Summary
The muscle-specific long noncoding RNA linc-MD1 was shown to be expressed during early phases of muscle differentiation and to trigger the switch to later stages by acting as a sponge for miR-133 and miR-135. Notably, linc-MD1 is also the host transcript of miR-133b, and their biogenesis is mutually exclusive. Here, we describe that this alternative synthesis is controlled by the HuR protein, which favors linc-MD1 accumulation through its ability to bind linc-MD1 and repress Drosha cleavage. We show that HuR is under the repressive control of miR-133 and that the sponging activity of linc-MD1 consolidates HuR expression in a feedforward positive loop. Finally, we show that HuR also acts in the cytoplasm, reinforcing linc-MD1 sponge activity by cooperating for miRNA recruitment. An increase in miR-133 synthesis, mainly from the two unrelated miR-133a coding genomic loci, is likely to trigger the exit from this circuitry and progression to later differentiation stages.
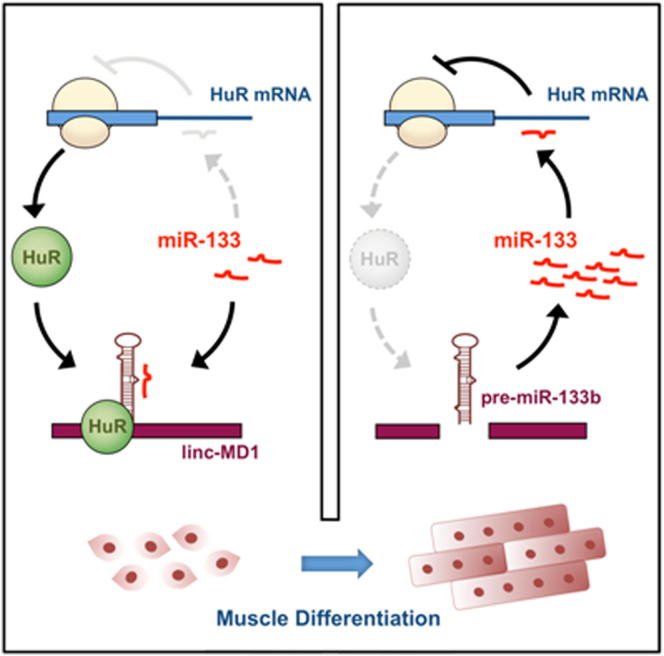
Graphical Abstract
Highlights
-
•
A feedforward positive loop exists between linc-MD1 and HuR during myogenesis
-
•
HuR controls the relative biogenesis of miR-133b and its host linc-MD1 RNA
-
•
Linc-MD1, by sponging miR-133, alleviates its repression on HuR expression
-
•
Cytoplasmic HuR reinforces linc-MD1 activity by cooperating for miRNA recruitment
linc-MD1 and miR-133 are alternatively processed from the same precursor RNA. These RNAs play opposing roles in early phases of myogenesis. Legnini et al. now show that the balance between the RNAs is regulated by HuR, which inhibits generation of miR-133 by inhibiting microprocessor activity on the precursor RNA.
Introduction
Our knowledge about the complexity of posttranscriptional control of gene expression is continuously expanding due to findings of new effectors of mRNA splicing, stability, and translation, as well as the discovery of the large extent of posttranscriptional regulation of noncoding RNAs. Several examples of nuclear and cytoplasmic control of miRNA maturation have been described (Guil and Cáceres, 2007, Zisoulis et al., 2012, Choudhury et al., 2013). More recently, with the discovery of a huge number of long noncoding RNAs (lncRNAs), the repertoire of mechanisms that control RNA biogenesis is expected to expand even further.
lncRNAs have been implicated in a large number of transcription regulatory processes (Rinn and Chang, 2012). Moreover, they have been shown to take part in posttranscriptional events such as stability and translational control of mRNAs (Gong and Maquat, 2011, Wang et al., 2013a, Yoon et al., 2012), as well as sponging activity for miRNAs (Poliseno et al., 2010, Cesana et al., 2011, Wang et al., 2013b, Kallen et al., 2013). The latter activity suggests that coding and noncoding RNAs may be part of common regulatory circuitries in which they can control one another through their ability to compete for miRNA binding, suggesting the term “competing endogenous RNA” (ceRNA; Salmena et al., 2011).
Linc-MD1 was the first lncRNA to be shown to play a relevant role in muscle differentiation by regulating specific myogenic factors required for the onset of late muscle gene transcription. In the cytoplasm, linc-MD1 was shown to act as a ceRNA for miR-135 and miR-133 (Cesana et al., 2011). By this mode of action, linc-MD1 impacts the distribution of these miRNAs on their natural mRNA targets, imposing an additional level of posttranscriptional control. Maml1 and Mef2C, two relevant factors in activating late muscle gene expression, were identified as natural targets of miR-133 and miR-135, which in turn are controlled by the “sponge” activity of linc-MD1. Given this tripartite regulatory network (mRNA, miRNA, and lncRNA), it is expected that a number of different factors that share target sites for common miRNAs could belong to the same circuitry.
Another interesting aspect of linc-MD1 relates to its biogenesis. Since it harbors the pri-miR-133b sequence, it can act alternatively as a miRNA precursor, if Drosha cleaves it in the nucleus, or as a sponge for the encoded miR-133b, if it is exported to the cytoplasm as an unprocessed species. Therefore, a major point related to how the alternative fate of the linc-MD1 primary transcripts is controlled awaited clarification.
With the aim of identifying members of the linc-MD1-regulated network, we found an additional component of this circuitry, the HuR protein, which is already known for its crucial role in myogenesis (von Roretz et al., 2011).
This protein, which is known to interact with a large number of RNA substrates (Lebedeva et al., 2011, Mukherjee et al., 2011), was initially described as an adaptor for mRNA export (Gallouzi and Steitz, 2001) and subsequently was reported to have a stabilizing effect on several important myogenic factors, such as MyoD, myogenin, and p21 (Figueroa et al., 2003). Over the past few years, evidence has also accumulated regarding its cooperative (Kim et al., 2009) and competitive (Kundu et al., 2012) interplay with miRNAs in controlling translation and stability of specific mRNAs. More recently, other contributions pointed to the role of HuR in controlling the processing and stability of pri-miRNAs (Choudhury et al., 2013) and lncRNAs (Yoon et al., 2012).
