Abstract
Objective
Cervical cancer is the second most common female cancer worldwide, and it remains a challenge to manage preinvasive and invasive lesions. Food-based cancer prevention entities, such as black raspberries and their derivatives, have demonstrated a marked ability to inhibit preclinical models of epithelial cancer cell growth and tumor formation. Here, we extend the role of black raspberry-mediated chemoprevention to that of cervical carcinogenesis.
Methods
Three human cervical cancer cell lines, HeLa (HPV16−/HPV18+, adenocarcinoma), SiHa (HPV16+/HPV18−, squamous cell carcinoma) and C-33A (HPV16−/HPV18−, squamous cell carcinoma), were treated with a lyophilized black raspberry ethanol extract (RO-ET) at 25, 50, 100 or 200 μg/ml for 1, 3 and 5 days, respectively. Cell proliferation was measured by WST1 (tetrazolium salt cleavage) assays. Flow cytometry (propidium iodide and Annexin V staining) and fluorescence microscopy analysis were used to measure apoptotic cell changes.
Results
We found that non-toxic levels of RO-ET significantly inhibited the growth of human cervical cancer cells, in a dose-dependent and time-dependent manner to a maximum of 54%, 52% and 67%, respectively (p<0.05). Furthermore, cell growth inhibition was persistent following short-term withdrawal of RO-ET from the culture medium. Flow cytometry and fluorescence microscopy demonstrated RO-ET-induced apoptosis in all cell lines.
Conclusion
Black raspberries and their bioactive components represent promising candidates for future phytochemical-based mechanistic pathway-targeted cancer prevention strategies.
Keywords: Black raspberry, Cervical cancer, Chemoprevention
Introduction
Cervical carcinoma, the second most common female cancer worldwide, is the seventh leading cause of cancer death in women. It is estimated that 12,200 new cases and about 4210 deaths will be attributed to cervical cancer in the United States in 2010 [1]. Although cervical cytology screening has helped reduce mortality rates, managing preinvasive and invasive cervical lesions remains a challenge.
Cancer chemoprevention is classically defined as the use of natural or synthetic agents, in combination or alone, to inhibit, delay, or reverse the carcinogenic process and is a promising area of current cancer research [2–7]. Using a food-based cancer risk reduction strategy, we and others have demonstrated the dramatic cancer prevention properties of whole freeze-dried black raspberries and strawberries on colon, esophagus, and oral cavity tumorigenesis in different chemical-induced experimental carcinogenesis animal models [8–11]. Similarly, various “berry” extracts have been shown to significantly reduce the growth rates of human oral cancer cells in vitro [12–14]. Given the similar epidemiologic basis of oral and cervical cancers, we hypothesized that bioactive food components present in black raspberries and their simple derivatives could inhibit cell growth and pro-carcinogenic processes in human cervical cells. Therefore, in this study, we investigated the growth inhibitory and chemopreventive potential of a black raspberry extract (RO-ET) in human cervical cancer cells.
Materials and methods
Cell culture and reagents
Human cervical cancer cell lines, HeLa, SiHa and C-33A, were purchased from the American Type Cell Collection (ATCC; Manassas, VA). Cells were maintained in complete growth medium containing advanced DMEM/F-12 supplemented with 5% fetal bovine serum (FBS), GlutaMAX (2 mmol/L), penicillin (100 units/mL), and streptomycin (100 μg/mL) (Invitrogen; Carlsbad, CA). Cells were switched to assay medium containing 1% FBS and 5 U/mL catalase for RO-ET administration [15]. Hoechst 33342 was purchased from Invitrogen and the Annexin-V-FLUOS Staining Kit from Roche Diagnostics Corporation (Indianapolis, IN).
Black raspberry extract
RO-ET was prepared essentially utilizing a standardized extraction protocol as previously reported by Hecht et al. [16]. Briefly, a dedicated black raspberry (Rubus occidentalis, RO) cultivar was obtained from Stokes Fruit Farm (Wilmington, OH) and freeze-dried. Lyophilized black raspberry powder was extracted with ethanol (ET)/water (80:20) to generate a residue (RO-ET), which was subsequently resuspended at 200 mg/mL in cell culture grade dimethyl sulfoxide (DMSO)(Sigma-Aldrich; St. Louis, MO). Aliquots of this minimally derived extract were stored in amber vials at −80 °C until needed for in vitro assays. Analysis of the extract by high performance liquid chromatography revealed that the most abundant compounds in the extract are the four anthocyanins in black raspberries [11].
