Abstract
Emerging clinical evidence now suggests dyslipidemia may be strongly linked with the development and progression of neuropathy in diabetic patients, and dyslipidemia is considered an important risk factor for the development of diabetic neuropathy. However, because of important species differences, current animal models fall short of accurately replicating human diabetic dyslipidemia. Rodents resist expansion in low-density lipoprotein cholesterol (LDL-C) and typically maintain or increase high-density lipoprotein cholesterol (HDL-C), despite prolonged high-fat feeding. Here, we discuss the findings of Hinder et al., in which they utilized novel genetic experimental approaches to develop a diabetic mouse model with human-like dyslipidemia. The authors created a mouse with an apolipoprotein E (ApoE) knockout in conjunction with a leptin receptor mutation. A triple mutant mouse with both ApoE and apolipoprotein B48 knockout and leptin deficiency was also created in an effort to generate a model of diabetic dyslipidemia that better mimics the human condition. The long-term goal of these studies is to develop more faithful models to address how hyperglycemia and hyperlipidemia may drive the development and progression of neuropathy. Hinder and colleagues were successful at creating a diabetic mouse model with severe hypertriglyceridemia, hypercholesterolemia, and a significant increase in the total cholesterol to HDL-C ratio. This work was successful in establishing a model of diabetic dyslipidemia that more closely emulates the poor lipid profile observed in human diabetic patients with neuropathy. This commentary will also review current models used to study the effects of dyslipidemia on diabetic neuropathy and highlight a proposed mechanism for the role of dyslipidemia in the pathogenesis of diabetic neuropathy.
Dyslipidemia is An Independent Risk Factor for Diabetic Neuropathy
The majority of diabetic patients will develop diabetic neuropathy, which is the most common and debilitating complication of diabetes (Rutkove, 2009; Vincent et al., 2009b; Zochodne, 2008). Hyperglycemia plays a key role in the development and progression of diabetic neuropathy (Edwards et al., 2008; Feldman, 2008; Figueroa-Romero et al., 2008; Sinnreich et al., 2005; Sumner et al., 2003), and a combination of multiple etiologies, each stemming from the initial insult of hyperglycemia, are likely responsible for the dying-back type axonal degeneration that underlies neuropathic symptoms (Edwards et al., 2008; Feldman, 2008; Figueroa-Romero et al., 2008). In light of long withstanding evidence that hyperglycemia is the leading cause of diabetic neuropathy (1988; 1993; 1999; Feldman et al., 1997; Franklin et al., 1990; Greene et al., 1999), evidence from several large clinical studies indicate metabolic derangements such as a poor lipid profile are linked with neuropathy development and progression, independent of glycemic control (Leiter, 2005; Lyons et al., 2004; Tesfaye, 2007; Tesfaye et al., 2005; Wiggin et al., 2009). Consequently, dyslipidemia has recently been identified as a major independent risk factor for the development of neuropathy [reviewed in (Vincent et al., 2009b)].
Poor lipid profiles correlate with the onset of symptoms type 2 diabetic patients (Clemens et al., 2004). In addition, elevated triglycerides correlate with the progression of diabetic neuropathy independent of disease duration, age, glycemic control, or body mass index (BMI) (Wiggin et al., 2009). Furthermore, nondiabetic patients with idiopathic neuropathy with and without impaired glucose tolerance had a significantly higher rate of dyslipidemia compared to diabetic patients without neuropathy (Smith et al., 2008). Despite the growing body of clinical literature that suggests diabetic patients with a poor lipid profile are at increased risk for developing neuropathy, few rodent models of diabetic neuropathy have incorporated dyslipidemia.