In this paper, we describe that HuR is negatively controlled by miR-133, and that linc-MD1, through miR-133 sponging, positively controls HuR levels during early stages of muscle differentiation. In turn, HuR controls linc-MD1 by favoring its accumulation in the cytoplasm at the expense of miR-133b synthesis. Finally, we show that HuR is also required in the cytoplasm to ensure efficient lincMD1 sponge activity by facilitating miRNA recruitment. Altogether, these data show the existence of a specific circuitry in which the timely controlled expression of linc-MD1 and HuR is reciprocally regulated in order to establish correct progression of in vitro muscle differentiation.
Results
HuR Expression Is Sustained by the miR-133 Decoy Activity of linc-MD1
In order to expand the collection of possible linc-MD1-ceRNA interactions, we performed in silico analysis of miR-133 and miR-135 target predictions and searched for putative targets involved in muscle differentiation. Putative targets were predicted using the miRanda algorithm (Enright et al., 2003). The list of all putative target mRNAs was then filtered for expression in skeletal muscle according to the Gene Expression Barcode data set (McCall et al., 2011). Among the top 20 predictions of each miRNA (Table S1 available online), the miR-133 putative target HuR was selected for further validation due to its well-described role in muscle differentiation (von Roretz et al., 2011; see Figure S1A). The HuR locus is known to give rise to different transcripts due to the usage of alternative polyadenylation sites (Al-Ahmadi et al., 2009). Therefore, before validating it as a miR-133 target, we checked the isoforms expressed in the murine myogenic C2C12 cell line by using RNA sequencing (RNA-seq) data (V. Cazzella, personal communication). We found that the major species contains one putative miR-133 responsive element (133-MRE), whereas a second, less abundant species, with an extended 3′ UTR, contains two 133-MREs.
Luciferase constructs carrying the 3′ UTR sequence of HuR corresponding to the short and long isoforms (short and long Luc-HuR wild-type [Luc-HuRWT]; Figure 1A), together with derivatives depleted of the miR-133 sites (short and long Luc-HuRmut), were used to assess the responsiveness to the miR-133/linc-MD1 circuitry. Figure 1B shows that in C2C12 cells, locked nucleic acid (LNA) oligos against miR-133 derepress the luciferase activity of both the short and long Luc-HuRWT constructs. In line with this, the luciferase levels of the Luc-HuRWT constructs increased when linc-MD1, which is known to sponge miR-133, was cotransfected (Figure 1C, pMD1). This effect was stronger with the long Luc-HuRWT, which has two 133-MREs. No derepression of luciferase activity was observed when a linc-MD1 construct depleted of the miR-133 binding site was cotransfected (Figure 1C, pMD1Δ133). As control, the short and long Luc-HuRmut constructs did not show any sensitivity to linc-MD1.
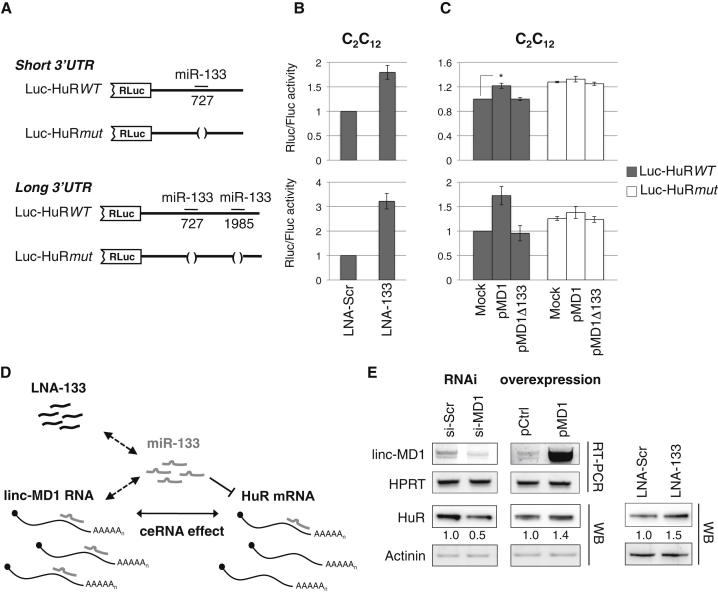
Figure 1.
linc-MD1 Regulates HuR Expression by Sponging miR-133
(A) The short and long 3′ UTRs of HuR mRNA containing one or two miR-133 MREs were cloned downstream of the Renilla luciferase coding region (Luc-HuRWT) in parallel with mutant derivatives the lacking MREs (Luc-HuRmut). The position of the MREs is indicated.
(B) Luciferase activity of short (upper panel) and long (lower panel) Luc-HuRWT transfected in C2C12 cells in the presence of control (LNA-Scr) or anti-miR-133 (LNA-133) LNA oligonucleotides.
(C) Luciferase activity of short and long Luc-HuRWT and the mutant derivatives cotransfected with pMD1 or pMD1mut (corresponding to pMD1-ΔDrosha and pMD1-ΔDrosha-Δ133 described in Cesana et al., 2011) in C2C12 cells. The level of pMD1 and pMD1mut expression is shown in Figure S1C. Luciferase activity was tested 2 days after the shift to DM. FLuc/RLuc RQ is shown with respect to a control sample set to a value of one. Data were derived from at least three independent experiments, error bars represent SE, and asterisk corresponds to two-tailed Student’s t test p < 0.05.
(D) Schematic representation of the ceRNA circuitry linking linc-MD1, HuR, and miR-133.
(E) RT-PCR (upper panels) and western (lower panels) analyses of linc-MD1 and HuR levels following linc-MD1 knockdown (RNAi – siMD1) or overexpression (pMD1) in C2C12 cells at 2 days of differentiation. Control siRNAs (si-Scr) and empty vector (pCtrl) were used as controls together with HPRT mRNA and Actinin. Right panel: western blot of HuR levels at 2-day treatment in differentiation conditions with control (LNA-Scr) and anti-miR-133 (LNA-133) LNA oligonucleotides. Below each panel, relative quantifications derived from at least three independent experiments are indicated with respect to control samples set to a value of one.