Cell growth inhibition assay
Cells were seeded in 96-well cell culture plates and after 24 h, the complete growth medium was replaced with fresh assay medium containing dilutions of RO-ET (25, 100 or 200 μg/mL) or 0.1% DMSO final concentrations, respectively. At least four replicate wells were used for each RO-ET concentration. For RO-ET administration, the culture medium was removed and replaced with fresh assay medium containing corresponding RO-ET or DMSO on assay day 2 (3-day exposure protocol), or on assay day 2 and day 4 (5-day exposure protocol). At the indicated time points, viable cell numbers were determined using WST-1 following the manufacturer’s protocol. Data are expressed as the ratio of RO-ET-treated cells versus DMSO-treated cells.
Apoptosis analysis by flow cytometry
Apoptotic cells were assessed using the Annexin-V-FLUOS Staining Kit. Briefly, cells were seeded in 35 mm culture plates, and exposed to RO-ET (200 μg/mL) or DMSO. Cells were detached using 0.25% trypsin and washed with 1× PBS. Cells were incubated with 100 μL HEPES containing 2 μL Annexin-V-FLUOS and 2 μL propidium iodide (PI, 1 mg/mL) for 15 min. Single cell suspension data (10,000 cells) were acquired using a FACScan Flow Cytometer (Becton-Dickinson; Franklin Lakes, NJ) and data processed with Cell Quest software (Becton-Dickinson).
Apoptosis analysis by Hoechst 33342 staining
Cervical cancer cells were seeded onto Nunc Lab-Tek chamber slides (ThermoFisher Scientific) and treated with RO-ET (200 μg/mL) for 3 days. Cells were fixed in 4% paraformaldehyde for 20 min. The fixed cells were washed with 1× PBS and stained with Hoechst 33342 (10 μg/mL) at 37 °C for 20 min. Cells were washed with 1× PBS and viewed under a Zeiss Axioskop (Thornwood, NY) microscope at 400× magnification.
Statistic analysis
Statistical comparisons of RO-ET-exposed versus DMSO vehicle control-treated cervical cancer cells were performed using an ANOVA followed by an unpaired t test. Differences were considered significant at p<0.05.
Results
RO-ET inhibits human cervical cancer cell growth in a dose- and time-dependent manner
To assess the potential growth inhibitory activity of RO-ET on HeLa, SiHa, and C-33A human cervical cancer cells, we first identified the optimal cell seeding density. Cell viability and growth were characterized using the WST-1 assay, and optimal cell densities were selected for HeLa (<5000/well), SiHa (<15,000/well), and C-33A (<15,000/well) cervical cells in 96-well culture plates. These cell densities were used for all subsequent growth inhibition assays unless specifically noted otherwise. Cervical cancer cells were seeded and exposed to RO-ET at 25, 100 or 200 μg/mL for 1-, 3- and 5-days, respectively. RO-ET at concentrations of 25–200 μg/mL did not result in detectable cytotoxicity as evidenced by lactate dehydrogenase (LDH) assay or trypan blue staining (data not shown).