Cellular Mechanisms of Dyslipidemia in Diabetic Neuropathy
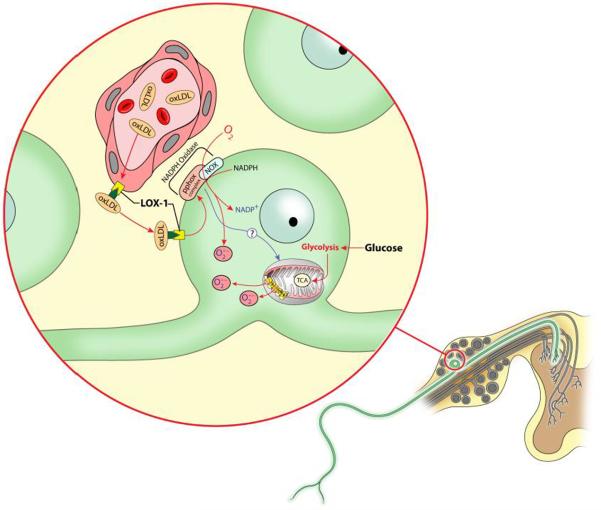
Although the association of dyslipidemia and neuropathy has been identified in clinical studies, the mechanisms by which lipids damage sensory neurons and contribute to pathogenesis of diabetic neuropathy are unclear. It is possible that increased high-density lipoproteins (HDLs), despite an otherwise poor lipid profile, may reduce peripheral lipid deposits and interfere with the influence of other lipoproteins on sensory neurons in diabetic patients. Vincent et al. (Vincent et al., 2009b) proposed a mechanism suggesting that elevated low-density lipoproteins (LDLs) have increased susceptibility to oxidation and oxidized LDLs (oxLDLs) induce cellular effects that lead to neuronal injury in the dorsal root ganglia (DRG) by binding the oxLDL receptor (LOX-1) receptor expressed on DRG neurons in a similar manner to oxLDL binding to its receptor in vascular endothelial cells (Chen et al., 2007) and renal tubular cells (Kelly et al., 2008). Vincent et al. also reported that oxLDLs are increased in the plasma of mice fed a high-fat diet and confirmed that the LOX-1 receptor is expressed on DRG neurons. Exposure of cultured rat DRG neurons to oxLDLS also increased LOX-1 expression and dose dependently increased oxidative stress via LOX-1 (Vincent et al., 2009a). In addition, these studies suggested that oxLDL is involved with LOX-1 induced neuron injury primarily by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation that results in increased superoxide production (Vincent et al., 2009a). Figure 1, adapted from Vincent et al. 2009a highlights this novel mechanism for dyslipidemia-induced sensory neuron damage in which oxLDL binding to LOX-1 induces NADPH oxidase activation and results in superoxide generation in DRG neurons. Because activation of LOX-1 on endothelial cells has also been reported to induce oxidative stress and inflammation (Li et al., 2004; Mehta et al., 2004), it plausible to suggest that oxLDLs could doubly contribute to neuropathy via both vascular and neuronal injury.
Figure 1. Putative mechanisms for dyslipidemia-induced sensory neuron injury.
Schematic diagram illustrating the DRG, with an enlarged inset of a DRG neuron and adjacent blood vessel. The oxidized form of LDL (oxLDL) binds to the LOX-1 receptor in vascular endothelial cells and DRG neurons and is subsequently endocytosed or transcytosed. oxLDL is thought to activate NADPH oxidase via interactions with the LOX-1 receptor, resulting in non-mitochondrial superoxide generation. In addition, NADPH oxidase may increase mitochondrial production of reactive oxygen species. Finally, glucose may independently induce mitochondrial superoxide production and increase endothelial LOX-1 expression. This schematic illustration was adapted from Vincent et al. (Vincent et al., 2009b) with permission from John Wiley and Sons, Ltd.
Demyelination resulting from lipid dysregulation is another potential mechanism of lipid induced neuronal injury. Segmental demyelination is a key feature in human patients with diabetic neuropathy, and myelin breakdown with focal demyelination has been shown to occur in high-fat-fed rodents (Xie et al., 2013). Thus, it is plausible to suggest that genetically or high-fat diet induced dyslipidemia may negatively impact myelination status in peripheral nerves and contribute to sensorimotor deficits.
Dyslipidemic Animal Models of Diabetic Neuropathy
To date, few studies have evaluated neuropathy and the lipid profile in rodents (Coppey et al., 2012; Guilford et al., 2011; Kumar et al., 2009; Obrosova et al., 2007; Vincent et al., 2009a) and most of these studies have been performed in high-fat-fed rodents. Although high-fat-fed mice develop neuropathy-like symptoms, including sensory and motor nerve conduction velocity deficits, reduced epidermal innervation, mechanical allodynia, thermal hypoalgesia, and mild hyperlipidemia (Guilford et al., 2011; Vincent et al., 2009a), the current dyslipidemic models fail to acquire all facets of the poor lipid profile observed in human dyslipidemia.