The same responsiveness to miR-133 was observed in nonmyogenic cells, such as HeLa cells in which miR-133 was produced from a transfected plasmid (Figure S1B).
In order to validate the crosstalk between HuR and linc-MD1 through competition for miR-133 (Figure 1D), we measured endogenous HuR levels upon RNAi or overexpression of linc-MD1 in C2C12 cells. Figure 1E shows that HuR decreased when linc-MD1 was downregulated, and increased when linc-MD1 was overexpressed. In C2C12 cells treated with anti-miR-133 LNA oligonucleotides, HuR increased similarly to cells overexpressing linc-MD1.
These results show that miR-133 controls HuR and that this repressing activity is modulated by linc-MD1, indicating that, similarly to Maml1 (Cesana et al., 2011), HuR also participates in the crosstalk with the lncRNA through competition for miR-133.
HuR Physically Interacts with linc-MD1
In order to analyze the interaction between linc-MD1 and the RNA-binding protein HuR, we performed RNA immunoprecipitation (RIP) assays with anti-HuR antibodies and isotype-matched immunoglobulin G (IgG). The IP specificity was tested by western blot (Figure S2A). Figure 2A shows that, similarly to MyoD mRNA (Figueroa et al., 2003), linc-MD1 coimmunoprecipitates with HuR. Notably, in the IP RNA fraction, miR-133 and miR-135 were also found (Figure S2B). The interaction of HuR with linc-MD1 appears to be evolutionarily conserved, since the human counterpart of linc-MD1, linc-hMD1 (Twayana et al., 2013), was also detected in HuR-IPs in human myoblasts (Figure 2A, lower panel). In line with its ability to bind miRNAs, linc-MD1 was also found in Ago2 immunoprecipitates (Figures 2B and S2C).
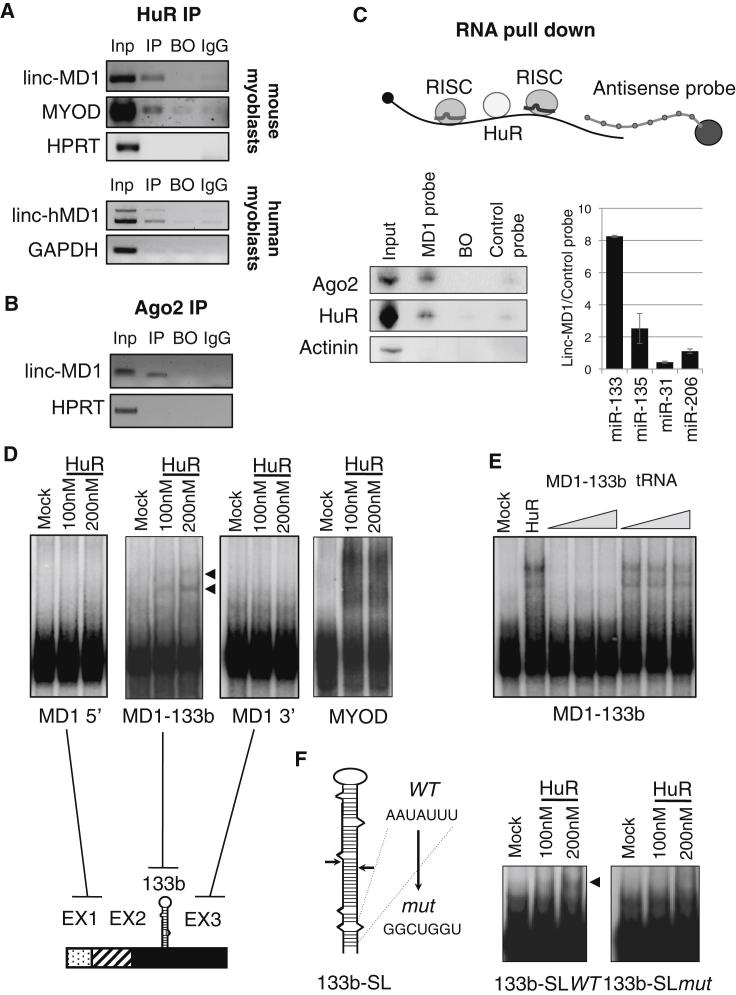
Figure 2.
HuR Associates with linc-MD1
(A) RIP was performed on cytoplasmic extracts from C2C12 cells and human myoblasts shifted to DM for 2 and 5 days, respectively. The extracts were incubated with anti-HuR (IP) or control antibodies (IgG). Beads-only were used as control (BO). The eluted RNA was analyzed by RT-PCR. IP efficiency was assessed by western blot (see Figure S2A).
(B) RIP was performed as in (A) by using anti-Ago2 antibodies. IP efficiency was assessed by western blot (see Figure S2C).
(C) Biotinylated antisense RNA complementary to the 3′ region of linc-MD1 was incubated with cytoplasmic extracts of C2C12 cells (2 days of DM). RNA pull-down was performed with streptavidin-linked beads (schematic representation in the top panel). Bottom panel: western blot of HuR, Ago2, and Actinin from input (Inp), specific probe-bound fraction (MD1 probe), beads-only (BO), and unspecific RNA probe (Control probe). The recovered linc-MD1 is shown in Figure S2D (left panel). The graph shows the ratio of miR-133 and miR-135 between the fraction bound to the specific probe versus the control one. miR-31 and miR-206 are used as negative controls. Error bars represent SE. Pull-down efficiency was assessed by RT-PCR (Figure S2E, left). Input represents 10% of the extract used for the pull-down. Extract from GM cells, where linc-MD1 is not expressed, was used as negative control (Figure S2E, right).
(D) EMSA using in vitro 32P-labeled transcripts and purified flag-HuR protein (Figure S2F). The amounts of recombinant protein utilized are reported above the panels. Mock samples with 200 nM flag peptide were used as control. The right panel shows a schematic representation of the three linc-MD1 regions (MD1 5′, MD1-133b, and MD1 3′) that were tested for HUR binding. The 3′ UTR of MyoD mRNA was used as positive control. The arrows indicate the specific shifts in MD1-133b.
(E) EMSA using in vitro 32P-labeled MD1-133b transcript and 200 nM of purified flag-HuR protein in the presence of specific (MD1-133b) and unspecific (tRNA) cold RNA competitors (from 10 to 100 molar excess).