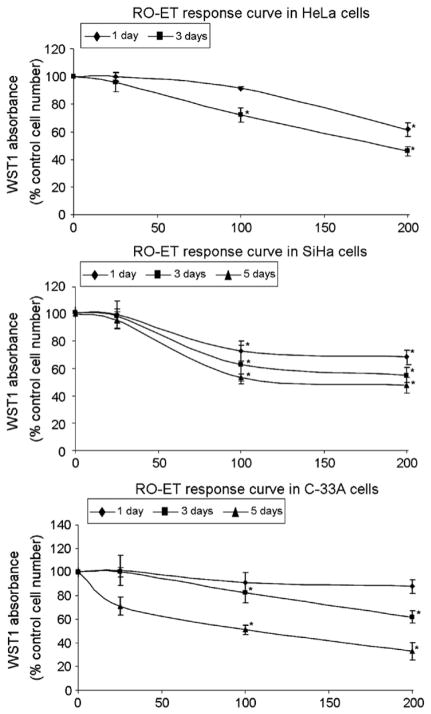
As shown in Fig. 1, RO-ET inhibited cell growth in HeLa, SiHa, and C-33A cells in both time- and dose-dependent manners. RO-ET exposure demonstrated a maximum decrease in cell growth of 54% (HeLa), 52% (SiHa) and 67% (C-33A) compared to DMSO control treatment (p<0.005). In HeLa cells, RO-ET concentrations of 25, 100 or 200 μg/mL demonstrated a reduction in proliferation of 5%, 28% and 54%, respectively, following 3 days of exposure. Similarly, in SiHa or C-33A cells these RO-ET concentrations demonstrated inhibition of 5%, 47%, and 52%, or 29%, 49%, and 67%, respectively, following 5 days of exposure. Thus, a readily observed dose–response in cell growth inhibition was seen in all cervical cell lines as RO-ET concentration increased. Furthermore, at a fixed concentration, growth inhibition varied with length of exposure. HeLa cells demonstrated a 39% and 54% decrease in cell growth following 1-day and 3-day RO-ET exposures, respectively. Once again, these growth decreases were recapitulated in SiHa (32%, 45%, and 52%) and C-33A (13%, 38%, 67%) cells following 1-, 3-, and 5-day RO-ET exposures, respectively. Overall, these data demonstrate a dose-dependent and time-dependent growth inhibition response to RO-ET in all cervical cells tested.
Fig. 1.
Dose- and time-dependent inhibition of cell growth in HeLa, SiHa and C-33A cells by RO-ET. RO-ET was added to HeLa, SiHa and C-33A cells to final concentrations of 25, 100, or 200 μg/mL. Cells were cultured for 1-day, 3-days (culture medium/LBR extract change on assay day 2), and 5-days (culture medium/LBR extract change on assay day 2 and day 4), and subjected to WST-1 assay. Control cells were treated with an equivalent volume and concentration of delivery vehicle (DMSO). Assay points were performed in quadruplicate and data are expressed as percentage of DMSO-treated control cells, mean±SD. Data shown are the represents of at least four different experiments. *p<0.05, significantly different from vehicle treated controls.
RO-ET-dependent growth inhibition is persistent in cervical cancer cells
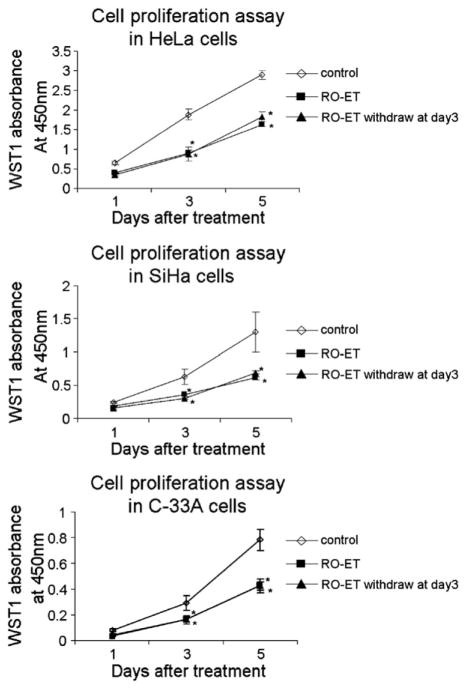
To investigate whether the growth inhibitory activity of RO-ET was transient, persistent, or reversible, cervical cancer cells were exposed to RO-ET for 3 days and subsequently allowed to grow for an additional 2 days in the absence of RO-ET. After 5 days, HeLa, SiHa and C-33A cells were assessed for changes in growth using the WST-1 assay. A 5-day continuous exposure to RO-ET demonstrated a decrease in cell growth of 43% (HeLa), 53% (SiHa) and 45% (C-33A) compared to DMSO control treatments. Interestingly, a 3-day LBR extract exposure followed by 2 days of RO-ET-free culturing demonstrated comparable growth inhibitions of 38% (HeLa), 50% (SiHa) and 46% (C-33A), compared to 5-day RO-ET exposure (Fig. 2). These results indicate that in all three human cervical cancer cell lines the growth inhibitory effects initiated by RO-ET exposure are persistent, and are not significantly reversed following short-term withdrawal of RO-ET.
