijssen MR, van der Schoot CE, Voermans C, Zwaginga JJ. The (patho) physiology of megakaryocytopoiesis: from thrombopoietin in diagnostics and therapy to ex vivo generated cellular products. Vox Sang. 2004;87(Suppl 2):52–5. doi: 10.1111/j.1741-6892.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 21.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–98. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 22.Mitsiades CS, Mitsiades NS, Munshi NC, Richardson PG, Anderson KC. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: interplay of growth factors, their receptors and stromal interactions. Eur J Cancer. 2006;42:1564–73. doi: 10.1016/j.ejca.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Fu P, Bagai RK, Meyerson H, Kane D, Fox RM, Creger RJ, Cooper BW, Gerson SL, Laughlin MJ, Koc ON, Lazarus HM. Pre-mobilization therapy blood CD34+ cell count predicts the likelihood of successful hematopoietic stem cell mobilization. Bone Marrow Transplant. 2006;38:189–96. doi: 10.1038/sj.bmt.1705431. [DOI] [PubMed] [Google Scholar]
- 24.Young JC, Bruno E, Luens KM, Wu S, Backer M, Murray LJ. Thrombopoietin stimulates megakaryocytopoiesis, myelopoiesis, and expansion of CD34+ progenitor cells from single CD34+Thy-1+Lin- primitive progenitor cells. Blood. 1996;88:1619–31. [PubMed] [Google Scholar]
- 25.Somlo G, Sniecinski I, ter Veer A, Longmate J, Knutson G, Vuk-Pavlovic S, Bhatia R, Chow W, Leong L, Morgan R, Margolin K, Raschko J, Shibata S, Tetef M, Yen Y, Forman S, Jones D, Ashby M, Fyfe G, Hellmann S, Doroshow JH. Recombinant human thrombopoietin in combination with granulocyte colony-stimulating factor enhances mobilization of peripheral blood progenitor cells, increases peripheral blood platelet concentration, and accelerates hematopoietic recovery following high-dose chemotherapy. Blood. 1999;93:2798–806. [PubMed] [Google Scholar]