Summary
It has proved difficult to classify viruses unless they are closely related since their rapid evolution hinders detection of remote evolutionary relationships in their genetic sequences. However, structure varies more slowly than sequence, allowing deeper evolutionary relationships to be detected. Bacteriophage P23-77 is an example of a newly identified viral lineage, with members inhabiting extreme environments. We have solved multiple crystal structures of the major capsid proteins VP16 and VP17 of bacteriophage P23-77. They fit the 14 Å resolution cryo-electron microscopy reconstruction of the entire virus exquisitely well, allowing us to propose a model for both the capsid architecture and viral assembly, quite different from previously published models. The structures of the capsid proteins and their mode of association to form the viral capsid suggest that the P23-77-like and adeno-PRD1 lineages of viruses share an extremely ancient common ancestor.
Highlights
-
•
High-resolution structures of the two major capsid proteins of bacteriophage P23-77
-
•
P23-77 capsid proteins exhibit a conserved single β-barrel core fold
-
•
P23-77 is an ancient relative of the double β-barrel lineage of viruses
-
•
Capsid model illustrates that P23-77 uses a novel method of organization
Rissanen et al. propose a model for the architecture and assembly of bacteriophage P23-77 quite different from those previously published. The capsid proteins and their mode of association to form the virus particle suggest that P23-77 share a common evolutionary origin with the PRD1/Adenovirus lineage.
Introduction
All life on Earth is under pressure from the staggering number of viruses present in the biosphere (Bergh et al., 1989; Wommack and Colwell, 2000), and the ensuing evolutionary race has shaped host organisms since the dawn of life (Forterre, 2005; Koonin et al., 2006), to the extent that the remains of viruses litter the genomes of cellular organisms, forming a substantial part of eukaryotic noncoding genetic material (Pritham et al., 2007). Conversely, the varied morphologies, genetic systems, and life cycles of viruses make them the most diverse group of evolving agents known. The rapidity of genetic change in viruses has hindered the use of traditional genome-sequence-based methods for detecting relationships, so that tracing back the evolutionary history of viruses beyond the family level has proved very difficult. Fortunately it has become clear that structural virology may shed light on the origins of the modern virosphere: “viral self” elements, most notably, conserved capsid protein structures, can be used to group viruses with scant genetic similarity, revealing that even viruses infecting hosts from different domains of life can belong to the same lineage (Bamford et al., 2002; Benson et al., 1999). One such lineage, exemplified by adenovirus and the PRD1 bacteriophage, is characterized by a capsid protein fold containing a pair of β-barrels sitting upright within the capsid. This so-called double β-barrel lineage contains members infecting hosts from all three domains of life (Benson et al., 2004). Many members of the lineage also share a similar DNA packaging ATPase and icosahedral morphology. These features appear to be critical to the survival of the virus and are strongly conserved, whereas there are great variations in the mode of replication, genome organization, and host range, consistent with the assumption that they have diverged from a last common ancestor over 2 billion years ago (Krupovic and Bamford, 2008).
To investigate the origins of this ancient lineage in the primordial world and to hopefully gain insight into the early virosphere, we have attempted to identify members that inhabit extreme habitats, which, to our knowledge, are previously unidentified. Recently, several viruses isolated from extreme conditions have been found to have striking similarity with those of the double β-barrel lineage (Happonen et al., 2010; Jäälinoja et al., 2008; Jaatinen et al., 2008; Khayat et al., 2005). We present here work on one of these, bacteriophage P23-77, which was isolated from alkaline hot springs in New Zealand (Yu et al., 2006) and infects Gram-negative bacterium Thermus thermophilus (ATCC 33923). Cryo-electron microscopy (cryo-EM) revealed that P23-77 has an icosahedral capsid, spikes on the vertices, and an internal lipid membrane enclosing the 17 kilo-base pair (kbp) circular dsDNA genome (Jaatinen et al., 2008; Jalasvuori et al., 2009). The major capsid proteins (MCPs) VP16 (∼20 kDa) and VP17 (32 kDa) are present in the capsid in approximately equal proportions. The bulk of the capsid is built from pseudohexagonal capsomers. In this respect, the capsid structure is similar to that seen in the double β-barrel lineage, which is also built from pseudohexameric structures, which are very similar in size to those of P23-77. However, there is a fundamental difference: in the double β-barrel architecture, the capsomers comprise three subunits, each with two β-barrels, leading to the pseudohexameric appearance, whereas in P23-77, the capsomers possess each two turrets, producing a crenellated appearance. The presence of only two turrets rules out the possibility of an underlying 3-fold structure and means that this virus cannot formally belong to the double β-barrel lineage. A closely related capsid structure has also been found in haloarchaeal virus SH1 where the assembly conundrum was addressed by postulating that the capsomers consist of a hexameric base, composed of six copies of the smaller MCP decorated with a smaller number of copies of the larger protein that builds the turrets (Jäälinoja et al., 2008). Based on the structural similarity of the capsomers of P23-77 and SH1, it was assumed that P23-77 capsomers have the same organization: a hexameric base of VP16 complemented by the turret protein VP17.
There is, however, a further connection between the double-β barrel lineage and the crenellated: the putative packaging ATPase of P23-77 and SH1 has a motif similar to that of PRD1 (Jalasvuori et al., 2009). These similarities have led to the proposal that the crenellated viruses possess MCPs composed of single β-barrels reminiscent of those found paired in the double β-barrel lineage. This would suggest that a common ancestor might have existed with a capsid built from proteins with the single β-barrel fold and possessing an ATPase to deliver the genome (Jalasvuori et al., 2009). This ancestor then diverged to produce the lineage of crenellated viruses (P23-77 and SH1) and the double β-barrel viruses (PRD1). If this were true, P23-77 and SH1 would form the earliest branch of the vertical β-barrel viral superlineage, and a detailed knowledge of their structures might illuminate the origin of viruses during the early era of life before the present domains of life formed (Krupovic and Bamford, 2008; Koonin et al., 2006; Woese, 2002). Here, we report the determination of three-dimensional structures for isolated small-MCP VP16 and large-MCP VP17 and of a complex of the two, representing an assembly intermediate. The small MCP contains a single β-barrel, while the large one contains two. These structures fit the EM reconstruction exquisitely well, demonstrating that the previous model for the organization of the capsid is incorrect and allowing us to propose a side-by-side dimer-driven model for the assembly of the capsid radically different to that expected. Comparison with the EM reconstructions of SH1 suggests that these two viruses share the same fundamental assembly mechanism. This indicates that P23-77 and SH1 are related and form, to our knowledge, a new branch of the superlineage of viruses that includes the well-established adeno-PRD1 lineage.