Fig. 2.
RO-ET administration induces growth inhibition that is persistent following short-term RO-ET withdrawal. HeLa, SiHa and C-33A cells were exposed to RO-ET for 1-, 3-, and 5-days, and analyzed using the WST-1 assay. Culture medium was removed and replaced with fresh assay medium containing RO-ET or DMSO on assay day 2 (3-day exposure protocol), or on assay day 2 and day 4 (5-day exposure protocol). To assess the transient or persistent nature of RO-ET-induced growth inhibition, cells were exposed to RO-ET for 3 days, subjected to a culture medium change, and allowed to grow in RO-ET-free conditions for an additional 2 days. Control cells were treated with an equivalent volume and concentration of RO-ET or DMSO. Assay points were performed in quadruplicate and data are shown in mean±SD. Data shown are representative of at least three different experiments. *p<0.05, significantly different from vehicle treated controls.
RO-ET induces apoptosis in human cervical cancer cells
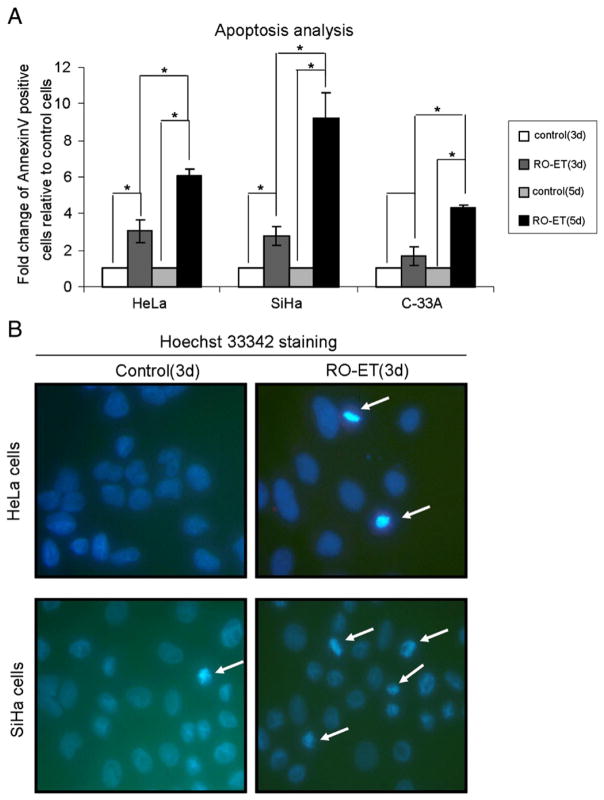
To explore the molecular mechanisms underlying the growth inhibitory effects of RO-ET in cervical cancer cells, we characterized RO-ET exposed cells for apoptotic cell populations by double-staining with Annexin V and propidium iodide using flow cytometry. RO-ET (3-day exposure) induced an increase in apoptotic cell populations in all cervical cell lines, ranging from 1.7-fold to 3.1-fold compared to controls. Exposure to RO-ET for 3 days increased apoptosis 3.1-fold in HeLa, 2.8-fold in SiHa, and 1.7-fold in C-33A cells compared to controls as indicated by bound Annexin V (Fig. 3A). The induction of apoptosis in each cervical cell line is consistent with the growth inhibitory effects measured by WST-1 assay. When exposed to RO-ET for 3 days, HeLa cells demonstrated −54% proliferation/+210% apoptosis, SiHa cells showed −45% proliferation/+180% apoptosis, and C-33A cells revealed −38% proliferation/+70% apoptosis. We noticed that the apoptosis induced by RO-ET was relatively lower in C-33A cells compared to HeLa and SiHa cells. Additionally, following a 3-day exposure to RO-ET, HeLa and SiHa cells exhibited hallmark apoptotic nuclear features, including chromatin condensation, as observed using Hoechst 33342 staining and fluorescent microscopy (Fig. 3B). We did not observe obvious apoptotic nuclear features in C-33A cells after a 3-day RO-ET exposure (data not shown), consistent with the flow cytometry analysis. A 5-day RO-ET exposure dramatically increased apoptosis 6.1-fold in HeLa, 9.3-fold in SiHa, and 4.3-fold in C-33A cells compared to controls (Fig. 3A), which is consistent with the time-dependent cell growth inhibition by RO-ET. Interestingly, the contribution of apoptosis in growth inhibition by RO-ET was different among HeLa, SiHa and C33A cells, which may be due to the cell genetic background difference. Overall, the Annexin V and PI staining results in conjunction with the observed nuclear condensation provide experimental evidence that RO-ET-mediated growth inhibition is associated with increased apoptotic biomarkers in human cervical cells.