(F) Left panel: schematic representation of the pri-miR-133b structure. The sequence corresponding to the HuR putative binding site is indicated together with the nucleotide substitutions of the mutant derivative. Arrows indicate the Drosha cleavage sites. Right panel: EMSA of in vitro 32P-labeled 133b-SLWT and 133b-SLmut transcripts with purified flag-HuR protein. The amounts of recombinant protein utilized are reported above the panels. The arrow indicates the specific shift due to HuR binding.
These interactions were also tested by a parallel approach in which C2C12 cell extracts were loaded on streptavidin columns prebound with biotinylated antisense RNAs complementary to linc-MD1 (Figure 2C, top). HuR and Ago were specifically recovered from the fraction bound to the MD1 probe together with linc-MD1 (Figure S2E), miR-133, and miR-135 (Figure 2C, bottom). A biotinylated control RNA complementary to the multiple cloning site of pCDNA3.1 vector was used as control (Figures 2C and S2D). These results demonstrate that HuR binds linc-MD1, and confirm linc-MD1’s ability to interact in vivo with both miR-133 and miR-135, associated with the Ago protein. The absence of any pull-down species in growth medium (GM), where linc-MD1 is not expressed (Figure S2E, right panel), confirms the specificity of the interaction observed in differentiation medium (DM).
In order to further analyze the direct interaction with HuR, we performed electrophoretic mobility shift analysis (EMSA) with a Flag-HuR protein purified from C2C12 cells (Figure S2F) incubated with different portions of in vitro transcribed, P32-labeled linc-MD1 (Figure 2D). With respect to the high affinity of the MyoD mRNA binding site (MYOD lanes), a lower but specific interaction with the pri-miR133-containing region (MD1-133b lanes) of linc-MD1 was detected. The specificity of this interaction was verified by competition with specific and unspecific RNAs (MD1-133b and tRNA; Figure 2E). Moreover, a mutation of the sequence AAUAUUU, which is located at the base of the pri-miR-133b stem-loop region and was identified by PARCLIP analysis in Lebedeva et al. (2011) as one of the most common HuR-binding sites, produced a strong decrease in HuR binding when compared with the WT counterpart (Figure 2F).
HuR Controls the Balance between linc-MD1 and miR-133 Biogenesis
HuR was previously described to have a peculiar nuclear/cytoplasmic distribution during muscle differentiation and to be involved in shuttling RNA from these two compartments (von Roretz et al., 2011). In early phases of C2C12 differentiation, HuR is predominantly nuclear (Figure S3A). Western analysis indicated that it peaks at 48 hr after induction to differentiation, and decreases after 72 hr together with linc-MD1 (Figure 3A). A parallel northern analysis shows that miR-133, which is low in the initial stages of differentiation, progressively increases, reaching maximum levels after 72 hr when both HuR and linc-MD1 are downregulated.
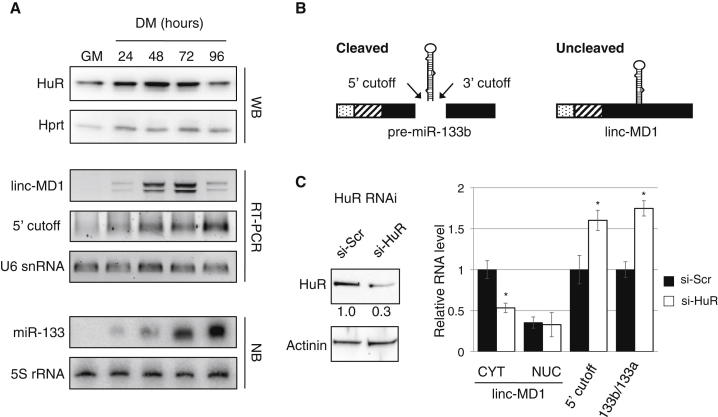
Figure 3.
Effects of HuR on linc-MD1 and miR-133b
(A) Proteins and RNAs were extracted from C2C12 cells in GM or DM for the indicated hours. HuR was analyzed by western blot (WB), linc-MD1 and 5′ cutoff products were analyzed by RT-PCR, and miR-133 was analyzed by northern blot (NB). Hprt, U6 small nuclear RNA (snRNA), and 5SRNA were used as controls.
(B) Schematic representation of linc-MD1, pre-miR-133b, and 5′ and 3′ cutoff products originating from Drosha cleavage (see also Figure S3B).
(C) Left panel: WB of HuR in C2C12 cells (day 2 of DM) upon treatment with either scramble (si-Scr) or anti-HuR (si-HuR) siRNAs. Actinin was used as control. Below each panel, relative quantifications derived from at least three independent experiments are indicated with respect to control samples set to a value of one. Right panel: bar graphs showing linc-MD1, 5′ cutoff, and miR-133b/miR-133a levels. Linc-MD1 was measured by RT-PCR in nuclear (NUC) and cytoplasmic (CYT) fractions of cells treated as in the left panel, and 5′ cutoff products were analyzed only in the nuclear fraction. Linc-MD1 and 5′ cutoff were normalized on HPRT mRNA and U6 snRNA, respectively. A schematic representation of the oligonucleotides used is provided in Figure S3B. miRNA relative levels were measured by LNA-based quantitative PCR detection (see also Figure S3C). Data were derived from at least three independent experiments, error bars represent SE, and the asterisk corresponds to two-tailed Student’s t test p < 0.05.
Since two isoforms contribute to the miR-133 pool (miR-133b and the two miR-133a members, a-1 and a-2), we initially tested, as a sensor for miR-133b production, the accumulation of 5′ cutoff products originating from Drosha cleavage (Figure 3B). To analyze these species, we treated nuclear RNA with polyA terminal transferase that was retrotranscribed with oligo-dT primers and amplified with oligonucleotides upstream of the Drosha cleavage site (see Table S2; Figure S3B). Quantification of the RT-PCR products indicated a continuous increase of their accumulation, opposite to the trend of linc-MD1 and HuR (Figure 3A). Therefore, after 72 hr of differentiation, a clear inverse correlation between Drosha cleavage and linc-MD1 production is observed, and this parallels the HuR decrease.