Human diabetic patients with dyslipidemia typically exhibit increased triglycerides, elevated LDL-C and reduced HDL-C. In mice, Vincent et al. reported a 100% increase in HDL that was accompanied by increased cholesterol uptake by HDL in high-fat fed mice compared to mice fed a control diet (Vincent et al., 2009a). Even though high-fat fed mice exhibit significant increases in LDL-C and triglycerides (Guilford et al., 2011; Vincent et al., 2009a), Hinder et al. makes a strong case that increased HDL may potentially counteract the negative metabolic effects of increased LDL-C on neurons and the vasculature. In addition, Guilford et al. reported mild increases in total cholesterol, LDL-C, and triglycerides in nondiabetic or diabetic mice fed a high-fat diet, but neither of these groups had a significant elevation in all three of the lipid variables (Guilford et al., 2011). Consistent with these findings, other high-fat feeding studies suggest that although high-fat fed rodents develop mild hyperlipidemia, rodents are resistant to robust increases LDL-C and the total cholesterol/HDL-C ratio, and do not exhibit significant reductions in HDL-C (Kobayashi et al., 2000; Seo et al., 2012; Zaman et al., 2011).
Furthermore, rodent models of diabetic neuropathy consistently lack demyelination, which is an important clinical component of human diabetic neuropathy (Sullivan et al., 2008). A plausible advantage of developing novel animal models that combine hyperglycemia and “human-like” dyslipidemia would be to develop more accurate animal models of the human phenotype. This would include models in which segmental demyelination and other lipid disorders of myelin may develop.
Genetic Manipulations in Mice to Mimic Human Dyslipidemia
In this paper, Hinder and colleagues work to remedy this problem by using novel genetic experimental approaches to reproduce human dyslipidemia in a diabetic mouse model. An ApoE null mutant mouse combined with leptin receptor mutation (ApoE−/− db/db) and a triple knockout incorporating both ApoE and ApoB48 knockout in the ob/ob mouse were developed to generate a rodent model (ApoE−/− ApoB100only ob/ob) with hyperglycemia and hyperlipidemia with a lipid profile that more closely resembles a human diabetic patient.
Db/db and ob/ob mice are models of type 2 diabetes that exhibit profound obesity, hyperphagia, hyperglycemia, glucose intolerance, hyperinsulinemia, and develop neuropathy (Coleman, 1982; Drel et al., 2006; Houseknecht and Portocarrero, 1998; Robertson and Sima, 1980; Sima and Robertson, 1978; Sullivan et al., 2007). In addition, ob/ob mice fed a standard diet develop dyslipidemia characterized by elevated triglycerides and LDL-C (Kobayashi et al., 2000). Importantly, HDL-C levels were increased in this type 2 model of diabetic dyslipidemia (Kobayashi et al., 2000). Although these are suitable models for type 2 diabetes, they still fall short of emulating human diabetic dyslipidemia.
Apolipoproteins are proteins associated with lipoproteins that bind lipids and are associated with many diseases, especially cardiovascular disease. There are several classes of apolipoproteins (A, B, C, D, E, H, J). A single molecule of apolipoprotein B (ApoB) is found on all beta lipoproteins (chylomicrons, very low-density lipoproteins [VLDLs], intermediate-density lipoproteins [IDLs], and LDLs) and there are two types of ApoB. ApoB48 containing lipoproteins are the primary carriers of cholesterol in mice, while cholesterol in humans is predominantly carried by LDLs that contain ApoB100 (Lloyd et al., 2008). ApoB100 is found on VLDLs, IDLs, and LDLs (Irshad and Dubey, 2005) and is the primary protein of LDL and is part of the LDL molecule that binds the LDL receptor (Thomas and LaFontaine, 2001). LDL binding to the LDL receptor is an important event for cholesterol uptake from the blood; thus, ApoB100 is a key protein involved in cholesterol removal from the blood in humans (Boren et al., 1998). Familial defective ApoB100 in human patients results in poor LDL receptor mediated cholesterol binding and elevated circulating cholesterol (Genest and Cohn, 1995), further emphasizing the importance of ApoB100 in human cholesterol homeostasis.