Fig. 3.
Induction of apoptotic activity by RO-ET in HeLa, SiHa and C-33A cervical cancer cells. (A) RO-ET administration induced apoptosis in HeLa, SiHa and C-33A cells. Cells were exposed to RO-ET for 3- or 5-days, stained with AnnexinV/PI, and characterized using flow cytometry. Data shown are the average of at least two experiments and mean±SD. *p<0.05, significantly different from vehicle treated controls. (B) Nuclear condensation in HeLa and SiHa cervical cells following delivery of RO-ET. Cells were administered RO-ET for 3 days and stained with Hoechst 33342. Nuclear condensation was assessed at 400× magnification using UV epifluorescence. Arrows point to regions representing apoptotic nuclear condensation.
Discussion
Various cervical cancer risk reduction strategies have been investigated, including immune modulation of cytokines, individual micronutrient supplementation, polyamine synthesis inhibitors, and pharmaceutical agents, and all have demonstrated a limited success [17,18]. Most recently, HPV vaccine strategies have been implemented that hold the promise of significant cervical cancer risk reduction; however, the actual impact of these initiatives remains years away. Thus, novel and broadly applicable approaches to cervical cancer prevention, such as those described herein, remain essential and necessary.
Fruit and vegetable consumption has been shown to be inversely related to cancer mortality [19], and provides the foundation for food-based approaches to cancer prevention. It has been observed that a diet rich in plant-based nutrients is important in reducing the risk of cervical cancer [20]. Black raspberries are of particular interest due to their large complement of compounds with demonstrated chemo-preventive activity. Whereas isolated phytonutrients have failed to demonstrate appreciable chemopreventive activities in clinical trials, whole-food-based and minimally derived extracts have not been systematically investigated in the cervix. In the present study we found that RO-ET inhibited the cell growth of all cervical cancer cells examined: HeLa, SiHa and C-33A. Our demonstration of RO-ET time-and dose-dependent growth inhibition of cervical cancer cells extends other “berry” bioactive component studies [21–23], and provides evidence for an RO-ET-mediated mechanism of apoptosis.
Historically, chemoprevention approaches rely on lifestyle changes and persistent exposure to a cancer prevention intervention strategy. Continuous exposure to RO-ET over 5 days in vitro resulted in the significant inhibition of cell growth in all cervical cancer cells examined. Interestingly, we observed that following a 3-day RO-ET administration, growth inhibition persisted at levels comparable to a continuous 5-day exposure for an additional 2 days if RO-ET was removed from the culture conditions. There was no immediate reversibility in growth suppression following this short-term withdrawal of RO-ET. Although longer periods of RO-ET-free growth are not possible in the existing in vitro model conditions due to cell confluency limitations, it will be interesting to further study the transient and persistent aspects of growth inhibition following RO-ET exposure and removal.