Modulation of HuR expression was used to test whether it had any direct effect on the alternative production of linc-MD1 and miR-133b. RNAi against HuR produced a consistent decrease in cytoplasmic linc-MD1 accumulation in parallel with a remarkable increase of 5′ cutoff products (Figure 3C). In order to demonstrate a direct effect on miR-133b processing, we set up specific conditions to distinguish miR-133a from miR-133b by LNA-based quantitative RT-PCR (qRT-PCR; Figure S3C). We observed that upon HuR downregulation, the miR-133b/miR-133a ratio increased about 2-fold, paralleling the effect observed for 5′ cutoff products. To further confirm these data, we also performed small RNA-seq on C2C12 cells treated with HuR small interfering RNAs (siRNAs). The results again indicate a relative increase of miR-133b with respect to miR-133a (Figure S3D; Table S3). Altogether, these data show that HuR is able to trigger linc-MD1 formation and accumulation in the cytoplasm in a pathway alternative to miR-133b biogenesis; moreover, they indicate that this process is regulated during differentiation.
HuR overexpression was performed in HeLa cells, where HuR is less abundant in the nucleus and linc-MD1 is not expressed. Figure S3F shows that although HuR overexpression represses miR-133b release from a transfected linc-MD1 coding plasmid (pMD1WT), it has no effect on the mutant pMD1ΔHuR, which lacks the HuR-binding site described in Figure 2F.
Since HuR was described to participate in RNA export to the cytoplasm, we tested whether the cytoplasmic levels of linc-MD1 were affected by leptomycin B, which is known to interfere specifically with HuR transport (Gallouzi and Steitz, 2001). No change of cytoplasmic linc-MD1 was observed (Figure S3E), excluding this possibility.
One obvious assumption to explain HuR activity was then the competition for Drosha cleavage. In vitro processing extracts were produced from HeLa cells treated with either scramble or anti-HuR siRNAs. Figure S3H shows that pri-miR-133b displayed a very slight but reproducible increase of pre-miR-133b processing when HuR was downregulated by RNAi (Figure S3G), indicating again the repressive effect of HuR on miR-133b biogenesis. It is important to underline that this very inefficient processing phenotype (for comparison, see the positive control pri-miR-9-2 in Figure S3H), is likely to be due to the presence in pri-miR-133b of a 60-nt-long stem (Figure S3I), which makes it a very poor microprocessor substrate (Han et al., 2006).
HuR Helps miRNA Recruitment in the Cytoplasm
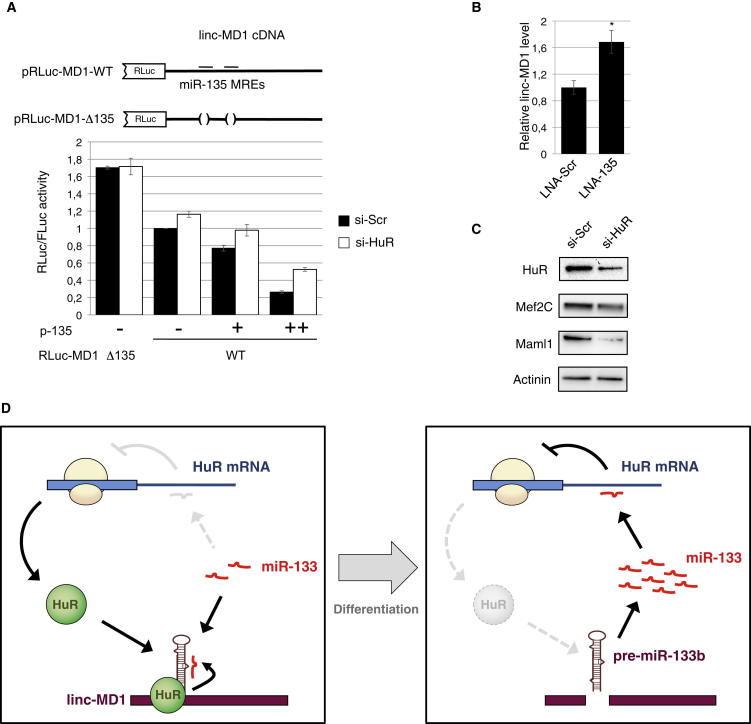
Since HuR was previously described to modulate miRNA-target recognition in the cytoplasm, we tested whether it could affect the sponging activity of linc-MD1. The effect of miR-135 overexpression in combination with HuR depletion was analyzed in C2C12 cells on the pRLuc-MD1-WT sensor construct and on its mutant derivative, pRLuc-MD1-Δ135, lacking the miR-135 MREs (Figure 4A, top). We chose to use miR-135 instead of miR-133 because it has two binding sites in two positions independently of HuR binding, and to exclude the superimposing negative effect of miR-133 on HuR synthesis (see Figure 1B). Moreover, since both pRLuc-MD1 constructs harbor a mutation that abolishes Drosha cleavage (see Cesana et al., 2011), we were able to exclude the nuclear activity of HuR and specifically analyze possible cytoplasmic effects. Figure 4A shows that with respect to pRLuc-MD-Δ135, the luciferase levels of Luc-MD1-WT decreased in the presence of endogenous levels of miR-135 (− samples, black bars). When RNAi against HuR was applied, a slight but reproducible increase of luciferase was observed for pRLuc-MD1-WT, but not for pRLuc-MD1-Δ135 (− samples, white bars). Moreover, increasing amounts of p135 further reduced pRLuc-MD1-WT luciferase activity (+ and ++, black bars), and this reduction was partially lessened when HuR expression was depleted by RNAi (white bars). Notably, the levels of luciferase mRNA paralleled the levels of luciferase activity, indicating that miRNA repression is also accompanied by RNA degradation (Figure S4A). Along this line, we observed that the endogenous levels of linc-MD1 were upregulated when LNA against miR-135 was transfected into C2C12 cells (Figure 4B).
Figure 4.
HuR Regulates linc-MD1 ceRNA Activity
(A) Upper panel: pRLuc-MD1-WT and Δ135 constructs carrying the linc-MD1 sequence downstream to the Renilla luciferase open reading frame (Cesana et al., 2011). The two miR-135 MREs, which are deleted in the Δ135 construct, are indicated. Lower panel: differentiating C2C12 cells were treated with scramble (si-Scr, black bars) or anti-HuR (si-HuR, white bars) siRNAs and transfected with RLuc-MD1-WT and Δ135, combined with empty vector (−) or increasing amounts of miR-135 coding plasmid (p-135). Luciferase activity was measured as RLuc/FLuc RQ after 48 hr. RNA from the “+” experiment was also analyzed for relative luciferase mRNA levels (see Figure S4A). Data were derived from at least three independent experiments, error bars represent SE, and the asterisk corresponds to two-tailed Student’s t test p < 0.05.
