Abstract
Kminek, G., Bada, J. L., Pogliano, K. and Ward, J. F. Radiation-Dependent Limit for the Viability of Bacterial Spores in Halite Fluid Inclusions and on Mars. Radiat. Res. 159, 722–729 (2003).
When claims for the long-term survival of viable organisms are made, either within terrestrial minerals or on Mars, considerations should be made of the limitations imposed by the naturally occurring radiation dose to which they have been exposed. We investigated the effect of ionizing radiation on different bacterial spores by measuring the inactivation constants for B. subtilis and S. marismortui spores in solution as well as for dry spores of B. subtilis and B. thuringiensis. S. marismortui is a halophilic spore that is genetically similar to the recently discovered 2-9-3 bacterium from a halite fluid inclusion, claimed to be 250 million years old (Vreeland et al., Nature 407, 897–900, 2000). B. thuringiensis is a soil bacterium that is genetically similar to the human pathogens B. anthracis and B. cereus (Helgason et al., Appl. Environ. Microbiol. 66, 2627–2630, 2000). To relate the inactivation constant to some realistic environments, we calculated the radiation regimen in a halite fluid inclusion and in the Martian subsurface over time. Our conclusion is that the ionizing dose of radiation in those environments limits the survival of viable bacterial spores over long periods. In the absence of an active repair mechanism in the dormant state, the long-term survival of spores is limited to less than 109 million years in halite fluid inclusions, to 100 to 160 million years in the Martian subsurface below 3 m, and to less than 600,000 years in the uppermost meter of Mars.
INTRODUCTION
Bacterial spores exhibit a remarkable resistance to adverse environmental conditions like desiccation, temperature extremes, pH variation, UV radiation and ionizing radiation. They have developed strategies to deal with the threat of irreversible damage to their DNA by reducing the water content in the cell, binding their DNA to small, acid-soluble proteins and using an efficient repair mechanism that operates during spore germination (1).
The isolation of an ancient viable halotolerant bacterial spore from a halite fluid inclusion in the Permian Salado Formation in New Mexico sparked new interest and controversy around the question of how long bacterial spores can survive in adverse environments here on Earth, and potentially on Mars (2, 3). At the moment, no upper limit for the survival of bacterial spores has been established. The ultimate limit for their survival might be the accumulation of damage that overwhelms the repair mechanism when germination is induced. We propose that the ionizing radiation dose in different environments could well be the Achilles heel for the long-term survival of bacterial spores. To test this hypothesis, we have investigated how the radiation environment in halite fluid inclusions and in the Martian subsurface would affect the survival of viable bacterial spores. The work we present here consists of two parts: experiments to measure the radiation inactivation constant of bacterial spores, and calculations to evaluate the radiation dose in fluid inclusions and on Mars.
MATERIALS AND METHODS
Bacterial Strains Used
The bacterial strains used in this investigation were Bacillus subtilis (PY79), Salibacillus marismortui (ATCC No. 700626T), and Bacillus thuringiensis subsp. kurstaki (BGSC No. 4D11). S. marismortui, formerly Bacillus marismortui, is a moderately halophilic spore former that was first isolated from Dead Sea water samples by Volcani in 1936 (4, 5). Based on the 16S rDNA phylogenetic tree, S. marismortui is very similar (99% S) to the isolate 2-9-3 that has been cultivated from a halite fluid inclusion (2, 6). Moderately halophilic bacteria initiate sporulation when the salt concentration of the brine reaches saturation. B. thuringiensis is a soil bacterium that is genetically close to B. anthracis and B. cereus. The divergence in DNA between B. thuringiensis, B. anthracis and B. cereus is less than 5% (7). B. thuringiensis is used as insecticidal toxin, while B. anthracis is the cause of the disease anthrax and is a potent biological warfare agent, and B. cereus is a common soil bacterium that causes food poisoning (7). We used a soil bacterium as a model because this class of bacterium usually is more resistant to desiccation, which coincidentally makes them also more resistant to ionizing radiation (8). B. subtilis was used to compare the radiation resistance of S. marismortui and B. thuringiensis with a well-known standard bacterium.
Spore Preparation
Single colonies of B. subtilis and B. thuringiensis were suspended in 3 ml of DSM [Difco sporulation agar, autoclaved and supplemental with 1 mM FeSO4, 1 M Ca(NO3)2 and 10 mM MnCl2] sporulation medium at 37°C. B. subtilis produced spores in 24 h, B. thuringiensis in about 1 week. Single colonies of S. marismortui were suspended in 500 ml K medium (750 ml filtered natural sea water, 250 ml distilled water, 0.5 g yeast extract, 2 g peptone per liter; autoclaved, 20 ml Hepes added and pH adjusted to 7.7–7.8) at room temperature. S. marismortui produced spores in about 2 weeks. Sporulation was verified by phase-contrast microscopy. The spore count was in the range of 5 × 108 spores/ml. After sporulation, the spores were cleaned with double-distilled water rinses (five times) and centrifugation (14,000 rpm for 5 min each). B. subtilis and B. thuringiensis were assayed on LB (Luria-Bertani) plates (10 g Bacto-tryptone, 5 g yeast extract, 5 g NaCl, 1 ml 1 M NaOH, 16 g agar per liter), S. marismortui was assayed on K-medium plates (same as liquid K medium, plus 15 g agar), all at 30°C. B. thuringiensis showed colonies after 7–10 h, B. subtilis after about 1 day, and S. marismortui after about 3 days.