Apoptosis is a tightly controlled mechanism of programmed cell death and recognized as an important target for potential cancer prevention strategies. It has been shown that black raspberry extracts stimulated apoptosis in colon cancer cells as well as oral squamous cell carcinoma cells and rat esophageal squamous cell carcinoma cells (see paper by Zikri et al. [12,13,24]); however, the role of RO-ET on cervical cancer cells remains largely uncharacterized. We found that RO-ET induced apoptosis in all cervical cell lines with varying degrees of potency. As mentioned previously, the variation in apoptotic response to RO-ET may be in part a reflection of known genetic background differences between HeLa (HPV16−/HPV18+, wild-type p53), SiHa (HPV16+/HPV18−, wild-type p53) and C-33A (HPV18−HPV16−, mutant p53). Bernard et al. have shown that HPV status and p53 functionality can influence the susceptibility of staurosporine-induced apoptosis in various cervical cancer cell lines [25]. They reported that wild type p53 cells such as HeLa demonstrated an enhanced apoptotic collapse of the mitochondrial membrane potential and greater DNA fragmentation compared to mutant p53 cells such as C-33A [25]. The time-dependent apoptotic responses by the human cervical cancer cells are mirrored by the growth inhibition induced by RO-ET in these cell lines, providing evidence that the growth inhibitory effects of RO-ET are in part attributed to activation of apoptotic mechanisms.
As an important target for potential cancer prevention strategies, apoptosis has been demonstrated to contribute to other nutrient-based intervention strategies evaluated in cervical cancer prevention. The alkaloid compound (−)-anonaine derived from Michelia alba trees [26], the isothiocyanate sulforaphane (SFN) [27], and the combination of SFN with eugenol [28] have all been reported to induce apoptosis in HPV18+ HeLa cervical cancer cells. Similarly, an aqueous extract from the bark of Cinnamomum cassia promoted apoptosis in HPV16+ SiHa human cervical cancer cells [29]. Furthermore, both the green tea derived flavonoid epigallocatechin gallate (EGCG) and a commercial formulation of green tea polyphenols (Polyphenon E) inhibited the growth of HPV18+ Me180 and HeLa cells, as well as cervical epithelial cells (TCL1) immortalized with HPV18 viral DNA, through apoptotic mechanisms [30].
Some studies have focused on identification of the individual bioactive components responsible for black raspberry-mediated chemoprevention. These reductionist approaches target specific common compounds present in the black raspberry extract and characterize which of these isolated compounds are associated with particular chemopreventive effects. Han et al. reported that two major components of black raspberries, ferulic acid and beta-sitosterol, inhibited the growth of premalignant and malignant human oral cell lines [14]. Zikri et al. reported that two black raspberries anthocyanins, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside, inhibited proliferation and induced apoptosis in vitro in tumorigenic rat esophageal epithelial cells [24], while Wang et al. reported that black raspberry anthocyanins delivered in vivo prevented esophageal tumors in rats [11]. Although some bioactive components of black raspberries have been identified for their cancer chemopreventive potential, many other components remain unknown and/or untested for cancer chemopreventive activity. Furthermore, we hypothesize that bioactive components within the complex matrix of the intact or minimally-extracted black raspberries inhibit carcinogenesis through additive or synergistic mechanisms. The ability of a complex mixture of chemopreventive agents within the minimally-derived extract to act upon multiple pathways of growth inhibition (and carcinogenesis) underlies the rationale for using a “crude” black raspberry extract in this study.
The K14-HPV16 mouse model is commonly used in studies involving cervical intraepithelial neoplasias and cancer, and provides the essential transition from in vitro to in vivo pre-clinical studies [31,32]. Topical application (locoregional) and dietary administration (systemic) of black raspberries and/or their bioactive components using the K14-HPV16 mouse model would assess the potential efficacy of black raspberries on the histopathology of progressive cervical lesions present in the transformation zone. These studies would be essential to establish a foundation and rationale for subsequent future Phase I clinical trials focused on black raspberry-mediated intervention strategies in human cervical cancer prevention.
In conclusion, a minimally derived ethanol extract of black raspberries demonstrated significant growth inhibitory and apoptotic activating activities in human cervical cancer cells in a dose- and time-dependent manner. Overall, these data suggest that black raspberries and their bioactive components represent promising candidates for food-based chemoprevention strategies for cervical cancer. This study provides an excellent foundation for future preclinical studies and strengthens the rationale and justification for the inclusion of black raspberry bioactive components in human cervical cancer prevention strategies.