Results
P23-77 Capsid Protein Structures Determined to High Resolution
Dissociation experiments have shown that the P23-77 capsid consists of roughly equal proportions of two soluble MCPs (Jaatinen et al., 2008). We established protocols for the soluble expression and purification of both of these, VP16 and VP17, expressed in E. coli. In line with the thermophilic nature of P23-77, the proteins are thermostable, withstanding temperatures over 80°C. Highly pure protein was obtained after a sequence of heat incubation and ion-exchange chromatography. In addition, we isolated VP16 from virus particles. Protein purification and crystallization are detailed in our recent publication (Rissanen et al., 2012).
Well-diffracting crystals of VP16, VP17, and the complex of VP16 and VP17 were obtained by hanging- and sitting-drop vapor diffusion. Recombinant VP16 crystallized in two forms: VP16-type-1 and VP16-type-2, while virus-derived VP16 crystallized in a third form, VP16-virus-derived (Rissanen et al., 2012). Diffraction data were collected at MX beamlines of the Diamond Light Source synchrotron, Didcot, UK. The structures of VP16-type-1 (1.80 Å resolution) and VP17 (2.26 Å resolution) were solved by isomorphous replacement using lead and mercury derivatives, respectively. Structures of VP16-type-2 (1.26 Å resolution), VP16-virus-derived (2.36 Å resolution), and the VP16/VP17 complex (1.53 Å resolution) were solved by molecular replacement using the coordinates of VP16-type-1 and VP17 as search models. Details of the crystallographic analyses are given in Table 1. The structures are well refined, judged by the currently accepted metrics.
Table 1.
Data Collection and Refinement Statistics
| VP16-Type-1 | VP16-Type-2 | VP16-Virus-Derived | VP17 | VP16/VP17 Complex | |
|---|---|---|---|---|---|
| Data Collection | |||||
| Space group | P6222 | C2 | P212121 | P6122 | C2 |
| Cell dimensions | |||||
| a, b, c (Å) | 61.85, 61.85, 251.22 | 76.60, 68.57, 31.58 | 41.41, 77.05, 403.00 | 107.21, 107.21, 233.78 | 76.78, 69.61, 81.62 |
| α, β, γ (°) | 90, 90, 120 | 90, 96.44, 90 | 90, 90, 90 | 90, 90, 120 | 90, 104.99, 90 |
| Resolution (Å) | 62.8–1.80 (1.85–1.80) | 34.3–1.26 (1.30–1.26) | 41.1–2.36 (2.42–2.36) | 59.7–2.26 (2.32–2.27) | 39.6-1.53 (1.57–1.53) |
| Rmerge | 0.075 (1.045) | 0.053 (0.626) | 0.114 (1.137) | 0.082 (0.917) | 0.064 (1.015) |
| I/σI | 30.7 (3.3) | 23.1 (2.6) | 7.5 (1.3) | 38.6 (5.3) | 17.7 (2.9) |
| Completeness (%) | 100 (100) | 85.8 (41.4) | 99.1 (99.6) | 100 (100) | 98.4 (83.3) |
| Redundancy | 28.5 (21.0) | 7.5 (6.5) | 3.2 (3.3) | 35.5 (36.6) | 6.3 (4.4) |
| Refinement | |||||
| Resolution (Å) | 53.56–1.80 | 17.79–1.26 | 42.16–2.36 | 43.94–2.26 | 22.40–1.53 |
| Number of reflections | 27,503 | 37,133 | 53,974 | 37,808 | 61,425 |
| Rwork/Rfree | 0.189/0.215 | 0.156/0.184 | 0.193/0.234 | 0.186/0.213 | 0.172/0.197 |
| Number of atoms | |||||
| Protein | 1,350 | 1,237 | 10,375 | 3,623 | 2,937 |
| Ligand/ion | 24 | 14 (1 citrate) | 4 | 0 | 2 |
| Water | 213 | 255 | 348 | 424 | 395 |
| B factors | |||||
| Protein | 41.9 | 13.2 | 52.4 | 50.6 | 27.3 |
| Ligand/ion | 63.8 | 15.6 | 43.3 | – | 27.9 |
| Water | 54.1 | 29.0 | 47.7 | 58.8 | 40.8 |
| RMSDs | |||||
| Bond lengths (Å) | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 |
| Bond angles (°) | 1.03 | 1.08 | 1.07 | 1.14 | 1.07 |
Values in parentheses are for highest resolution shell. Each data set was collected from one crystal.