(B) RT-PCR analysis performed on linc-MD1 RNA in conditions of LNA-mediated miR-135 inhibition (LNA-135) with respect to control (LNA-Scr).
(C) Western blot analysis of HuR, Maml1 and Mef2C performed on differentiating C2C12 cells treated with scramble (si-Scr) or anti-HuR (si-HuR) siRNAs.
(D) Schematic representation of the positive feedforward control loop linking linc-MD1, miR-133, and HuR in early phases of muscle differentiation. The exit from the circuitry is obtained through miR-133 upregulation, mainly from the two unrelated miR-133a-1 and miR-133a-2 coding loci (Figure S4D).
According to the ceRNA hypothesis, the effect of HuR on linc-MD1 levels and sponge activity should indirectly affect the circuitry controlled by linc-MD1. Therefore, the accumulation of the known components of the linc-MD1 circuitry (Cesana et al., 2011) was tested in conditions of HuR depletion. Figure 4C shows that upon RNAi of HuR, both Maml1 and Mef2C were downregulated. Since HuR also acts by stabilizing certain classes of transcripts, such as its own mRNA and Myogenin, we tested whether Maml1 and Mef2C mRNAs could be found in HuR IPs. Maml1 was completely absent and Mef2C was only present in traces (∼12-fold less than HuR mRNA; Figure S4B), suggesting that their accumulation is more likely to be controlled by the linc-MD1 ceRNA circuitry than simply by a stabilizing effect of their mRNAs.
Altogether, these data indicate that HuR facilitates the linc-MD1-miRNA interaction, enhancing its sponge activity with effects on the ceRNA circuitry.
Discussion
The commitment of precursor muscle cells to initiate and progress into the differentiation path relies on the temporally orderly expression of specific myogenic factors. The linc-MD1-regulated network, which was initially defined on the basis of transcriptional control, was recently enlarged when muscle-specific miRNAs (Williams et al., 2009) and lncRNAs (Cesana et al., 2011, Wang et al., 2013a) came to the scene.
In particular, the miRNA sponge activity of linc-MD1 resulted in the upregulation of two myogenic factors, Maml1 and Mef2C. The expectation, according to the ceRNA hypothesis, is that a large number of coding and noncoding RNAs can crosstalk with each other through competition for common miRNAs (Salmena et al., 2011).
In this work, we identified the HuR protein as another component of the linc-MD1-regulated circuitry. We showed that HuR is repressed by miR-133, and that linc-MD1 alleviates this effect in early phases of differentiation.
HuR was particularly attractive because it is known to contribute in a relevant manner to muscle differentiation (von Roretz et al., 2011); however, due to its many interactors and functions, a simple classification of its activity is still difficult to obtain. Transcriptome-wide analysis revealed that HuR interacts with many mRNAs, as well as with numerous noncoding RNAs, indicating its pleiotropic RNA-binding activity (Lebedeva et al., 2011, Mukherjee et al., 2011).
Besides describing HuR as a component of the linc-MD1 circuitry, we show that HuR binds linc-MD1 and favors its accumulation at the expense of miR-133b biogenesis, thus establishing a positive feedforward control. This regulatory circuitry is reinforced in the cytoplasm by the positive effect of HuR on miRNA recruitment on linc-MD1, thus contributing to its sponging activity.
Based on the inverse correlation between HuR levels and Drosha cleavage products, the most straightforward hypothesis to explain HuR’s mode of action is that the two factors compete for the linc-MD1 substrate, as previously shown for the processing of tissue-specific miRNAs (Keniry et al., 2012, Choudhury et al., 2013). We found that when HuR was downregulated, the levels of miR-133b and the 5′ cutoff products originating from Drosha cleavage indeed increased. Moreover, despite the inefficiency of the in vitro processing assays, we observed a slight but reproducible increase of pre-miR133b release when HuR was downregulated. The low efficiency of in vitro processing is likely due to the structure of pri-miR-133b, which is a very poor Drosha substrate and thus hampers the visualization of strong regulatory effects. Moreover, since Drosha is known to act cotranscriptionally (Morlando et al., 2008, Ballarino et al., 2009), it is likely that the in vitro system does not allow proper reconstitution of the process.
Finally, the mapping of the HuR-binding site at the base of the pri-miR-133b stem loop corroborated the conclusion that HuR physically interferes with microprocessor activity.
The positive loop linking HuR and linc-MD1 operates in a specific window of time, finely controlling the relative abundance of the two factors. A possible trigger for exit from the positive HuR/linc-MD1 loop is the establishment of sustained miR-133 expression at later stages of differentiation. Notably, miR-133b, harbored in the linc-MD1 locus, contributes by only to a minor extent to the cellular miR-133 levels, which instead are mainly represented by the miR-133a-1 and miR-133a-2 species (Figure S4C).
The miR-133 family is composed of three members clustered into bicistronic pairs with miR-1 and miR-206. The three clusters most likely arose from two genomic duplications on different chromosomes (Hertel et al., 2006; Figure S4D). The miR-206/133b gene cluster is the most recent addition and it is only present in vertebrates, whereas miR-1 and miR-133a are both conserved in fly and worm. The origin of the miR-133b locus correlates with the increase in complexity of vertebrate skeletal muscle. In fact, fly and worm do not have satellite cells, nor do they possess different fiber types, such as slow-twitch and fast-twitch fibers, as found in vertebrate skeletal muscle. Therefore, the hypothesis that the three miR-133 loci have differentiated their activity to meet the unique regulatory demands of vertebrates is very attractive. Although the 133a-1 and 133a-2 loci would provide most of the functional miRNA molecules, the locus encoding for miR-133b and its host linc-MD1 transcript would have acquired more dedicated regulatory functions. It is possible to suggest that the role of linc-MD1 in fine-tuning the timing control of differentiation is critical only in organisms that require specific control of muscle regeneration or more specific muscle specializations.