Spore Irradiation
The spores were not stored but were used immediately for the different kinds of radiation experiments. For the radiation experiments with liquid samples (B. subtilis and S. marismortui), the spores were suspended in 1 ml double-distilled water with the pH adjusted to either 7 or 4. Aliquots of 200 μl were placed in thin-walled glass vials (inner diameter 4 mm, wall thickness 1 mm). For the experiments with dry samples (B. subtilis and B. thuringiensis), the spores were suspended in 1 ml of double-distilled water. Aliquots of 200 μl were placed in glass vials and dried down at room temperature and 1.3 kPa pressure. The glass vials with the liquid and dry samples were flushed with nitrogen overnight and flame-sealed under constant nitrogen flow.
The samples were irradiated with γ rays from a 137Cs source at a dose rate of 13.3 Gy/min to a total dose from 1–8 kGy at the Radiation Medicine Resource of the UCSD Cancer Center. At least three samples of each bacterial strain were exposed to each radiation dose (1, 2, 4, 6, 8 kGy). All experiments were performed twice, so that each data point consists of six individual measurements.
Irradiated spores and nonirradiated standards were diluted in 10 ml 1xT-Base. A dilution series from 2 × 10−2 to 1 × 10−6 covered the surviving fractions for all bacterial strains. The numbers of surviving spores were assayed after plating and growth for 1 day on LB plates (B. subtilis and B. thuringiensis) and for 3 days on K-medium plates (S. marismortui).
RESULTS
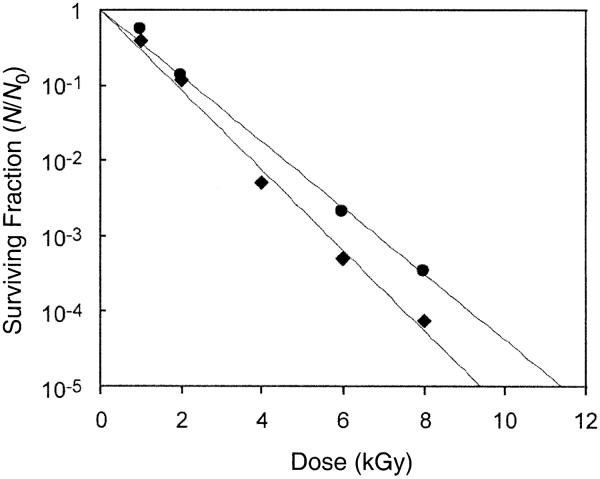
We measured the loss of colony-forming ability of spores irradiated with γ rays from a 137Cs source with a total dose between 1–8 kGy. Dose–survival curves for dry B. thuringiensis spores and S. marismortui spores in pH 4 solution are shown in Fig. 1. The curve characteristics are shown in Table 1. Regression curves are based on a first-order exponential response:
where N is the number of colonies after irradiation, N0 is the number of colonies prior to irradiation, k is the inactivation constant (kGy−1), D is the radiation dose (kGy), and n is the extrapolation number.
FIG. 1.
Dose–survival curves for dry B. thuringiensis spores (●) and S. marismortui spores (◆) in a pH 4 solution.
TABLE 1.
Radiation Inactivation Constants for B. subtilis, S. marismortui and B. thuringiensis Spores
| Radiation inactivation constant |
B. subtilis
|
S. marismortui
|
|||
|---|---|---|---|---|---|
| Wet pH 7 |
Dry | Wet pH 7 |
Wet pH 4 |
B. thuringiensis
Dry |
|
| k (kGy−1) | 1.22 ± 0.04 | 1.01 ± 0.03 | 1.23 ± 0.02 | 1.14 ± 0.03 | 1.00 ± 0.01 |
The results show that the largest difference in radiation inactivation constants is between dry and wet samples. The ratio of the inactivation constants of liquid and dry spores (kliquid/kdry) of B. subtilis strain PY79 is 1.21. Differences between B. subtilis and S. marismortui spores in a pH 7 solution are within the statistical error. The same is true for differences in the inactivation constant of dry B. subtilis and B. thuringiensis spores. Noteworthy is the reduced radiation sensitivity at low pH. A comparison of S. marismortui in solutions of pH 7 and pH 4 shows a ratio of inactivation constants of 1.08 (kpH 7/kpH 4).
To put the radiation inactivation constant of spores into a geochemical context, we calculated the effect of natural radiation on the viability of spores in halite fluid inclusions and on Mars.
Halite Fluid Inclusions
Halite fluid inclusions can be used to study the composition of sea or lake water through time (9). Essential for the interpretation of the chemical composition of fluid inclusions is an understanding of the sedimentary and diagenetic environment in which the evaporate evolved. In addition, any halite crystal that contains fluid inclusions has to show features of primary evaporates so that more recent exchange with ground water through dissolution and recrystallization processes can be excluded (10, 11). Only then will the age of the deposit be equal to the age of a fluid inclusion and everything it contains.