Major Capsid Protein Structures Identify P23-77 as a Relative of the Double β-Barrel Lineage
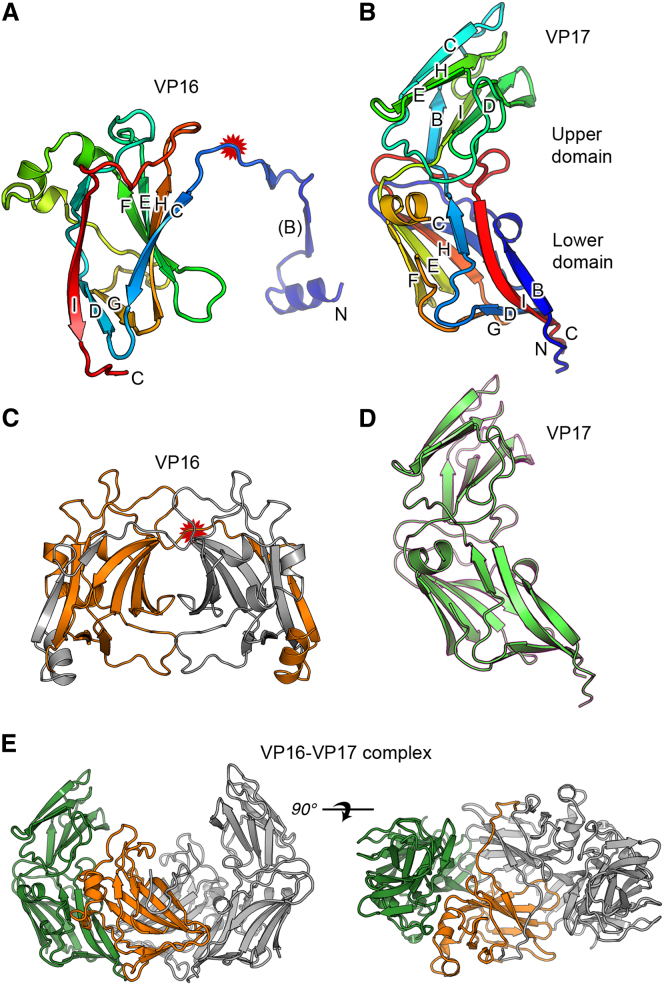
The structures are illustrated in Figure 1. For VP16-type-1, essentially all of the structure is well defined in the electron density map, the structure only lacking the N-terminal methionine (M1). In all crystal forms, VP16 exists as dimers of entwined subunits, with a very large surface area of interaction between the two subunits (3,152 Å2 for VP16-type-1), which is judged biologically significant by PISA (see Supplemental Experimental Procedures available online) (Krissinel, 2010; Krissinel and Henrick, 2007). The subunit comprises a β-barrel, seven strands of which are contributed by one subunit and an eighth by the other subunit in the dimer. The structure therefore resembles a strand-swapped dimer, such as can sometimes be formed by misfolding of β-proteins such as immunoglobulin folds (Sonnen et al., 2010). However, we also find this strand-swapping in all four copies of VP16 dimers in crystals derived from virion material, indicating that the strand-swapped dimer of VP16 is the native state of VP16 in the virus. Pairwise comparison of the VP16 subunits shows that they are all extremely similar (root-mean-square deviations [RMSDs] range from 0.2 to 1.2 Å). All VP16 structures showed the presence of a Cl− ion at the junction between the two subunits (Figure S1), and we note that an ion at this position may be a general mechanism of facilitating strand swaps in β-barrels as a similar observation has been made for CD47 (Hatherley et al., 2008). For the purposes of comparison with other β-barrel structures, we will rewire the subunit of VP16-type-1 by cutting across residues 33–35 to form an intact chimeric subunit (Figure 2). Analysis of this rewired VP16 with the Structure Homology Program (Abrescia et al., 2012; Stuart et al., 1979) reveals that it has a viral jelly roll similar to those seen in the adeno-PRD1 lineage, with marked similarity to the individual β-barrels of the MCP of marine bacteriophage PM2 (Abrescia et al., 2008). Does the VP16 dimer form one third of a pseudohexameric capsid building block? To test this, we superposed the VP16 dimer onto the β-barrels of the MCP of PM2. Remarkably, there is a major rotation (∼45°) of the β strands in the second β-barrel when the first is superposed (see Figure S2). This puzzle is explained in the context of the VP16/VP17 complex, discussed later.
Figure 1.
P23-77 MCPs
(A–E) High-resolution X-ray structures of (A) VP16-type-1 and (B) VP17, colored blue to red from the N terminus to the C terminus, (C) VP16 dimer (subunits colored orange and gray), (D) VP17 (green), and (E) heterotetramer complex of VP16 (orange) and VP17 (green). VP16 rewiring site (relates to Figure 2) is highlighted with a red star in (A) and (C). Symmetry molecules are shown in gray.
See also Figure S1.
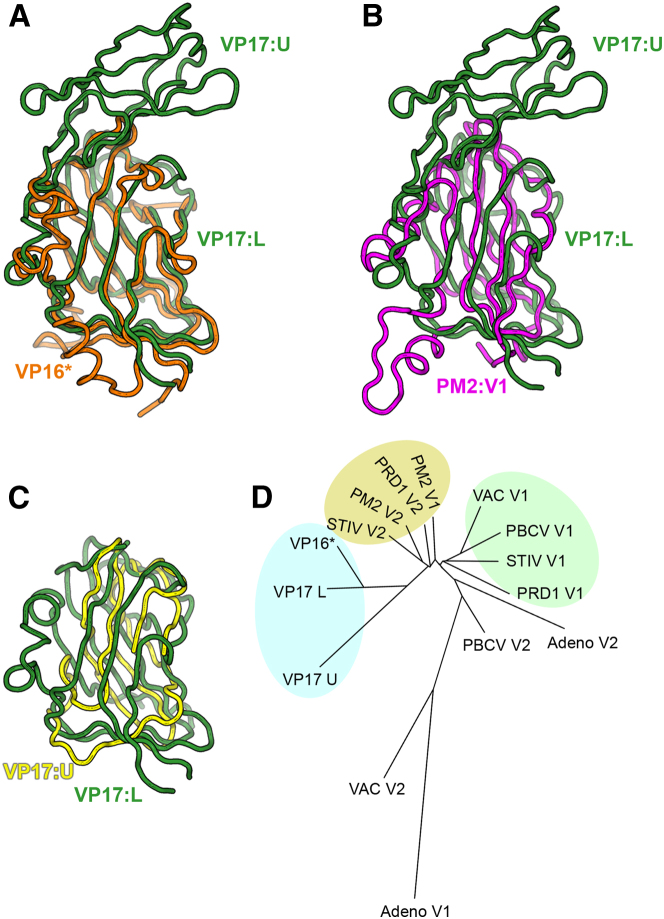
Figure 2.
Phylogeny of P23-77 Capsid Proteins in Relation to the Double β-Barrel Lineage
(A–D) Structure-based superimpositions and phylogenetic tree of the capsid proteins of P23-77 and members of the double β-barrel lineage using the Structure Homology Program (SHP) (Abrescia et al., 2012; Stuart et al., 1979) and individual V1 and V2 domains. To enable comparison with other β-barrel structures, subunit of VP16-type-1 was rewired across residues 33–35 (see Figure 1) to form an intact chimeric subunit.
(A) VP16 (orange) superposed on VP17 (green).
(B) PM2 V1 (purple) superposed on VP17 (green).
(C) VP17 upper domain (yellow) superposed on VP17 lower domain (green).
(D) Phylogenetic tree illustrating the evolutionary distance of the MCPs of double β-barrel lineage members and P23-77 (light blue group), showing that the closest relatives to VP16 and VP17 are mostly V2 domains (yellow group), V1 domains being further diverged (green group).