LDL receptor knockout mice are a common model used in dyslipidemia and atherosclerosis research despite the fact that Apo B48 containing VLDLs and chylomicron remnants carry the majority of plasma cholesterol in these mice. This is a significant issue as this problem renders this model less relevant to human conditions (Lloyd et al., 2008). Elevated plasma ApoB100 levels are associated with atherosclerosis in humans (Krauss, 1998; Segrest, 2002). Interestingly, ApoB48 knockout mice have increased ApoB100-expressing LDLs, which is more similar to lipid profiles in humans. Consequently, ApoB48 knockout mice were utilized in the recent studies by Hinder et al. in conjunction with an ApoE knockout in an ob/ob mouse to model human diabetic dyslipidemia (Lloyd et al., 2008).
ApoE is associated with VLDLs, HDLs, and chylomicrons and similar to ApoB proteins, ApoE interacts with the LDL receptor but also interacts with a liver receptor for VLDL remnants (Mayes, 1996). ApoE has numerous functions in the body, including playing an essential role in VLDL remnant and chylomicron clearance by the liver (Mahley, 1988). ApoE also has been identified as an antioxidant, a modulator of neurotropic factors, and participates in lipid transport to the central nervous system (Burtis et al., 2001). There are four major alleles of ApoE, and select alleles have been associated with increased risk for developing Alzheimer's disease (Kim et al., 2009; Poirier, 2000; Sheng et al., 1996) and atherosclerosis (Couderc and Bailleul, 1998; Davignon et al., 1999; Ilveskoski et al., 2000). The ApoE knockout mouse is a common model in lipid and atherosclerosis fields (Osada et al., 2000) that develops total cholesterol levels that are five times higher that of normal mice (Zhang et al., 1992), elevated LDL-C, reduced HDL-C, moderate increases in triglycerides, and atherosclerotic lesions similar to those in humans (Pendse et al., 2009; Plump et al., 1992; Zhang et al., 1992).
Building a Better Model of Human Dyslipidemia in Mice
Although the data did not support the authors' hypothesis, the new animal models developed by Hinder et al. break new ground for studies on the effects of dyslipidemia relative to diabetic neuropathy. Hinder and colleagues proposed “that lack of ApoE would exacerbate the development of diabetic microvascular complications, including neuropathy, i.e., that dyslipidemia and hyperglycemia together aggravate the onset of diabetic complications”. However, peripheral nerve dysfunction was not exacerbated by the addition of an ApoE knockout to the leptin receptor mutation (ApoE−/− db/db) despite that fact that these mice exhibited hyperglycemia and profound dyslipidemia compared to db/db mice with wild-type ApoE (ApoE+/+ db/db) that were similarly obese and hyperglycemic, but not dyslipidemic. Importantly, the addition of ApoE knockout to the leptin receptor mutant resulted in a lipid profile that more closely resembles human dyslipidemia than previous dyslipidemic neuropathy models. ApoE−/−db/db mice had elevated triglycerides, total cholesterol, LDL-C, and VLDL-C accompanied by reduced HDL-C and a total cholesterol/HDL-C ratio that exceeded the threshold for cardiovascular risk. Although ApoE−/− db/db displayed deficits in thermal sensitivity, nerve conduction velocity and epidermal innervation, these negative changes were not different or worsened compared to ApoE+/+ db/db that displayed similar neuropathy signs and hyperglycemia, but not were not dyslipidemic.
Although the Apo E knockout with wild-type leptin receptor (ApoE−/− db/+) also had a total cholesterol/HDL-ratio above 6.0, these mice did not display any behavioral, electrophysiological, or pathological characteristics of neuropathy, suggesting that this lipid parameter may not be a key mediator driving neuropathy in this mouse model. Hinder et al. discuss the possibility that a lack of increased triglycerides in the ApoE−/− db/+ could be responsible for the absence of neuropathy in this mouse model. However, the db/db mouse with wild-type ApoE exhibited significant neuropathy despite lack of hypertriglyceridemia.
As discussed by Hinder and colleagues, the triple mutant with ApoE and ApoB48 knockout with leptin deficiency (ApoE−/− ApoB100only ob/ob) displayed a surprising and perhaps disappointing lipid profile. Although the authors predicted the combination of these genetic manipulations to produce more profound dyslipidemia, this model had elevated total cholesterol with expansion of LDL-C and VLDL-C, but HDL-C was also increased and there were minimal effects on triglycerides. However, despite the disappointing lipid phenotype, this triple mutant still exhibited significant characteristic signs of neuropathy including reduced thermal sensitivity, nerve conduction velocity deficits, and reduced epidermal innervation.