Acknowledgments
This work is supported by funding from Perinatal Resources, Incorporated, The American College of Obstetricians and Gynecologists (both to David E. Cohn), P50 CPHHD P50CA105632 (Electra D. Paskett), and P30CA016058 (The Ohio State University Comprehensive Cancer Center).
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.American Cancer Society. Cancer facts & figures 2010. Atlanta (GA): American Cancer Society; 2010. [Google Scholar]
- 2.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–8. [PubMed] [Google Scholar]
- 3.Kelloff GJ, Sigman CC, Greenwald P. Cancer chemoprevention: progress and promise. Eur J Cancer. 1999;35:2031–8. doi: 10.1016/s0959-8049(99)00164-1. [DOI] [PubMed] [Google Scholar]
- 4.Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–7. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 5.Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2000;78:199–334. doi: 10.1016/s0065-230x(08)61026-x. [DOI] [PubMed] [Google Scholar]
- 6.Lippman SM, Lee JJ, Sabichi AL. Cancer chemoprevention: progress and promise. J Natl Cancer Inst. 1998;90:1514–28. doi: 10.1093/jnci/90.20.1514. [DOI] [PubMed] [Google Scholar]
- 7.William WN, Heymach JV, Kim ES, Lippman SM. Molecular targets for cancer chemoprevention. Nat Rev Drug Discov. 2009;8:213–25. doi: 10.1038/nrd2663. [DOI] [PubMed] [Google Scholar]
- 8.Casto BC, Kresty LA, Kraly CL, Pearl DK, Knobloch TJ, Schut HA, Stoner GD, Mallery SR, Weghorst CM. Chemoprevention of oral cancer by black raspberries. Anticancer Res. 2002;22:4005–15. [PubMed] [Google Scholar]
- 9.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, Blackwood M, Stoner GD. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–9. [PubMed] [Google Scholar]
- 10.Harris GK, Gupta A, Nines RG, Kresty LA, Habib SG, Frankel WL, LaPerle K, Gallaher DD, Schwartz SJ, Stoner GD. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr Cancer. 2001;40:125–33. doi: 10.1207/S15327914NC402_8. [DOI] [PubMed] [Google Scholar]
- 11.Wang L-S, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, McIntyre C, Rocha C, Lechner JF, Stoner GD. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res (Phila) 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 2006;54:9329–39. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigo KA, Rawal Y, Renner RJ, Schwartz SJ, Tian Q, Larsen PE, Mallery SR. Suppression of the tumorigenic phenotype in human oral squamous cell carcinoma cells by an ethanol extract derived from freeze-dried black raspberries. Nutr Cancer. 2006;54:58–68. doi: 10.1207/s15327914nc5401_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han C, Ding H, Casto B, Stoner GD, D’Ambrosio SM. Inhibition of the growth of premalignant and malignant human oral cell lines by extracts and components of black raspberries. Nutr Cancer. 2005;51:207–17. doi: 10.1207/s15327914nc5102_11. [DOI] [PubMed] [Google Scholar]
- 15.Liu RH, Sun J. Antiproliferative activity of apples is not due to phenolic-induced hydrogen peroxide formation. J Agric Food Chem. 2003;51:1718–23. doi: 10.1021/jf026162r. [DOI] [PubMed] [Google Scholar]
- 16.Hecht SS, Huang C, Stoner GD, Li J, Kenney PM, Sturla SJ, Carmella SG. Identification of cyanidin glycosides as constituents of freeze-dried black raspberries which inhibit anti-benzo[a]pyrene-7,8-diol-9,10-epoxide induced NFkappaB and AP-1 activity. Carcinogenesis. 2006;27:1617–26. doi: 10.1093/carcin/bgi366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell MC, Alvarez RD. Chemoprevention and vaccines: a review of the nonsurgical options for the treatment of cervical dysplasia. Int J Gynecol Cancer. 2005;15:4–12. doi: 10.1111/j.1048-891X.2005.15002.x. [DOI] [PubMed] [Google Scholar]