For monomeric VP17, 42 N-terminal and 18 C-terminal amino acids are disordered. Helical wheel analysis of the N-terminal residues of VP17 shows that the first 21 (1–21) residues are mainly hydrophobic, especially on one face of the helix, while the following 21 (22–42) residues are nearly all hydrophilic. This suggests an N-terminal membrane-binding domain connected to the ordered protein via proline-rich helix (see Supplemental Experimental Procedures; Figure S3). In contrast to VP16, in neither of the crystal forms which contain VP17 does the protein form homotypic interactions. VP17 contains two β-barrels, but these are not arranged sequentially along the polypeptide chain as seen in classic double β-barrel structure of the adeno-PRD1 lineage; instead, the second domain is formed as an insertion in the primary β-barrel and sits atop it, as opposed to beside it (Figure 1B). The primary lower β-barrel of VP17 also resembles the MCP of PM2 but is much more similar to VP16. The secondary upper β-barrel of VP17 has only six strands rather than the typical eight, and while it is less similar to either VP16 or the primary β-barrel of VP17 than those are to each other, it is more similar to them than to any other known β-barrel. These results are consistent with the superlineage concept for the origins of the virus as seen from the structure-based phylogeny (Figure 2), which illustrates the degree of similarity that the individual β-barrels of VP16 and VP17 have to other lineage members, showing that they tend to be most similar to the V2 barrels of relatives (the two barrels of the double-barrel proteins are traditionally known as V1 and V2 according to their position in the sequence), except in the case of PM2 where both barrels are similar to each other and to those of the P23-77 proteins. That the similarity to the adeno-PRD1 lineage reflects divergence is supported by the observation that the point where the secondary β-barrel is inserted (the DE loop) is frequently a site of major insertions in the adeno-PDR1 lineage (Bahar et al., 2011). In addition, the core β-barrel structures of both VP16 and VP17 have extensions, including an α helix loop (residues 96–116 in VP16), which is also found at a similar place in the β-barrels of other lineage members.
The complex of VP16-VP17 contains a VP16 dimer, very similar to that seen for the isolated VP16, with each subunit engaging a subunit of VP17, to form an extended heterotetramer (Figure 1E). A surface area of 733 Å2 is occluded for each interface between VP16 and VP17 in this complex (see Supplemental Experimental Procedures) (Krissinel and Henrick, 2007). The mode of VP16-VP17 engagement is lateral—as would be expected if the two subunits packed side by side around the pseudohexameric base structure—rather than stacked. This structure is not compatible with the current model for the architecture of these viruses—that one protein forms the hexameric base while the other sits on top of it (Jäälinoja et al., 2008; Jaatinen et al., 2008). The structure does, however, support a new arrangement where the crenellated structures observed in the virus are formed from the VP17 secondary upper β-barrels. In this arrangement, six highly similar β-barrels are arranged around a pseudo-6-fold axis in a fashion analogous to that seen in the trimeric MCPs of the adeno-PRD1 lineage. This explains the tremendous similarity between the primary β-barrel of VP17 and the VP16 β-barrel (Figure 2A) and predicts that adjacent β-barrels of VP16 and VP17 in the complex will be superimposable on adjacent β-barrels for the MCP trimers of viruses of the double β-barrel lineage. This is rather precisely true, with the centroids of the β-barrels and their orientations being essentially indistinguishable (see Figure S2), suggesting that the VP16-VP17 complex seen in the crystal truly represents the cognate complexes assembled on the virus. We are fortunate that the cryo-EM structure of P23-77 has been determined, at 14 Å resolution, which allows a rigorous test of this hypothesis (Jaatinen et al., 2008).
Capsid Model Provides Insight into P23-77 Virion Architecture and Assembly
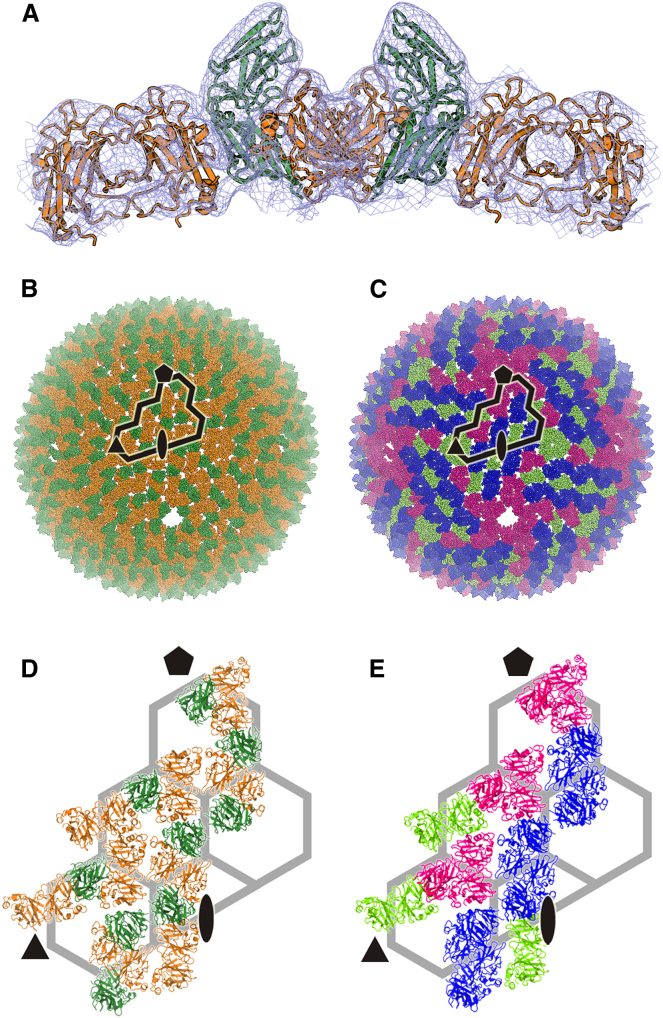
We initially fitted the capsid protein structures to the cryo-EM-derived electron density map of the P23-77 capsid in Coot (Emsley and Cowtan, 2004; Jaatinen et al., 2008). Afterward, the structures were refined into the density using the Visual Environment for Docking Algorithms (VEDA; http://mem.ibs.fr/VEDA), a new graphical version of URO (Navaza et al., 2002), to assess various packing models objectively. The details are presented in the Supplemental Experimental Procedures, however, the results were unambiguous: the final refinement to the 14 Å map gives the values CC (correlation coefficient) = 74.1% and R = 50.2% (these correspond to the values from URO-based fitting without map masking; Navaza et al., 2002). The whole capsid can be assembled from three basic building blocks: a VP16 dimer, a VP16-VP17 heterotrimer, and a VP16-VP17 heterotetramer, all of which fit very well with no significant change from the subassemblies derived directly from the crystal structures (Figure 3). In light of this, later we suggest an assembly pathway for the virus (see Discussion). There can be no doubt that the assembly mechanism for the crenellated viruses differs radically from that of the double β-barrel lineage. In the latter, the building block is a homotrimer, which forms pseudohexameric capsomers that pack together to form a close-packed lattice of P3 symmetry with center-to-center spacing between trimers