Taken together, the genetic approaches used by Hinder and colleagues place us closer to achieving the goal of successfully creating a mouse model (Apo E−/− db/db) with increased LDL-C and triglycerides in conjunction with reduced HDL-C, the phenotype that best resembles human diabetic dyslipidemia. Collectively, these data suggest dyslipidemia using these genetic approaches does not have a significant impact on peripheral nerve dysfunction and neuropathy in mice.
Interestingly, the pattern of neuropathy appears to follow the pattern of hyperglycemia, since two experimental groups (ApoE+/+ db/db and ApoE−/− db/db) with hyperglycemia and increased glycated hemoglobin both displayed the clearest signs of peripheral neuropathy. Although glucose was not significantly elevated in the triple mutant compared to ApoE−/− ApoB100only ob/+ mice, glycated hemoglobin was significantly elevated and these mice displayed clear signs of neuropathy including reduced thermal sensitivity, nerve conduction velocity deficits, and reduced epidermal innervation compared to ApoE−/− ApoB100only ob/+ mice. If the predictions of the role of dyslipidemia related to neuropathy were correct, one would expect that mouse models with significant dyslipidemia would develop significant neuropathy. However, based on the studies thus far, neuropathy appears to be more closely linked with hyperglycemia.
Although neuropathy appears to be more closely associated with hyperglycemia rather than dyslipidemia in this study, this issue brings up some additional and important questions. It is plausible to suggest that genetically-induced dyslipidemia in mice additionally requires other lifestyle features present in humans, including but not limited to sedentary lifestyles and excess dietary energy and fat intake. An additional key component may be the tools used to measure neuropathy, and a lack of behavioral testing for mechanical sensitivity may be one example. High-fat fed mice exhibit neuropathy characterized by robust mechanical allodynia, reduced thermal sensitivity, and reduced epidermal innervation (Guilford et al., 2011; Obrosova et al., 2007; Xie et al., 2013). In addition, a biochemical and structural evaluation of myelination status may have been a useful tool to study the impact of genetically induced “human-like” dyslipidemia on features of neuropathy.
In high-fat feeding studies, it is difficult to tease out whether neuropathy in high-fat fed mice is due to dyslipidemia or the high-fat composition of the diet. This novel study of genetically-induced dyslipidemia allows the effects of dyslipidemia to be assessed apart from high-fat feeding. Interestingly, these results suggest that dietary fat composition may have a more profound effect on neuropathy than the secondary effect of dyslipidemia. Finally, increased body weight is also a confounding factor in dyslipidemic high-fat fed rodent models. In the current study, genetically obese mice with wild type ApoE developed neuropathy, and the ApoE−/− db/db mice also developed neuropathy, but body weight was not significantly increased compared to wild type ApoE littermate controls (ApoE+/+ db/+). Thus, genetic obesity may not be a key factor driving neuropathy in these mouse models.
Possible Future Directions
Studies using treatments such as omega 3 fatty acids that specifically improve the lipid profile could yield some important insights into the role of dyslipidemia in neuropathy development and progression. It may be very important in future clinical studies to rigorously document the dietary habits of diabetic patients with dyslipidemia and neuropathy. Perhaps these approaches may elucidate possible relationships between dietary composition and/or excess energy intake and neuropathy in diabetic patients.
Concluding Remarks
Modeling human conditions in mice is extremely challenging. However, these are key hurdles to overcome as we seek to identify mechanisms that explain links between dyslipidemia and neuropathy progression. The current studies by Hinder and colleagues are important steps necessary to improve upon current models of diabetic neuropathy. The establishment of these new and novel models of diabetic dyslipidemia with lipid profiles that emulate the human condition have provided interesting new insights into this problem, and have led to important new questions that are clearly associated with a very complex problem. This genetic approach proved fruitful, as the authors were the first to create a diabetic mouse with severe hypertriglyceridemia and hypercholesterolemia, expansion of VLDL-triglycerides, increased LDL-C, and a robust increase in the total cholesterol to HDL-C ratio. Although neuropathy was not exacerbated in conjunction with dyslipidemia, this model will likely be beneficial for studies of cardiovascular disease and for testing lipid-lowering pharmacotherapeutics.