Experimental Procedures
Cell Cultures and Treatments
C2C12 murine myoblasts were cultured and transfected as previously described Cacchiarelli et al. (2010), with the exception that 0.5% fetal bovine serum was used in the DM. Transient transfection of plasmid, siRNA (QIAGEN; 40 nM final concentration), and LNA oligos (EXIQON; 100 nM final concentration) was carried out with the use of Lipofectamine-2000 (Invitrogen) according to the manufacturer’s specifications. Human primary myoblasts were cultured according to Cazzella et al. (2012). HeLa cells were cultured and transfected as previously described (Morlando et al., 2008).
RIP
RIP was performed by incubating 20 μg of antibody or isotypic IgGs to 30 μl of Protein A/G salmon sperm agarose beads (Millipore) for 2 hr at 4°C. C2C12 lysates were prepared with cells cultured for 2 days in DM with 100 μl of passive lysis buffer (PLB); 200 μg of each lysate was used for each RIP assay. Samples were precleared for 1 hr at 4°C with 30 μl of beads, and the supernatant was then resuspended in 600 μl of NT2 buffer and added to antibody-coated beads for 4 hr at 4°C. Beads were washed with NT2 buffer five times and split for protein (1/3) and RNA analysis (2/3). One-fifth of the input lysate was used as control. PLB and NT2 buffers were prepared according to Tenenbaum et al. (2002).
Biotin-RNA Pull-Down Assay
A biotin-RNA pull-down assay was carried out by incubating 1.5 μg of biotinylated RNA antisense transcripts with 250 μg of cytoplasmic lysate prepared with Buffer A for 30 min at room temperature. Then 100 μl of Streptavidin MagneSphere paramagnetic particles (Promega) was saturated with 150 μg of tRNA and added to the extract for 15 min at room temperature. After three washings with the EMSA buffer, the bound RNA and protein complexes were eluted and subsequently analyzed by qRT-PCR and western blot, respectively. Biotinylated transcripts were obtained from PCR-generated templates (oligonucleotides are listed in Table S2) by using the MAXIscript in vitro transcription system (Life Technologies). All buffer compositions are described in Morlando et al. (2012).
Electrophoretic Mobility Shift Assay
EMSA was carried out as described in Kundu et al. (2012) unless the incubation was performed at 25°C for 30 min. RNA substrates were prepared by in vitro transcription using T7 RNA polymerase (Promega) from PCR-amplified templates (oligonucleotides are listed in Table S2) in the presence of [α-32P]UTP (Perkin-Elmer). Flag-HuR was purified by C2C12 cells transiently transfected with plasmid for HuR overexpression (kindly provided by G. Michlewsky), using anti-FlagM2 (Sigma) antibody and the Dynabeads Protein G Immunoprecipitation Kit (Invitrogen) according to the manufacturer’s instructions.
RNA Analyses
Northern Blot and Quantitative Real-Time PCR
Total RNA from C2C12 cells was isolated as described in Morlando et al. (2012) and nuclear/cytoplasmic RNA fractionation was carried out using the Paris Kit (Ambion) according to the manufacturer’s specifications. The northern blot assay was performed as described in Cacchiarelli et al. (2010). cDNA generation was carried out using the miScript Reverse Transcription Kit (QIAGEN). Real-time PCR detection of miRNAs and cutoff molecules was performed using the miScript SYBR-Green PCR Kit and DNA oligonucleotides (QIAGEN).
Protein Extraction and Western Blot
Total protein extract was obtained as described in Laneve et al. (2010) and nuclear/cytoplasmic fractionation was carried out by using the NE-Per Kit (Thermo Scientific-Pierce) according to the manufacturer’s specifications. The immunoblots were incubated with the following antibodies: anti-HuR (sc-5261; Santa Cruz) or anti-Ago2 (01822021; Wako), anti-Mef2C (ab79436; Abcam), anti-Maml1 (A300-673a; BETHYL), anti-Hprt (sc-20975; Santa Cruz), and anti-Actinin (sc-15335, Santa Cruz). The densitometric analysis was performed using Image Lab software (Bio-Rad).
The miRNA predictions pipeline, luciferase reporter assays, plasmid descriptions, enzymatic assays, RNA-seq methods, and a complete list of all oligonucleotides used in this work are available in the Supplemental Experimental Procedures.
Acknowledgments
We thank M. Cesana and D. Cacchiarelli for providing reagents. We thank O. Sthandier and M. Marchioni for technical support. This work was partially supported by grants from Telethon (GGP11149), Epigen, Parent Project Italia, AIRC, IIT “SEED,” FIRB, and PRIN.
Published: January 16, 2014
Footnotes
Supplemental Information includes four figures, three tables, and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2013.12.012.