We investigated what effect ionizing radiation in a halite fluid inclusion would have on the viability of bacterial spores. The only radionuclide of significance in a halite fluid inclusion is 40K, which accounts for 0.0117% of natural potassium today. 40K has a half-life of 1.28 × 109 years and decays by β-particle emission to calcium (〈E〉 = 0.455 MeV, 89.3%) and by an electron capture to argon (Eγ = 1.46 MeV, 10.7%). The size of a 10-μl fluid inclusion was chosen because it is large enough to determine the chemistry of the brine and to extract samples for cultivation. About 83% of the β electrons are emitted in the energy range from 0.1 to 1.3 MeV. Fluid inclusions with a density of 1.25 g/cm3 (12), and a dimension of 10 μl, will absorb most of the β-particle decay energy. However, only about 1% of the γ-ray energy emitted from inside the fluid inclusion is actually absorbed in the inclusion. Gamma radiation from the surrounding crystal might contribute to the internal radiation dose, but the amount is low compared to the internal radiation dose from the β-particle decay because the abundance of potassium in the halite crystal is usually two orders of magnitude smaller than its abundance in the brine. Therefore, we did not include the energy from the emitted γ radiation in our calculations. Potassium concentration in halite fluid inclusions can vary significantly (12-14). We calculated the dose for a lower potassium concentration of 2.2 g/liter, and an average potassium concentration of 7 g/liter. The isotopic abundance of 40K changes over time, from 0.0117% today to 0.0134% 250 million years ago. Because of the wide range of different potassium concentrations in halite fluid inclusions, this isotopic change is of minor significance and will not be considered in the radiation dose estimates.
The dose rate is proportional to the concentration of the radionuclide, the decay rate and the energy release per decay. The specific activity of 40K is 2.0905 × 105 Bq/g, and the energy released from the β-particle decay only (89.3%), after correction for the energy loss in neutrino emissions, is 0.455 MeV per decay or 2.6787 × 1012 MeV/year per gram of 40K. A concentration of 2.2 g/liter potassium (0.257 ppm 40K, with an isotopic abundance of 0.0117%) gives a dose rate of 110 μGy/year. A concentration of 7 g/liter potassium (0.819 ppm 40K) gives a dose rate of 350 μGy/year. The accumulated dose over 250 million years is between 28 kGy and 88 kGy, depending on the potassium concentration.
The accumulated dose over time can be compared to the radiation sensitivity of bacterial spores. We measured the radiation inactivation constant for the halophilic S. marismortui spore in a deoxygenated solution (Table 1). Most of the halite fluid inclusions have a pH of 4–5 (15). Therefore, we used the inactivation constant of S. marismortui in a pH 4 solution (k = 1.14 kGy−1), which is slightly different from the inactivation constant of S. marismortui in a pH 7 solution (k = 1.23 kGy−1) (Table 1). Radiation inactivation constants of B. subtilis, B. pumilus and B. cereus in solution (mostly around pH 7) measured by other authors are in the range of 1.32–2.15 kGy−1 (16).
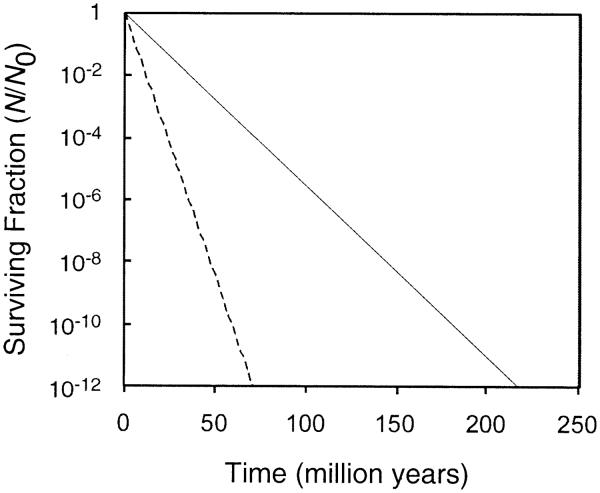
To calculate the loss of viability of bacterial spores in a fluid inclusion, we hypothetically put them in a 10-μl fluid inclusion. Assuming a number of 4000 CFU/ml in a brine (17) and an N/N0 = 10−6, the chances of finding viable spores is about 1 in 25,000 after 17–34 million years for 7 g/liter potassium and 55–109 million years for 2.2 g/liter potassium, depending on the radiation inactivation constant used (Fig. 2).
FIG. 2.
Calculated surviving fraction of S. marismortui spores in a pH 4 solution as a function of time in million years. Radiation dose is calculated for a low and average potassium concentration of 2.2 (—) and 7 (---) g/liter.
The situation of retrieving ancient viable bacterial spores in a halite fluid inclusion on Mars is similar to the terrestrial case. The abundance of potassium ions on Mars, based on analysis of the Martian meteorite Nakhla, is similar to that in terrestrial seawater (18). Hence it would be highly unlikely to find viable spores in fluid inclusions from an extinct early biosphere on Mars.
Mars
For centuries, scientists believed that Mars might be a planet almost like ours, habitable for life as we know it and occupied by an advanced civilization that struggled for their survival (19). However, in 1965, Mariner IV showed us a very different world (20). Mars today is a cold, dry desert planet, bathed in UV radiation and with an atmospheric pressure that is 1/100 that of the Earth. However, the climate on Mars is thought to have been more clement during the transition from the Noachian to the Hesperian epoch (21, 22), a transition period around 3.1 billion years ago (23). If life ever evolved on Mars, would it be possible to see the remnants of a long-extinct biosphere today? Or even more interesting, would it be possible to find Martian bacterial spores that survived for billions of years on Mars?
In the course of this study, we calculated the effect of the ionizing radiation environment on Mars on the survival of viable bacterial spores. There are two distinct radiation environments on Mars: The first few meters of the Martian subsurface is dominated by the effect of galactic cosmic radiation (GCR), while below about 3 m, the radiation environment is characterized by radiation from the radioactive isotopes 40K, 232Th, 235U and 238U.