Acknowledgements
The work of the authors on this publication is supported by Award Number T32HD057850 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. In addition, support was contributed by National Institutes of Health (NIH) Grant R01NS43313 and NIH Grant P20 RR016475 from the Idea Network of Biomedical Research Excellence (INBRE) program of the National Center for Research Resources. The authors thank Stanton Fernald for his contributions on the illustration.
Genotype Abbreviations
- Wild-type
ApoE+/+ db/+
- Leptin receptor mutation only
ApoE+/+ db/+
- ApoE knockout alone
ApoE−/− db/+
- ApoE knockout with leptin receptor mutation
ApoE−/− db/db
- ApoE knockout with ApoB48 knockout and wild-type leptin
ApoE−/− ApoB100only ob/+
- ApoE knockout with ApoB48 knockout and leptin mutation
ApoE−/− ApoB100only ob/ob
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Factors in development of diabetic neuropathy. Baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. Diabetes. 1988;37:476–481. [PubMed] [Google Scholar]
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boren J, Lee I, Zhu W, Arnold K, Taylor S, Innerarity TL. Identification of the low density lipoprotein receptor-binding site in apolipoprotein B100 and the modulation of its binding activity by the carboxyl terminus in familial defective apo-B100. Journal of Clinical Investigation. 1998;101:1084–1093. doi: 10.1172/JCI1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis CA, Ashwood ER, Border B, Tietz NW. Lipid, Lipoprotein, and Apolipoprotein. In: Burtis CA, Ashwood ER, Border B, Tietz NW, editors. Tietz Fundamentals of Clinical Chemistry. 5th ed. W.B. Saunders; Sydney-Marrickville: 2001. pp. 462–492. [Google Scholar]
- Chen XP, Xun KL, Wu Q, Zhang TT, Shi JS, Du GH. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoprotein-induced apoptosis in human umbilical vein endothelial cells: role of reactive oxygen species. Vascul Pharmacol. 2007;47:1–9. doi: 10.1016/j.vph.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Clemens A, Siegel E, Gallwitz B. Global risk management in type 2 diabetes: blood glucose, blood pressure, and lipids--update on the background of the current guidelines. Exp Clin Endocrinol Diabetes. 2004;112:493–503. doi: 10.1055/s-2004-821306. [DOI] [PubMed] [Google Scholar]
- Coleman DL. Diabetes-obesity syndromes in mice. Diabetes. 1982;31:1–6. doi: 10.2337/diab.31.1.s1. [DOI] [PubMed] [Google Scholar]
- Coppey LJ, Holmes A, Davidson EP, Yorek MA. Partial replacement with menhaden oil improves peripheral neuropathy in high-fat-fed low-dose streptozotocin type 2 diabetic rat. J Nutr Metab. 2012;2012:950517. doi: 10.1155/2012/950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc R, Bailleul S. [Apolipoprotein E and its alleles in healthy subjects and in atherosclerosis] Ann Biol Clin (Paris) 1998;56:651–659. [PubMed] [Google Scholar]
- Davignon J, Cohn JS, Mabile L, Bernier L. Apolipoprotein E and atherosclerosis: insight from animal and human studies. Clin Chim Acta. 1999;286:115–143. doi: 10.1016/s0009-8981(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, Obrosova IG. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–3343. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EL. Diabetic neuropathy. Curr Drug Targets. 2008;9:1–2. doi: 10.2174/138945008783431709. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Stevens MJ, Greene DA. Pathogenesis of diabetic neuropathy. Clin Neurosci. 1997;4:365–370. [PubMed] [Google Scholar]
- Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin GM, Kahn LB, Baxter J, Marshall JA, Hamman RF. Sensory neuropathy in non-insulin-dependent diabetes mellitus. The San Luis Valley Diabetes Study. Am J Epidemiol. 1990;131:633–643. doi: 10.1093/oxfordjournals.aje.a115547. [DOI] [PubMed] [Google Scholar]