- 18.Follen M, Meyskens FL, Alvarez RD, Walker JL, Bell MC, Storthz KA, Sastry J, Roy K, Richards-Kortum R, Cornelison TL. Cervical cancer chemoprevention, vaccines, and surrogate endpoint biomarkers. Cancer. 2003;98:2044–51. doi: 10.1002/cncr.11674. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296:695–8. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh C, Baker JA, Moysich KB, Rivera R, Brasure JR, McCann SE. Dietary intakes of selected nutrients and food groups and risk of cervical cancer. Nutr Cancer. 2008;60:331–41. doi: 10.1080/01635580701861769. [DOI] [PubMed] [Google Scholar]
- 21.Wedge DE, Meepagala KM, Magee JB, Smith SH, Huang G, Larcom LL. Antic-arcinogenic activity of strawberry, blueberry, and raspberry extracts to breast and cervical cancer cells. J Med Food. 2001;4:49–51. doi: 10.1089/10966200152053703. [DOI] [PubMed] [Google Scholar]
- 22.Ross HA, McDougall GJ, Stewart D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry. 2007;68:218–28. doi: 10.1016/j.phytochem.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Jung J, Son MY, Jung S, Nam P, Sung JS, Lee SJ, Lee KG. Antioxidant properties of Korean black raspberry wines and their apoptotic effects on cancer cells. J Sci Food Agric. 2009;89:970–7. [Google Scholar]
- 24.Zikri NN, Riedl KM, Wang LS, Lechner J, Schwartz SJ, Stoner GD. Black raspberry components inhibit proliferation, induce apoptosis, and modulate gene expression in rat esophageal epithelial cells. Nutr Cancer. 2009;61:816–26. doi: 10.1080/01635580903285148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard B, Fest T, Prétet JL, Mougin C. Staurosporine-induced apoptosis of HPV positive and negative human cervical cancer cells from different points in the cell cycle. Cell Death Differ. 2001;8:234–44. doi: 10.1038/sj.cdd.4400796. [DOI] [PubMed] [Google Scholar]
- 26.Chen CY, Liu TZ, Tseng WC, Lu FJ, Hung RP, Chen CH, Chen CH. (−)-Anonaine induces apoptosis through Bax- and caspase-dependent pathways in human cervical cancer (HeLa) cells. Food Chem Toxicol. 2008;46:2694–702. doi: 10.1016/j.fct.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Sharma C, Sadrieh L, Priyani A, Ahmed M, Hassan AH, Hussain A. Anti-carcinogenic effects of sulforaphanein association with its apoptosis-inducing and anti-inflammatory properties in human cervical cancer cells. Cancer Epidemiol. 2011;35:272–8. doi: 10.1016/j.canep.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Hussain A, Priyani A, Sadrieh L, Brahmbhatt K, Ahmed M, Sharma C. Concurrent sulforaphane and eugenol induces differential effects on human cervical cancer cells. Integr Cancer Ther. 2011 doi: 10.1177/1534735411400313. [DOI] [PubMed] [Google Scholar]
- 29.Koppikar SJ, Choudhari AS, Suryavanshi SA, Kumari S, Chattopadhyay S, Kaul-Ghanekar R. Aqueous cinnamon extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer. 2010;10:210–1. doi: 10.1186/1471-2407-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou C, Liu H, Feugang JM, Hao Z, Chow HH, Garcia F. Green tea compound in chemoprevention of cervical cancer. Int J Gynecol Cancer. 2010;20:617–24. doi: 10.1111/IGC.0b013e3181c7ca5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sepkovic DW, Stein J, Carlisle AD, Ksieski HB, Auborn K, Raucci L, Nyirenda T, Bradlow HL. Results from a dose–response study using 3,3′-diindolylmethane in the K14-HPV16 transgenic mouse model: cervical histology. Cancer Prev Res (Phila) 2011;4:890–6. doi: 10.1158/1940-6207.CAPR-10-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Immel TA, Groth U, Huhn T, Öhlschläger P. Titanium salan complexes displays strong antitumor properties in vitro and in vivo in mice. PLoS One. 2011;6:e17869. doi: 10.1371/journal.pone.0017869. [DOI] [PMC free article] [PubMed] [Google Scholar]