We will address the radioactivity of the Martian subsurface below 3 m first. The abundance of potassium, thorium and uranium on Mars is about two orders of magnitude smaller than on Earth. This reflects the lower degree of differentiation of the Martian crust. Based on the SPB (Shergotti Parent Body) model, the abundance of potassium, thorium and uranium is 315 ppm, 5.6 × 10−2 ppm and 1.6 × 10−2 ppm, respectively (24). The isotopic abundance today is 0.0117% for 40K, 100% for 232Th, 0.7200% for 235U, and 99.2745% for 238U. The decay energy corrected for neutrino loss is 0.71 MeV for 40K, 39.9 MeV for 232Th, 47.1 MeV for 238U, and 44.2 MeV for 235U (25).
The dose rate from internal radioactivity can be calculated from the concentration of each radionuclide and its decay parameters:
| (1) |
where D is the dose rate, c is the concentration of the radionuclide, E is the energy per decay corrected for neutrino loss in β-particle decay; λ is the decay constant, 6.022 × 1023 Avogadro’s constant in mol−1, A is atomic mass, and the conversion factor for 1 Gy is 6.24181 × 1015 eV/g.
The calculated dose rate using Eq. (1) from the decay of radionuclides ranges from 350 μGy/year at 3.1 billion years ago to 130 μGy/year today. The total accumulated dose over the last 3.1 billion years in the Martian subsurface is close to 740 kGy.
The accumulated dose over time can be compared to the radiation sensitivity of bacterial spores. We measured the radiation inactivation constant of dry B. thuringiensis soil spores in a nitrogen atmosphere. The inactivation constant, of B. thuringiensis is 1.00 kGy−1 (Table 1). The radiation inactivation constant of dry B. subtilis and B. pumilus spores measured by other authors is in the range of 0.66–0.92 kGy−1 (26). Dry bacterial spores show a temperature dependence in their radiation sensitivity, following a vant’Hoff-Arrhenius law above 130 K (27). The temperature-corrected inactivation constant for a Martian subsurface temperature of 220 K is 0.59–0.90 kGy−1 for the referenced and measured dry bacterial spores.
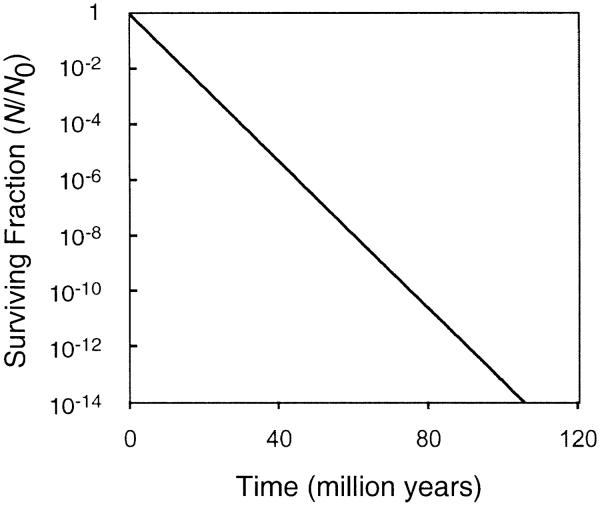
To calculate the loss of viability of bacterial spores, we hypothetically put them in the Martian subsurface 3.1 billion years ago (Fig. 3). The chances of finding viable bacterial spores in a 1-g sample of Martian soil is less than 1 in a million after 100–160 million years, depending on the radiation inactivation constant used, assuming an optimistic original bacterial concentration of 1 × 108/g of soil (28) and a reduction of the population by 14 orders of magnitude (D14).
FIG. 3.
Calculated surviving fraction of dry bacterial spores in the Martian subsurface as a function of time in million years. The starting point is 3.1 billion years ago. Variations in the radiation inactivation constant have no strong impact on the survival time.
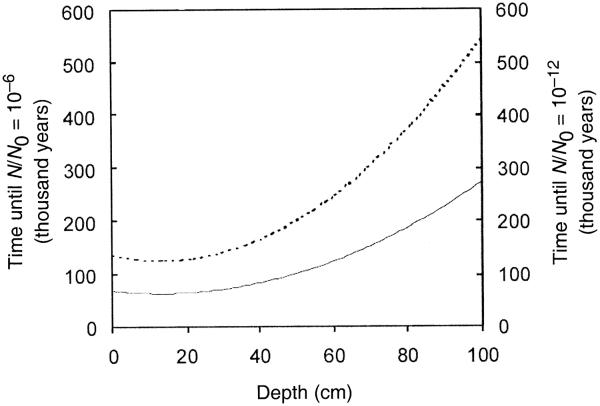
The probability is even lower when considering the Martian subsurface that is accessible to in situ landers (with present technology <3 m). The oldest Noachian terrain on Mars had an extremely low erosion rate during the subsequent Hesperian and Amazonian periods of just several 10−2 to 10−1 nm/year (29). At the same time, about 95% of all channel features on Mars are located on the old Noachian terrain (30). Thus over 3.1 billion years, or since the time that Mars had a more clement period, only several centimeters of material has been eroded away in Noachian net deflation areas. These areas represent the best location to access samples from this early time. Unfortunately, the first couple of meters of the Noachian terrain have been exposed to the GCR for the same period. The GCR field on Mars is derived from results of theoretical calculations describing the radiation attenuation of GCR behind Martian regolith (31). The model was generated by solving the Boltzmann equation using a HZETRN system with the 1977 Solar Minimum environmental model (31). With a density of 2.6 g/cm3 (32), the depth-dependent dose rate ranges from 0.19 Gy/year at the surface to 600 μGy/year at a depth of 3 m. The accumulated dose over 3.1 billion years within the first meter is of the order of 200–800 MGy. The probability of finding viable bacterial spores in a 1-g sample of Martian soil within the first meter is ≤1 in a million within 600,000 years using an average radiation inactivation constant for dry bacterial spores (Fig. 4).