- Genest J, Jr., Cohn JS. Clustering of cardiovascular risk factors: targeting high-risk individuals. Am J Cardiol. 1995;76:8A–20A. doi: 10.1016/s0002-9149(05)80010-4. [DOI] [PubMed] [Google Scholar]
- Greene DA, Stevens MJ, Obrosova I, Feldman EL. Glucose-induced oxidative stress and programmed cell death in diabetic neuropathy. Eur J Pharmacol. 1999;375:217–223. doi: 10.1016/s0014-2999(99)00356-8. [DOI] [PubMed] [Google Scholar]
- Guilford BL, Ryals JM, Wright DE. Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57BL/6 mice. Exp Diabetes Res. 2011;2011:848307. doi: 10.1155/2011/848307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseknecht KL, Portocarrero CP. Leptin and its receptors: regulators of whole-body energy homeostasis. Domest Anim Endocrinol. 1998;15:457–475. doi: 10.1016/s0739-7240(98)00035-6. [DOI] [PubMed] [Google Scholar]
- Ilveskoski E, Jarvinen O, Sisto T, Karhunen PJ, Laippala P, Lehtimaki T. Apolipoprotein E polymorphism and atherosclerosis: association of the epsilon4 allele with defects in the internal elastic lamina. Atherosclerosis. 2000;153:155–160. doi: 10.1016/s0021-9150(00)00388-9. [DOI] [PubMed] [Google Scholar]
- Irshad M, Dubey R. Apolipoproteins and their role in different clinical conditions: an overview. Indian Journal of Biochemistry and Biophysics. 2005;42:73–80. [PubMed] [Google Scholar]
- Kelly KJ, Wu P, Patterson CE, Temm C, Dominguez JH. LOX-1 and inflammation: a new mechanism for renal injury in obesity and diabetes. Am J Physiol Renal Physiol. 2008;294:F1136–1145. doi: 10.1152/ajprenal.00396.2007. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000;49:22–31. doi: 10.1016/s0026-0495(00)90588-2. [DOI] [PubMed] [Google Scholar]
- Krauss RM. Atherogenecity of triglyceride-rich lipoproteins. American Journal of Cardiology. 1998;81:13B–17B. doi: 10.1016/s0002-9149(98)00032-0. [DOI] [PubMed] [Google Scholar]
- Kumar NP, Annamalai AR, Thakur RS. Antinociceptive property of Emblica officinalis Gaertn (Amla) in high fat diet-fed/low dose streptozotocin induced diabetic neuropathy in rats. Indian J Exp Biol. 2009;47:737–742. [PubMed] [Google Scholar]
- Leiter LA. The prevention of diabetic microvascular complications of diabetes: is there a role for lipid lowering? Diabetes Res Clin Pract. 2005;68(Suppl 2):S3–14. doi: 10.1016/j.diabres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Li L, Sawamura T, Renier G. Glucose enhances human macrophage LOX-1 expression: role for LOX-1 in glucose-induced macrophage foam cell formation. Circ Res. 2004;94:892–901. doi: 10.1161/01.RES.0000124920.09738.26. [DOI] [PubMed] [Google Scholar]
- Lloyd DJ, McCormick J, Helmering J, Kim KW, Wang M, Fordstrom P, Kaufman SA, Lindberg RA, Veniant MM. Generation and characterization of two novel mouse models exhibiting the phenotypes of the metabolic syndrome: Apob48−/−Lepob/ob mice devoid of ApoE or Ldlr. Am J Physiol Endocrinol Metab. 2008;294:E496–505. doi: 10.1152/ajpendo.00509.2007. [DOI] [PubMed] [Google Scholar]
- Lyons TJ, Jenkins AJ, Zheng D, Lackland DT, McGee D, Garvey WT, Klein RL. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004;45:910–918. doi: 10.1167/iovs.02-0648. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mayes PA. Cholesterol synthesis, trasport, and excretion. In: Murray RK, Granner DK, Mayes PA, Rodwell VW, editors. Harpers Biochemistry. Appleton and Lange; Englewood Cliffs: 1996. pp. 249–260. [Google Scholar]