of ∼90 Å. In P23-77, there is a pseudo-P3 lattice of similar dimensions, although the two distinct patterns of crenellation of the pseudohexameric building block betrays the two ways that two subunits of VP17 populate these apparent rings, in a way that is not consistent with P3 symmetry. What is completely unexpected is that these rings are not building blocks for the assembly of the virus; instead, the strongest interactions are within the VP16 dimers that span adjacent rings. P23-77 has “capsomers” with four VP16s that always dimerize across “capsomer” borders and two VP17s that attach to the VP16s in the same “capsomer.” Regardless of the organizational complexity, there are two fundamental rules: VP16 always forms a homodimer, and VP17 always attaches to one copy of VP16 via a specific site. Flexibility to the model is provided by the fact that the VP17 attachment is not necessary in all instances: the VP16 dimer is sometimes present without the adjacent VP17s, and in the heterotrimer, VP17 is attached to only one of the dimerized VP16s. An assembly hierarchy based on strong VP16 dimerization and VP16-VP17 interaction is supported by experimental data from analytical gel filtration chromatography and yeast two-hybrid trials. Analytical gel filtration chromatography shows that purified VP17 is a monomer and VP16 is a dimer, and in yeast two-hybrid experiments, the strongest interaction is observed between two copies of VP16 and between VP17 and VP16 (see Supplemental Experimental Procedures; Table S1). The complete protein capsid built in the cryo-EM density and refined with VEDA is illustrated in Figure 3. The capsid is nearly spherical, with the RMSDs of the centers of mass of each subunit in the viral facet (defined as between three adjacent 5-fold axes) from a sphere being only 1.5% of the virus radius (RMSD, 5.6 Å with a virus radius of 368 Å), while their deviation from a plane is 23 Å. PRD1 is less spherical, and this is reflected in the RMSDs of the centers of mass of each subunit in the PRD1 viral facet from a sphere being 3.1% of the virus radius (RMSD, 9.1 Å with a virus radius of 296 Å), while their deviation from a plane is only 7 Å. The peculiarities of the P23-77 capsid include the distribution of VP17s, absent at the icosahedral 3-fold symmetry axes and surrounding the 5-folds. Most remarkable, however, is that the strong strand-swapped dimer of VP16s is at the core of assembling and keeping the capsid together.
Figure 3.
Capsid Organization of P23-77
Structures of VP16 (orange) and VP17 (green) fitted into the cryo-EM electron density map of the P23-77 virion (Jaatinen et al., 2008), refined in VEDA.
(A) Illustration of the fit of MCPs in capsid density.
(B) P23-77 capsid model colored according to protein species, showing borders of the asymmetric unit.
(C) P23-77 capsid model colored according to oligomeric state, with VP16 dimer in lime green, VP16-VP17 trimer in magenta, and VP16-VP17 tetramer in blue.
(D) The asymmetric unit of P23-77, colored according to protein species.
(E) The asymmetric unit of P23-77, colored according to oligomeric state.
See also Figure S3.
Archaeal Virus SH1 Shares Structural Features with P23-77
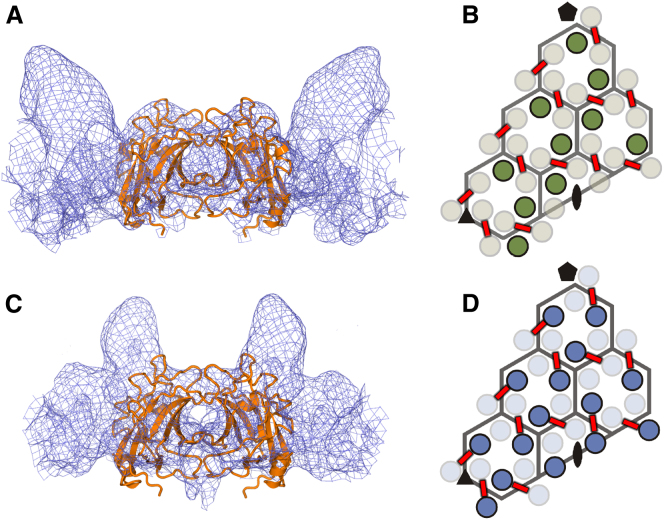
The cryo-EM analysis of the archaeal virus SH1 and bacteriophage P23-77 suggested that they might be related, since many aspects of their morphology appeared similar (same pseudo T-number and similar size and shape of most capsomers), although the arrangement of the crenellations differs between the two. While both have some capsomers with two turrets, symmetrically disposed, SH1 possesses other capsomers bearing three turrets, whereas the other capsomers of P23-77 possess two asymmetrically disposed turrets (Jäälinoja et al., 2008; Jaatinen et al., 2008). We used the same fitting methods that we applied to P23-77 to attempt to fit VP16 and VP17 into the SH1 capsid electron density map (Jäälinoja et al., 2008). There are strong similarities: the angle of the jelly-rolls is the same in the two viruses, and the bridges connecting the capsomers are identically positioned (Figure 4). The major capsid proteins of SH1 are believed to be VP4 and VP7, of 25.7 kDa and 20 kDA, respectively (Kivelä et al., 2006), and so, as for P23-77, it is reasonable to expect one protein to contain an additional “turret-like” domain. However, this additional domain is likely to be a little smaller in SH1 than P23-77 (molecular mass, 25.7 kDa in SH1 and 32 kDa in P23-77). It seems likely, in light of this and given the structure of P23-77 VP17, that the additional domain of VP4 will simply sit on top of the lower domain. This accords with the EM density, although the turret domains are found at different positions within the morphological units (Figure 4). We propose a reasonably simple explanation for this. In P23-77, the protein VP16 is the driver for dimer assembly, and all dimer building blocks are VP16-VP16 homodimers that do not have a turret domain. In contrast, in SH1, while the protein dimers are in positions analogous to those of VP16, these dimers always have at least one turret, while there are no turrets in positions analogous to VP17. This suggests that the protein that drives dimer formation is, in SH1, the turret-bearing subunit (VP4). Furthermore, in SH1, the dimer building blocks can be formed from either VP4 homodimers or VP4-VP7 heterodimers, but not VP7 homodimers. Thus, the underlying assembly principle appears to be conserved, but the details of the individual proteins forming the assembly and relative affinities of interaction are different.