FIG. 4.
The graph shows the calculated depth dependence of the time (in thousand years) until the viability of dry bacterial spores is reduced by a factor of 106 (—) or 1012 (---) by GCR on Mars. Variations in the radiation inactivation constant have no strong impact on the survival time.
DISCUSSION
It might be argued that the much higher dose rate used for the present experiments is not a good model for the long-term exposure at very low dose rates. To evaluate the validity of our experiment, it is necessary to briefly discuss the effect of water content and oxygen on the radiation damage of spores. There are three classes of damage to bacterial spores from ionizing radiation (33, 34): Class I damage is the direct damage to spores by short-lived radicals in the absence of oxygen, class II is the damage with short-lived radicals in the presence of oxygen during irradiation, and class III is the damage by long-lived radicals in the presence of oxygen after irradiation. Liquid water completely anneals class III damage so that any spores in anoxic solution will only show class I damage. In that sense, we suggest that our experiment can indeed simulate the long-term radiation exposure of spores in anoxic solutions. Any oxygen present in a real fluid inclusion would only increase the inactivation constant and shorten the survival time for viable spores (35). The situation is different in dry spores. Irradiated dry spores develop class I and III damages when they are exposed to oxygen after irradiation (33). In our experimental procedure, we measure the inactivation constant of class I and III damage. However, thermorestoration can prevent the latent oxygen effect by annealing the long-lived radicals, reducing the inactivation constant (36). This thermorestoration requires temperatures of 50°C or higher for maximal restoration. At lower temperatures and longer anaerobic storage times, the efficiency of this process is strongly reduced. In addition, the thermorestoration constant is strongly dependent on temperature. Thus we assume no substantial restoration effect at Martian subsurface temperatures of 220 K and below. Therefore, we suggest that we can simulate the long-term radiation exposure of spores in the anoxic dry state. Since the spores are dormant and damage will build up at both dose rates with no interaction with any enzymatic repair processes, no dose-rate effect should occur.
One obvious question is how and to what extent the radiochemistry of a brine could affect our interpretation. Radiolysis of deoxygenated pure water results in a 10−8–10−7 mol/liter steady-state concentration of hydrogen, hydrogen peroxide and oxygen (37), while oxygenated pure water shows a buildup of hydrogen peroxide and hydrogen in the 10−5 mol/liter range and a depletion of oxygen (37). Radiolysis of sea water leads to numerous radical reactions but is dominated by the radiation chemical reactivity of the chloride ion (38, 39). The cytotoxicity of irradiated sea water is most likely derived from hydrogen peroxide and hypochlorite (40-42). Bacterial spores are protected from the detrimental effect of hydrogen peroxide at low concentrations by a thick spore coat (43). The sporicidal effect of hypochlorite on bacterial spores, however, is much more pronounced (42). To avoid the sporicidal effect of hypochlorite, which is strongly dependent on concentration, we used pure water in our experiments. Deoxygenated pure water also limits the contribution of hydrogen peroxide (low concentration for a short time) in spore inactivation. Both cytotoxins will be present after irradiation of brine inclusions—hypochlorite from the chloride in the brine for a very short time and hydrogen peroxide from water hydrolysis at a low steady-state concentration. Therefore, the time we calculated to inactivate bacterial spores in a halite fluid inclusion can only be seen as an upper limit, ignoring any sporicidal effect of the irradiated medium.
Our results do not totally exclude the survival of ancient bacterial spores in halite fluid inclusions. They are valid to the extent that bacterial spores have indeed been dormant and unable to repair any damage in this state. However, it does set limits on the long-term viability of bacterial spores based on the chemistry of the fluid inclusion, which, if the inclusion is a primary feature, should have remained constant over time.