- Mehta JL, Chen J, Yu F, Li DY. Aspirin inhibits ox-LDL-mediated LOX-1 expression and metalloproteinase-1 in human coronary endothelial cells. Cardiovasc Res. 2004;64:243–249. doi: 10.1016/j.cardiores.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56:2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- Osada J, Joven J, Maeda N. The value of apolipoprotein E knockout mice for studying the effects of dietary fat and cholesterol on atherogenesis. Curr Opin Lipidol. 2000;11:25–29. doi: 10.1097/00041433-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Pendse AA, Arbones-Mainar JM, Johnson LA, Altenburg MK, Maeda N. Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J Lipid Res. 2009;50(Suppl):S178–182. doi: 10.1194/jlr.R800070-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E and Alzheimer's disease. A role in amyloid catabolism. Ann N Y Acad Sci. 2000;924:81–90. doi: 10.1111/j.1749-6632.2000.tb05564.x. [DOI] [PubMed] [Google Scholar]
- Robertson DM, Sima AA. Diabetic neuropathy in the mutant mouse [C57BL/ks(db/db)]: a morphometric study. Diabetes. 1980;29:60–67. doi: 10.2337/diab.29.1.60. [DOI] [PubMed] [Google Scholar]
- Rutkove SB. A 52-year-old woman with disabling peripheral neuropathy: review of diabetic polyneuropathy. JAMA. 2009;302:1451–1458. doi: 10.1001/jama.2009.1377. [DOI] [PubMed] [Google Scholar]
- Segrest JP. The role of non-LDL:non-HDL particles in atherosclerosis. Curr Diab Rep. 2002;2:282–288. doi: 10.1007/s11892-002-0096-0. [DOI] [PubMed] [Google Scholar]
- Seo EY, Ha AW, Kim WK. alpha-Lipoic acid reduced weight gain and improved the lipid profile in rats fed with high fat diet. Nutr Res Pract. 2012;6:195–200. doi: 10.4162/nrp.2012.6.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Apolipoprotein E distribution among different plaque types in Alzheimer's disease: implications for its role in plaque progression. Neuropathol Appl Neurobiol. 1996;22:334–341. doi: 10.1111/j.1365-2990.1996.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Sima AA, Robertson DM. Peripheral neuropathy in mutant diabetic mouse [C57BL/Ks (db/db)] Acta Neuropathol. 1978;41:85–89. doi: 10.1007/BF00689757. [DOI] [PubMed] [Google Scholar]
- Sinnreich M, Taylor BV, Dyck PJ. Diabetic neuropathies. Classification, clinical features, and pathophysiological basis. Neurologist. 2005;11:63–79. doi: 10.1097/01.nrl.0000156314.24508.ed. [DOI] [PubMed] [Google Scholar]
- Smith AG, Rose K, Singleton JR. Idiopathic neuropathy patients are at high risk for metabolic syndrome. J Neurol Sci. 2008;273:25–28. doi: 10.1016/j.jns.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KA, Lentz SI, Roberts JL, Jr., Feldman EL. Criteria for creating and assessing mouse models of diabetic neuropathy. Curr Drug Targets. 2008;9:3–13. doi: 10.2174/138945008783431763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- Tesfaye S. Advances in the management of painful diabetic neuropathy. Clin Med. 2007;7:113–114. doi: 10.7861/clinmedicine.7-2-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- Thomas TR, LaFontaine TP. Exercise, Nutritional Strategies, and Lipoproteins. In: Roitman JL, editor. ACSM's Resource Manual: Guildelines for Exercise Testing and Prescription. 4th ed. Lippincott Williams and Wilkins; Baltimore: 2001. pp. 308–316. [Google Scholar]
- Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009a;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009b;14:257–267. doi: 10.1111/j.1529-8027.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58:1634–1640. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Fu H, Hou JF, Jiao K, Costigan M, Chen J. High energy diets-induced metabolic and prediabetic painful polyneuropathy in rats. PLoS One. 2013;8:e57427. doi: 10.1371/journal.pone.0057427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman MQ, Leray V, Le Bloc'h J, Thorin C, Ouguerram K, Nguyen P. Lipid profile and insulin sensitivity in rats fed with high-fat or high-fructose diets. Br J Nutr. 2011;106(Suppl 1):S206–210. doi: 10.1017/S0007114511004454. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Zochodne DW. Diabetic polyneuropathy: an update. Curr Opin Neurol. 2008;21:527–533. doi: 10.1097/WCO.0b013e32830b84cb. [DOI] [PubMed] [Google Scholar]