Supplemental Information
References
- Al-Ahmadi W., Al-Ghamdi M., Al-Haj L., Al-Saif M., Khabar K.S. Alternative polyadenylation variants of the RNA binding protein, HuR: abundance, role of AU-rich elements and auto-Regulation. Nucleic Acids Res. 2009;37:3612–3624. doi: 10.1093/nar/gkp223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarino M., Pagano F., Girardi E., Morlando M., Cacchiarelli D., Marchioni M., Proudfoot N.J., Bozzoni I. Coupled RNA processing and transcription of intergenic primary microRNAs. Mol. Cell. Biol. 2009;29:5632–5638. doi: 10.1128/MCB.00664-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacchiarelli D., Martone J., Girardi E., Cesana M., Incitti T., Morlando M., Nicoletti C., Santini T., Sthandier O., Barberi L. MicroRNAs involved in molecular circuitries relevant for the Duchenne muscular dystrophy pathogenesis are controlled by the dystrophin/nNOS pathway. Cell Metab. 2010;12:341–351. doi: 10.1016/j.cmet.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Cazzella V., Martone J., Pinnarò C., Santini T., Twayana S.S., Sthandier O., D’Amico A., Ricotti V., Bertini E., Muntoni F., Bozzoni I. Exon 45 skipping through U1-snRNA antisense molecules recovers the Dys-nNOS pathway and muscle differentiation in human DMD myoblasts. Mol. Ther. 2012;20:2134–2142. doi: 10.1038/mt.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., Bozzoni I. Key ceRNA role for the long non-codingRNA linc-MD1 in the control of muscle differentiation. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury N.R., de Lima Alves F., de Andrés-Aguayo L., Graf T., Cáceres J.F., Rappsilber J., Michlewski G. Tissue-specific control of brain-enriched miR-7 biogenesis. Genes Dev. 2013;27:24–38. doi: 10.1101/gad.199190.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright A.J., John B., Gaul U., Tuschl T., Sander C., Marks D.S. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa A., Cuadrado A., Fan J., Atasoy U., Muscat G.E., Muñoz-Canoves P., Gorospe M., Muñoz A. Role of HuR in skeletal myogenesis through coordinate regulation of muscle differentiation genes. Mol. Cell. Biol. 2003;23:4991–5004. doi: 10.1128/MCB.23.14.4991-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallouzi I.E., Steitz J.A. Delineation of mRNA export pathways by the use of cell-permeable peptides. Science. 2001;294:1895–1901. doi: 10.1126/science.1064693. [DOI] [PubMed] [Google Scholar]
- Gong C., Maquat L.E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature. 2011;470:284–288. doi: 10.1038/nature09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guil S., Cáceres J.F. The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. Nat. Struct. Mol. Biol. 2007;14:591–596. doi: 10.1038/nsmb1250. [DOI] [PubMed] [Google Scholar]
- Han J., Lee Y., Yeom K.H., Nam J.W., Heo I., Rhee J.K., Sohn S.Y., Cho Y., Zhang B.T., Kim V.N. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Hertel J., Lindemeyer M., Missal K., Fried C., Tanzer A., Flamm C., Hofacker I.L., Stadler P.F., Students of Bioinformatics Computer Labs 2004 and 2005 The expansion of the metazoan microRNA repertoire. BMC Genomics. 2006;7:25. doi: 10.1186/1471-2164-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen A.N., Zhou X.B., Xu J., Qiao C., Ma J., Yan L., Lu L., Liu C., Yi J.S., Zhang The imprinted H19 LncRNA antagonizes Let-7 MicroRNAs. Mol Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keniry A., Oxley D., Monnier P., Kyba M., Dandolo L., Smits G., Reik W. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat. Cell Biol. 2012;14:659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.H., Kuwano Y., Srikantan S., Lee E.K., Martindale J.L., Gorospe M. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 2009;23:1743–1748. doi: 10.1101/gad.1812509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Fabian M.R., Sonenberg N., Bhattacharyya S.N., Filipowicz W. HuR protein attenuates miRNA-mediated repression by promoting miRISC dissociation from the target RNA. Nucleic Acids Res. 2012;40:5088–5100. doi: 10.1093/nar/gks148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneve P., Gioia U., Andriotto A., Moretti F., Bozzoni I., Caffarelli E. A minicircuitry involving REST and CREB controls miR-9-2 expression during human neuronal differentiation. Nucleic Acids Res. 2010;38:6895–6905. doi: 10.1093/nar/gkq604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva S., Jens M., Theil K., Schwanhäusser B., Selbach M., Landthaler M., Rajewsky N. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. Cell. 2011;43:340–352. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- McCall M.N., Uppal K., Jaffee H.A., Zilliox M.J., Irizarry R.A. The Gene Expression Barcode: leveraging public data repositories to begin cataloging the human and murine transcriptomes. Nucleic Acids Res. 2011;39(Database issue):D1011–D1015. doi: 10.1093/nar/gkq1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlando M., Ballarino M., Gromak N., Pagano F., Bozzoni I., Proudfoot N.J. Primary microRNA transcripts are processed co-transcriptionally. Nat. Struct. Mol. Biol. 2008;15:902–909. doi: 10.1038/nsmb.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlando M., Dini Modigliani S., Torrelli G., Rosa A., Di Carlo V., Caffarelli E., Bozzoni I. FUS stimulates microRNA biogenesis by facilitating co-transcriptional Drosha recruitment. EMBO J. 2012;31:4502–4510. doi: 10.1038/emboj.2012.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee N., Corcoran D.L., Nusbaum J.D., Reid D.W., Georgiev S., Hafner M., Ascano M., Jr., Tuschl T., Ohler U., Keene J.D. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol. Cell. 2011;43:327–339. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliseno L., Salmena L., Zhang J., Carver B., Haveman W.J., Pandolfi P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum S.A., Lager P.J., Carson C.C., Keene J.D. Ribonomics: identifying mRNA subsets in mRNP complexes using antibodies to RNA-binding proteins and genomic arrays. Methods. 2002;26:191–198. doi: 10.1016/S1046-2023(02)00022-1. [DOI] [PubMed] [Google Scholar]
- Twayana S., Legnini I., Cesana M., Cacchiarelli D., Morlando M., Bozzoni I. Biogenesis and function of non-coding RNAs in muscle differentiation and in Duchenne muscular dystrophy. Biochem. Soc. Trans. 2013;41:844–849. doi: 10.1042/BST20120353. [DOI] [PubMed] [Google Scholar]
- von Roretz C., Beauchamp P., Di Marco S., Gallouzi I.E. HuR and myogenesis: being in the right place at the right time. Biochim. Biophys. Acta. 2011;1813:1663–1667. doi: 10.1016/j.bbamcr.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Wang J., Gong C., Maquat L.E. Control of myogenesis by rodent SINE-containing lncRNAs. Genes Dev. 2013;27:793–804. doi: 10.1101/gad.212639.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu Z., Jiang J., Xu C., Kang J., Xiao L., Wu M., Xiong J., Guo X., Liu H. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev. Cell. 2013;25:69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Williams A.H., Liu N., van Rooij E., Olson E.N. MicroRNA control of muscle development and disease. Curr. Opin. Cell Biol. 2009;21:461–469. doi: 10.1016/j.ceb.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.H., Abdelmohsen K., Srikantan S., Yang X., Martindale J.L., De S., Huarte M., Zhan M., Becker K.G., Gorospe M. LincRNA-p21 suppresses target mRNA translation. Mol. Cell. 2012;47:648–655. doi: 10.1016/j.molcel.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisoulis D.G., Kai Z.S., Chang R.K., Pasquinelli A.E. Autoregulation of microRNA biogenesis by let-7 and Argonaute. Nature. 2012;486:541–544. doi: 10.1038/nature11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.