Several studies investigated the survival of bacterial spores under simulated Martian conditions (e.g. 44, 45). The conclusion of these studies is that bacterial spores show significant survival as long as they are protected from the incident UV radiation. However, no consistent upper limit for the long-term survival is given. Our results show that even in the absence of any other chemical or physical degradation process, ionizing radiation severely limits the long-term survival of viable bacterial spores in the Martian subsurface. If any cycle on Mars allows dormant life forms to awake from their dormant state, repair the accumulated damage, and multiply, then the period of this cycle must be shorter than the time until the viability of spores falls below a certain value. In that case, radiation will not limit the long-term viability of dormant life forms other than setting the upper limit for the time between dormant/active cycles. This period has to be shorter than about 100 to 160 million years for the deeper subsurface and less than about 600,000 years for the uppermost few meters, assuming a D14 until recovery of dormant spores. The only established quasi periodic changes on a global scale are the obliquity oscillations with a period of 120,000 years and an amplitude modulation of 2 million years (46, 47). The obliquity is the angle between the spin axis and the normal to the orbit plane. On Earth, the presence of the Moon has stabilized the rotation axis. Mars does not have a large moon, and so the obliquity can change from 13° to 42° (47). This strongly affects the climate on Mars. The thermal wave of these obliquity changes can penetrate several hundreds of meters into the subsurface and, depending on obliquity and on the latitude, can either lower or raise the temperature in the Martian subsurface (48). However, the peak temperatures where permafrost is stable close to the surface (>60° latitude) are still limited to those below 200 K. Although there are cryptoendolithic communities that can thrive at temperatures below freezing, this ability depends on a nanometer-thin layer of liquid water around them (49). The thickness of this water layer goes down steadily with decreasing temperature. It might be possible to have some extremely slow metabolic activity at temperatures of about 250 K, which, however, is still 30 to 50 K higher than the Martian subsurface temperature. Thus, although the period of obliquity changes are within the limit for D14, it is questionable whether they could change the environmental conditions enough to allow spores to germinate. Another possible source of periodic changes in the Martian environment would be local volcanic activity. Unlike Earth, hot spots on Mars are likely to be confined to the same locations for long periods (50). If such activity occurred throughout the history of Mars, it would allow very local ecological niches in the Martian subsurface. There are indications for recent (order of 100 million years) volcanism on Mars (51).
However, even if viable bacterial spores cannot be found on Mars, it should still be possible to detect their molecular remains. Amino acids would be stable enough to still give a detectable signal assuming about 108 cells/g of soil 3.1 billion years ago. For a detection limit of parts per billion at present, the search would have to drill to a depth of at least 1 m into the Martian subsurface. At shallower depth, amino acids are destroyed by the GCR over 3.1 billion years (Kminek, unpublished data).
Are there other models than bacterial spores that show similar long-term survival capabilities? The extremophile Deinococcus radiodurans might be a potential candidate. D. radiodurans has been described as a polyextremophile model organism for long-term survival on Mars (52). Based on its radiation resistance alone (53), dormant D. radiodurans could survive at least as long in the Martian subsurface as bacterial spores. However, because D. radiodurans does not form spores, it might be more prone to other physical and chemical effects. Although D. radiodurans is resistant to desiccation, a 6-log reduction in viability is expected after only 3–4 years, and the survival is even lower if alternating cycles of desiccation and partial rehydration are present (8). Bacterial spores show a better resistance to desiccation (54). Potential chemical agents are peroxides in the Martian regolith. There are indications from Viking measurements and theoretical models that at least the surface layer of Mars contains a variety of peroxides (55, 56). While spore-forming bacteria are well protected from peroxides by their spore coat (43), D. radiodurans is not. Therefore, bacterial spores are probably still a better model for the long-term survival of life forms on Mars.
CONCLUSION
In the absence of any possibility to repair damage over time, we conclude that the long-term survival of viable bacterial spores is limited by ionizing radiation from the environment. For halite fluid inclusions, the upper limit is less than 109 million years, depending on the potassium concentration. From a radiobiological point of view, it is very unlikely that a 250 million-year-old viable bacterial spore could be isolated from a halite fluid inclusion.
The long-term survival of viable bacterial spores on Mars is limited to less than 100 to 160 million years in the Martian subsurface deeper than 3 m and to less than 600,000 years within the uppermost meter of Mars. It is highly unlikely to find viable bacterial spores from an early Martian biosphere in the absence of frequent revivals of dormant spores through environmental changes.