Figure 4.
Structural Features Shared by P23-77 and SH1
Structure of VP16 dimer fitted into the cryo-EM electron density maps of the P23-77 virion (Jaatinen et al., 2008) and SH1 virion (Jäälinoja et al., 2008).
(A) VP16 in P23-77 virion density.
(B) Asymmetric unit of P23-77, showing the position of protruding VP17 turret proteins (green circles) and VP16 (pale beige circles).
(C) VP16 in SH1 density, at the same position as in (A).
(D) Asymmetric unit of SH1, showing the position of protruding equivalent turret proteins (blue circles). VP16 spans across capsomer borders in P23-77, causing bridges of density in the cryo-EM map. These bridges are found in identical position in P23-77 and SH1 and are illustrated here and in (B) as red bars. Note that, in SH1, every bridge connects a turreted protein to either a turreted or nonturreted protein.
See also Table S1.
Discussion
P23-77 Structures Shed Light on Primordial Virus Evolution
Extreme environments such as the hot springs inhabited by the host of P23-77, bacterial genus Thermus (optimal growth temperature from 65 to 75°C; Beffa et al., 1996), are dominated by a few organisms (Hacene et al., 2004; Oren, 2002; Skirnisdottir et al., 2000). However, viral communities in such environments are abundant and show a remarkable diversity of morphotypes that are not found in moderate habitats (Prangishvili, 2003; Prangishvili and Garrett, 2005; Rice et al., 2001; Sime-Ngando et al., 2011). Extreme environments appear to represent stable, nonoverlapping ecological niches in which close communities of a few highly adapted species thrive, changing extremely slowly (Drake, 2009; Friedman et al., 2004). These conditions might have allowed the survival of ancient viral forms; consequently, extreme environments like hot springs are a very appealing hunting ground in the search for ancient virus types, linked to the most primordial prokaryotic populations and perhaps to the last universal common ancestor of cells (Cavalier-Smith, 2006; Glansdorff et al., 2008; Shimizu et al., 2007).
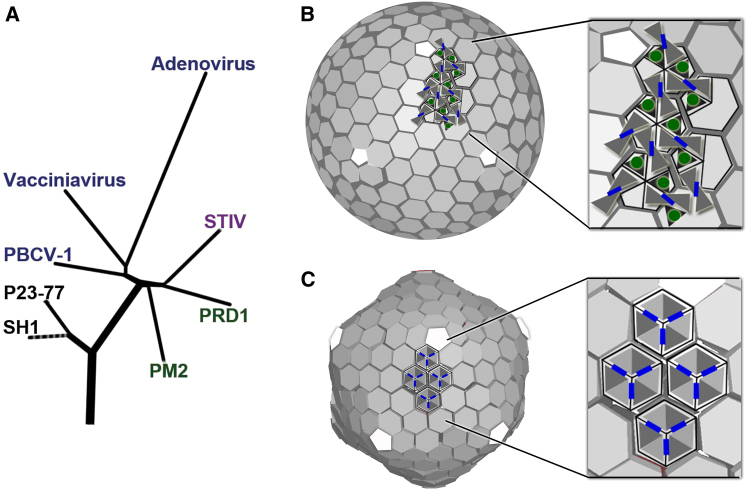
P23-77 may therefore give insights into the early stages of viral evolution. The core fold of both MCPs of P23-77 is a β-barrel that shows strong similarity to one half of the fold of the MCP of the double β-barrel lineage. The closest structural relative is the marine bacteriophage PM2, a virus considered the most ancient member of the double β-barrel lineage (Abrescia et al., 2008). We therefore propose that an ancestral virus, existing before the present domains of life formed, possessed a capsid protein with a single β-barrel that assembled in some unknown architecture presumably simpler than those we have observed. This ancestral virus utilized a single β-barrel MCP, arranged as capsomers that might be either pentameric, allowing the facile assembly of small icosahedral particles, or hexameric and pentameric, allowing the assembly of more complex particles, given a suitable mechanism to control assembly. It seems quite likely that an early gene duplication event allowed the specialization of the pentameric and hexameric proteins (pentons and hexons) and that this was the first step in the generation of more complex viruses. We propose that the present-day lineages had separate origins from this ancestor, both involving gene duplication. In one case, this led to a virus with two separate MCPs (a precursor of the P23-77/SH1 lineage), and in the other case to a tandem β-barrel structure (precursor of the double β-barrel lineage). Specialization of these two lineages would then lead to the strand-swap dimer to stabilize the virions of the P23-77 lineage and to the divergence of the two β-barrels seen in the double β-barrel lineage (Bahar et al., 2011). This hypothesis would be strengthened if it were possible to find a missing-link virus existing today with simple hexameric morphological units. Even in the absence of a missing link, the various levels of similarity between the two-protein and double β-barrel viruses suggests that they share a common ancestor. We propose a phylogeny for this superlineage in Figure 5.
Figure 5.
P23-77 Is a Member of the Vertical β-Barrel Superlineage
(A) Phylogenetic tree based on the known capsid protein structures of the vertical β-barrel superlineage members shows that P23-77 forms the earliest diverging branch in the lineage.
(B) P23-77 virion is icosahedrally ordered and utilizes single β-barrel proteins, presented with triangles, to build the capsid.
(C) Organization of bacteriophage PRD1, with individual domains of the double β-barrel capsid proteins shown as triangles. Most prominent interaction surfaces are shown in blue, revealing the difference between P23-77 and other lineage members that use compact trimers to form each capsomer. However, the capsomers of both P23-77 and PRD1 contain six β-barrels.
The structure-based phylogenetic tree was made using the SHP (Stuart et al., 1979) and the known double β-barrel structures of lineage members. For P23-77, single β- barrel capsid proteins were fused together to enable comparison.
See also Figure S2.