ACKNOWLEDGMENTS
We thank Joseph Aguilera for his help at the Radiation Medicine Resource Center and Marc Sharp and Brian Clement for their help with the spore lines. This research was supported by the NASA Specialized Center of Research and Training in Exobiology (NSCORT) at the Scripps Institution of Oceanography, University of California at San Diego.
REFERENCES
- 1.Setlow P. I will survive: Protecting and repairing spore DNA. J. Bacteriol. 1992;174:2737–2741. doi: 10.1128/jb.174.9.2737-2741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vreeland RH, Rosenzweig WD, Powers DW. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature. 2000;407:897–900. doi: 10.1038/35038060. [DOI] [PubMed] [Google Scholar]
- 3.Landis GA. Martian water: Are there extant halobacteria on Mars? Astrobiology. 2001;1:161–164. doi: 10.1089/153110701753198927. [DOI] [PubMed] [Google Scholar]
- 4.Arahal DR, Márquez MC, Volcani BE, Schleifer KH, Ventosa A. Bacillus marismortui sp. nov., a new moderately halophilic species from the Dead Sea. Int. J. Syst. Bacteriol. 1999;49:521–530. doi: 10.1099/00207713-49-2-521. [DOI] [PubMed] [Google Scholar]
- 5.Arahal DR, Márquez MC, Volcani BE, Schleifer KH, Ventosa A. Reclassification of Bacillus marismortui as Salibacillus marismortui. Int. J. Syst. Evol. Microbiol. 2000;50:1501–1503. doi: 10.1099/00207713-50-4-1501. [DOI] [PubMed] [Google Scholar]
- 6.Nickle DC, Learn GH, Rain MW, Mullins JI, Mittler JE. Curiously modern DNA for a “250 million-year-old” bacterium. J. Mol. Evol. 2002;54:134–137. doi: 10.1007/s00239-001-0025-x. [DOI] [PubMed] [Google Scholar]
- 7.Helgason E, Økstad OA, Caugant DA, Johansen HA, Fouet A, Mock M, Hegna I, Kolstø AB. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—One species on the basis of genetic evidence. Appl. Environ. Microbiol. 2000;66:2627–2630. doi: 10.1128/aem.66.6.2627-2630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattimore V, Battista JR. Radioresistance of Deinococcus radiodurans: Functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 1996;178:633–637. doi: 10.1128/jb.178.3.633-637.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowenstein TK, Timefeeff MN, Brennan ST, Hardie LA, Demicco RV. Oscillations in phanerozoic seawater chemistry: Evidence from fluid inclusion. Science. 2001;294:1086–1088. doi: 10.1126/science.1064280. [DOI] [PubMed] [Google Scholar]
- 10.Bein A, Hovorka SD, Fisher RS, Roedder E. Fluid inclusion in bedded permian halite, Palo Duro Basin, Texas: Evidence for modification of seawater in evaporate brine-pools and subsequent early diagenesis. J. Sediment. Petrol. 1991;61:1–14. [Google Scholar]
- 11.Hazen RM, Roedder E. How old are bacteria from the Permian age? Nature. 2001;411:155. doi: 10.1038/35075663. [DOI] [PubMed] [Google Scholar]
- 12.Horita J, Weinberg A, Das N, Holland HD. Brine inclusions in halite and the origin of the middle Devonian prairie evaporates of Western Canada. J. Sediment. Res. 1996;66:956–964. [Google Scholar]
- 13.Bukowski K, Galamay AR, Goralski M. Inclusion brine chemistry of the Badenian salt from Wieliczka. J. Geochem. Expl. 2000;69–70:87–90. [Google Scholar]
- 14.Khmelevska O, Kovalevych V, Peryt TM. Changes of seawater composition in the Triassic-Jurassic time as recorded by fluid inclusions in halite. J. Geochem. Expl. 2000;69–70:83–86. [Google Scholar]
- 15.Roedder E. The fluids in salt. Am. Min. 1984;69:413–439. [Google Scholar]
- 16.Borick PM, Fogarty MG. Effects of continuous radiation on microorganisms. Appl. Microbiol. 1967;15:785–789. doi: 10.1128/am.15.4.785-789.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vreeland RH, Piselli AF, Jr., McDonnough S, Meyers SS. Distribution and diversity of halophilic bacteria in a subsurface salt formation. Extremophiles. 1998;2:321–331. doi: 10.1007/s007920050075. [DOI] [PubMed] [Google Scholar]
- 18.Sawyer DJ, McGehee MD, Canepa J, Moore CB. Water soluble ions in the Nakhla Martian meteorite. Meteorit. Planet. Sci. 2000;35:743–747. [Google Scholar]
- 19.Lowell P. Mars. Houghton Mifflin; Boston: 1895. [Google Scholar]
- 20.Leighton RB, Murray BC, Sharp RP, Allen JD, Sloan RK. Mariner IV photography of Mars: Initial results. Science. 1965;149:627–630. doi: 10.1126/science.149.3684.627. [DOI] [PubMed] [Google Scholar]
- 21.Pollack JB, Kasting JF, Richardson SM, Poliakoff K. The case for a wet, warm climate on early Mars. Icarus. 1987;71:203–224. doi: 10.1016/0019-1035(87)90147-3. [DOI] [PubMed] [Google Scholar]
- 22.Squyres SW, Kasting JF. Early Mars: How warm and how wet? Science. 1994;265:744–749. doi: 10.1126/science.265.5173.744. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann WK, Neukum G. Cratering chronology and the evolution of Mars. Space Sci. Rev. 2001;96:165–194. [Google Scholar]
- 24.Dreibus G, Wänke H. Volatiles on Earth and Mars: A comparison. Icarus. 1987;71:225–240. [Google Scholar]
- 25.Urey HC. The cosmic abundance of potassium, uranium, and thorium and the heat balances of the Earth, the Moon, and Mars. Proc. Natl. Acad. Sci. USA. 1955;41:127–144. doi: 10.1073/pnas.41.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen EA, Holm NW. Inactivation of dried bacteria and bacterial spores by means of ionizing radiation. Ada Pathol. Microbiol. Scand. 1964;60:253–264. doi: 10.1111/apm.1964.60.2.253. [DOI] [PubMed] [Google Scholar]
- 27.Webb RB, Ehret CF, Powers EL. A study of the temperature dependence of radiation sensitivity of dry spores of bacillus mega-terium between 5°K and 309°K. Experientia. 1958;14:324–326. doi: 10.1007/BF02160387. [DOI] [PubMed] [Google Scholar]