P23-77/SH1 Lineage Capsid Organization Differs Radically from the Double β-Barrel Lineage
We suggest that SH1 has a capsomer composition similar to that of P23-77: six molecules of two different protein species that participate in forming the hexagonal base, some with upper domains that compose the turret structures. The MCPs of both viruses correspond roughly in size, and the strong similarity of the capsomer structure suggests organizational similarity. The bridges between different capsomers probably reflect similar dimers in both P23-77 and SH1.
The use of a trimeric double β-barrel capsid protein as exemplified by the members of the adeno-PRD1 lineage instead of six single β-barrel capsid proteins halves the number of proteins necessary for building up the capsomer and reduces the assembly error rate (Jäälinoja et al., 2008), while the use of preformed trimers introduces a further quality control point in the assembly process. Furthermore, double β-barrel trimers are likely more stable than single β-barrel hexamers. In contrast, P23-77 uses various building blocks (VP16 homodimer, VP16/VP17 heterotrimer, and VP16/VP17 heterotetramer) for capsid assembly, and the crenellations might have a stabilizing function and/or sterically block inappropriate oligomerization. This method of capsid assembly is complex and, therefore, probably more error prone and less efficient. However, the resultant capsid is clearly very robust, perhaps explaining why this form of capsid seems to be very successful in thermophilic bacteria and halophilic archaea (Jalasvuori et al., 2010).
Mechanism of P23-77 Capsid Assembly
Despite the proposed homology with the double β-barrel viruses, the assembly pathways are clearly radically different. Here, we propose a model for the assembly of P23-77 that explains the extraordinarily complex organization of the capsid by assigning specialized roles to the two MCPs VP16 and VP17. We suggest that VP17 is connected to the internal membrane via the N-terminal extension that contains a polyproline helix and hydrophobic residues (this is disordered in the crystal structures; see Figure S3), while VP16 exists as preformed soluble dimers. We also note that the capsid has an inherently uneven distribution of VP17s, which are enriched around the 5-fold axes of the icosahedron and are depleted around the 3-fold axes (Figure 3). We propose that the assembly begins at the 5-fold, where five VP17s surround an unidentified penton protein and form a strong connection to the membrane, as seen in the capsid cryo-EM (Jaatinen et al., 2008). VP17s then recruit VP16 dimers, which strengthen the structure and, in turn, organize additional VP17s which may migrate while remaining attached to the viral membrane. The assembly progresses toward the 3-fold, where the lack of VP17s is explained by the assembly frontiers meeting and meshing together, excluding VP17. A striking overall result of this process is that, while the virions of the double β-barrel viruses usually assemble as flat facets that need to be glued together by ancillary proteins (Abrescia et al., 2004), the P23-77-like viruses are able assemble robust, almost spherical, capsids by building curvature into the assembly process (Figure 5).
In conclusion, detailed atomic structures of the MCPs combined with earlier cryo-EM visualization of the entire capsid have revealed an unexpected, and novel, architecture for P23-77. Comparison of the protein structures suggests that viruses of this form, which are found in extreme environments, have changed little over billions of years and are vestiges of a branch of the upright β-barrel viruses that may have been wiped out in other organisms by the highly successful double β-barrel lineage viruses.
Experimental Procedures
Protein Expression and Purification
Expression plasmids containing genes for VP16 (ORF16) and VP17 (ORF17) ligated to pET22b(+) vector were used for high yield expression in E. coli HMS174(DE3) cells. Expression was induced with 1 mM IPTG, and proteins were extracted with French pressure cell treatment and purified by a sequence of optimized chromatography steps including anion exchange and size exclusion chromatography. Details of the purification process, crystallization, and subsequent diffraction experiments were reported recently (Rissanen et al., 2012).
Crystallization
Crystallization experiments with the recombinant proteins yielded four well-diffracting crystal types: VP16-type-1 (crystallized in 5% polyethylene glycol [PEG] 1000 and 5% PEG 8000), VP16-type-2 (crystallized in 20% PEG 6000, 0.1 M citrate, pH 4), VP17 monomer (crystallized in 1.9 M sodium formate, 0.1 M Bis-Tris, pH 7.0), and a VP16/VP17 complex (crystallized in 1.1 M di-ammonium tartrate, pH 7). VP16-virion-derived crystallized from 25% PEG 3350, 0.15 M NaCl, 5 mM MgCl2, 0.1 M citric acid, pH 3.5, and 20 mM Tris-HCl, pH 7.5.
Structure Determination and Refinement
Native data for crystals of VP16-type-1 were collected at beamline I03 at wavelength λ = 0.979 Å; of VP16-type-2, at I04 (λ = 1.000 Å); of VP16-virion-derived, at I24 (λ = 0.969 Å); of VP17, at I04 (λ = 1.071 Å); and of the VP16/VP17-complex, at I24 (λ = 1.071 Å). In some cases, crystals were exposed at several positions, but diffraction data from one crystal only were used for each data set. All data were processed with xia2/XDS (Kabsch, 1993; Winter, 2010).
The structures of VP16-type-1 and VP17 were solved by isomorphous replacement by soaking crystals in heavy metal solutions. For VP16-type-1 crystals, the original crystallization solution of 5% PEG 1000 and 5% PEG 8000 was found not to be suitable for these experiments. By adding 30% PEG 6000, 0.1 M HEPES, pH 7.0, at a ratio of 1/1 (v/v) to the original crystallization solution, crystals could be stabilized and soaking experiments could be carried out. A good derivative of VP16 was prepared by soaking a crystal in lead acetate at a concentration of more than 20 mM for 1 hr. Derivative data were collected at beamline I03 at λ = 0.940 Å, a wavelength close to the LIII edge of lead. Heavy metal binding sites were determined with SHELXD (Schneider and Sheldrick, 2002). Phases, calculated to 2.68 Å with the program autoSHARP (Vonrhein et al., 2007), resulted in an isomorphous phasing power of 1.28/1.42 for centric/acentric reflections and an anomalous phasing power of 0.96. The initial model built by ARP/wARP (Perrakis et al., 1999) within autoSHARP (Vonrhein et al., 2007) contained 95% of all VP16 residues.
For VP17, the mother liquor was changed to 4 M NaCl, 0.1 M HEPES buffer, pH 7.0, to increase the solubility of heavy metals. A crystal soaked in mercury acetate for 16 hr at a concentration of more than 20 mM was found to be a sufficiently good derivative to solve the structure. Derivative data were collected at beamline I02 at λ = 1.009 Å, the LIII peak wavelength for Hg in the crystal as determined by a fluorescent scan. Phases calculated to 3.10 Å with autoSHARP (Vonrhein et al., 2007) gave an isomorphous phasing power of 0.28/0.24 for centric/acentric reflections and an anomalous phasing power of 0.30. The program ARP/wARP (Perrakis et al., 1999) built more than 57% of the total number of residues automatically.