- 28.Gilichinsky DA. Permafrost microbiology. Permafr. Periglac. Process. 1995;6:281–291. [Google Scholar]
- 29.Golombek MP, Bridges NT. Erosion rates on Mars and implications for climate change: Constraints from pathfinder landing site. J. Geophys. Res. 2000;105:1841–1853. [Google Scholar]
- 30.Carr MH, Clow GD. Martian channels and valleys: Their characteristics, distribution, and age. Icarus. 1981;48:91–117. [Google Scholar]
- 31.Mileikowsky C, Cucinotta FA, Wilson JW, Gladman B, Horneck G, Lindgren L, Melosh J, Rickman H, Valtonen M, Zheng JQ. Natural transfer of viable microbes in space. Icarus. 2000;145:391–427. doi: 10.1006/icar.1999.6317. [DOI] [PubMed] [Google Scholar]
- 32.Moore HJ, Jakosky BM. Viking landing sites, remote-sensing observations, and physical properties of Martian surface materials. Icarus. 1989;81:164–184. [Google Scholar]
- 33.Powers EL, Webb RB, Ehret CF. Storage, transfer, and utilization of energy from X-rays in dry bacterial spores. Radiat. Res. 1960;(Suppl. 2):94–121. [PubMed] [Google Scholar]
- 34.Powers EL, Tallentire A. The roles of water in the cellular effects of ionizing radiations. Act. Chim. Biol. Radiat. 1968;12:3–27. [Google Scholar]
- 35.Tallentire A, Powers EL. Modification of sensitivity to X-irradiation by water in bacillus megaterium. Radiat. Res. 1963;20:270–287. [PubMed] [Google Scholar]
- 36.Webb RB, Powers EL, Ehret CF. Thermorestoration of radiation damage in dry bacterial spores. Radiat. Res. 1960;12:682–693. [PubMed] [Google Scholar]
- 37.Bjergbakke E, Draganic ZD, Sehested K, Draganic IG. Radiolytic products in waters: Part I: Computer simulation of some radiolytic processes in the laboratory. Radiochim. Acta. 1989;48:65–71. [Google Scholar]
- 38.Bjergbakke E, Draganic ZD, Sehested K, Draganic IG. Radiolytic products in waters: Part II: Computer simulation of some radiolytic processes in nature. Radiochim. Acta. 1989;48:73–77. [Google Scholar]
- 39.Draganic IG, Bjergbakke E, Draganic ZD, Sehested K. Decomposition of ocean waters by potassium-40 radiation 3800 Ma ago as a source of oxygen and oxidizing species. Precambrian Res. 1991;52:337–345. [Google Scholar]
- 40.Saran M, Bertram H, Bors W, Czapski G. On the cytotoxicity of irradiated media. To what extent are stable products of radical chain reactions in physiological saline responsible for cell death? Int. J. Radiat. Biol. 1993;64:311–318. doi: 10.1080/09553009314551461. [DOI] [PubMed] [Google Scholar]
- 41.Saran M, Bors W. Radiation chemistry of physiological saline reinvestigated: Evidence that chloride-derived intermediates play a key role in cytotoxicity. Radiat. Res. 1997;147:70–77. [PubMed] [Google Scholar]
- 42.Sagripanti JL, Bonifacino A. Comparative sporicidal effects of liquid chemical agents. Appl. Environ. Microbiol. 1996;62:545–551. doi: 10.1128/aem.62.2.545-551.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riesenman PJ, Nicholson WL. Role of the spore coat layers in bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl. Environ. Microbiol. 2000;66:620–626. doi: 10.1128/aem.66.2.620-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koike J, Oshima T, Kobayashi K, Kawasaki Y. Studies in the search for life on Mars. Adv. Space Res. 1995;3:211–214. doi: 10.1016/s0273-1177(99)80086-6. [DOI] [PubMed] [Google Scholar]
- 45.Hagen CA, Hawrylewicz EJ, Ehrlich R. Survival of microorganisms in a simulated Martian environment. Appl. Microbiol. 1964;12:215–218. doi: 10.1128/am.12.3.215-218.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laskar J, Robutel P. The chaotic obliquity of the planets. Nature. 1993;362:608–612. [Google Scholar]
- 47.Ward WR. Long term orbital and spin dynamics of Mars. In: Kieffer HH, Jakosky BM, Snyder C, Matthews MS, editors. Mars. University of Arizona Press; Tucson: 1992. pp. 298–320. [Google Scholar]
- 48.Fanale FP, Salvail JR, Banerdt WB, Saunders RS. The regolith-atmosphere-cap-system and climate change. Icarus. 1982;50:381–407. [Google Scholar]
- 49.Rivkina EM, Friedmann EI, McKay CP, Gilichinsky DA. Metabolic activity of permafrost bacteria below the freezing point. Appl. Environm. Microbiol. 2000;66:3230–3222. doi: 10.1128/aem.66.8.3230-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McSween HY., Jr. What we learned about Mars from SNC meteorites. Meteoritics. 1994;29:757–779. [Google Scholar]
- 51.Hartmann WK, Malin MC, McEwen A, Carr M, Soderblom L, Thomas P, Danielson E, James P, Veverka J. Recent volcanism on Mars from crater counts. Nature. 1999;397:586–589. [Google Scholar]
- 52.Richmond RC, Sridhar R, Zhou Y, Daly MJ. Physico-chemical survival pattern for the radiophile D. radiodurans: A polyextremophile model for life on Mars. Int. Soc. Opt. Engin. Conf. Proc. Instrum. Methods Missions Astrobiol. II. 1999;3755:210–222. [Google Scholar]
- 53.Battista JR. Against all odds: The survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 1997;51:203–224. doi: 10.1146/annurev.micro.51.1.203. [DOI] [PubMed] [Google Scholar]
- 54.Marshall BJ, Murrell WG, Scott WJ. The effect of water activity, solutes and temperature on the viability and heat resistance of freeze-dried bacterial spores. J. Gen. Microbiol. 1963;31:451–460. [Google Scholar]
- 55.Klein HP. The Viking mission and the search for life on Mars. Rev. Geophys. Space Phys. 1979;17:1655–1662. [Google Scholar]
- 56.Bullock MA, Stocker CR, McKay CP, Zent AP. A coupled soil-atmosphere model of H2O2 on Mars. Icarus. 1994;107:142–154. doi: 10.1006/icar.1994.1012. [DOI] [PubMed] [Google Scholar]