The structures of VP16-type-2, VP16-virion-derived, and the VP16/VP17 complex were solved by molecular replacement with PHASER (McCoy et al., 2007). The asymmetric unit of VP16-type-1, VP16-type-2, and the complex contains one molecule/complex, that of VP17 contains two molecules, and that of VP16-virion-derived contains eight molecules in the form of four dimers.
Initial models of all structures were improved by cycles of manual model building with Coot (Emsley and Cowtan, 2004), followed by refinement with Buster (Bricogne et al., 2011) and/or Phenix (Adams et al., 2010). TLS parameters were defined with the help of the TLSMD server (Merritt and Painter, 2006). Molprobity (Chen et al., 2010) was used for final corrections and structure validations. Ramachandran plots calculated with the final coordinates of VP16-type-1, VP16-type-2, VP16-virion-derived, VP17, and the VP16/VP17 complex showed that 100, 99.4, 99.9, 98.1, and 98.6% of residues lie in favored regions with 0.0, 0.0, 0.0, 0.5, and 0.6% outliers, respectively.
Capsid Model Building and Refinement
Atomic structures of VP16, VP17, and the VP16/VP17 complex were manually fitted to the cryo-EM density of P23-77 capsid in Coot to produce the asymmetric unit (see Supplemental Experimental Procedures). The asymmetric unit, composed of 18 copies of VP16 and nine copies of VP17 as illustrated in Figure 3, contained all proteins as separate molecules. These coordinates were then loaded to VEDA together with the capsid electron density map. After assigning icosahedral 2-fold axes to be along the axes of the Cartesian coordinate system, VEDA was able to build up the whole icosahedral capsid using 60 copies of the asymmetric unit and refine the coordinates to the density. VEDA analyzes the degree of the fit with CC and R values, which were 74.1% and 50.2%, respectively. Coordinates were exported from VEDA after refinement and compared to the crystal structures and used in producing molecular graphics with program PyMol (The PyMOL Molecular Graphics System, Version 1.5.0.5, Schrödinger, LLC, http://www.pymol.org).
Acknowledgments
We thank Dr. Tom Walter for help with crystallization, Petri Papponen for valuable assistance in protein purification, Jun Dong for information technology support, Diamond Light Source for access to beamlines I02, I03, I04, and I24 (Proposal Number 8423), and the staff at these beamlines for excellent technical support. In Oxford, this work has been supported by the Medical Research Council, and administrative support was provided by the Wellcome Trust (Grant 075491/Z/04). This work was also supported by the EU P-CUBE program (Grant 227764), the Oxford Instruct Centre, the Finnish Centre of Excellence (CoE) Program of the Academy of Finland 2006–2011 CoE in Virus Research (#1129648) and CoE in Biological Interactions 2012-2017 (#252411), an Academy of Finland personal grant to J.K.H.B. (#251106), and a Finnish Cultural Foundation personal grant to S.M. (Grant 00110600).
Contributor Information
Jaana K.H. Bamford, Email: jaana.bamford@jyu.fi.
David I. Stuart, Email: dave@strubi.ox.ac.uk.
Accession Numbers
The coordinates and structure factors reported in this paper have been deposited in the Protein Data Bank with ID codes 3ZMO (VP16-type-1), 3ZN4 (VP16-type-2), 3ZN5 (VP16-virus-derived), 3ZMN (VP17), and 3ZN6 (VP16/VP17 complex).
Supplemental Information
References
- Abrescia N.G., Cockburn J.J., Grimes J.M., Sutton G.C., Diprose J.M., Butcher S.J., Fuller S.D., San Martín C., Burnett R.M., Stuart D.I. Insights into assembly from structural analysis of bacteriophage PRD1. Nature. 2004;432:68–74. doi: 10.1038/nature03056. [DOI] [PubMed] [Google Scholar]
- Abrescia N.G., Grimes J.M., Kivelä H.M., Assenberg R., Sutton G.C., Butcher S.J., Bamford J.K., Bamford D.H., Stuart D.I. Insights into virus evolution and membrane biogenesis from the structure of the marine lipid-containing bacteriophage PM2. Mol. Cell. 2008;31:749–761. doi: 10.1016/j.molcel.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Abrescia N.G., Bamford D.H., Grimes J.M., Stuart D.I. Structure unifies the viral universe. Annu. Rev. Biochem. 2012;81:795–822. doi: 10.1146/annurev-biochem-060910-095130. [DOI] [PubMed] [Google Scholar]
- Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar M.W., Graham S.C., Stuart D.I., Grimes J.M. Insights into the evolution of a complex virus from the crystal structure of vaccinia virus D13. Structure. 2011;19:1011–1020. doi: 10.1016/j.str.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford D.H., Burnett R.M., Stuart D.I. Evolution of viral structure. Theor. Popul. Biol. 2002;61:461–470. doi: 10.1006/tpbi.2002.1591. [DOI] [PubMed] [Google Scholar]
- Beffa T., Blanc M., Lyon P.F., Vogt G., Marchiani M., Fischer J.L., Aragno M. Isolation of Thermus strains from hot composts (60 to 80 degrees C) Appl. Environ. Microbiol. 1996;62:1723–1727. doi: 10.1128/aem.62.5.1723-1727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S.D., Bamford J.K., Bamford D.H., Burnett R.M. Viral evolution revealed by bacteriophage PRD1 and human adenovirus coat protein structures. Cell. 1999;98:825–833. doi: 10.1016/s0092-8674(00)81516-0. [DOI] [PubMed] [Google Scholar]
- Benson S.D., Bamford J.K., Bamford D.H., Burnett R.M. Does common architecture reveal a viral lineage spanning all three domains of life? Mol. Cell. 2004;16:673–685. doi: 10.1016/j.molcel.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Bergh O., Børsheim K.Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Bricogne G., Blanc E., Brandl M., Flensburg C., Keller P., Paciorek W., Roversi P., Sharff A., Smart O.S., Vonrhein C. Global Phasing Ltd.; Cambridge, UK: 2011. BUSTER. [Google Scholar]
- Chen V.B., Arendall W.B., 3rd, Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., Murray L.W., Richardson J.S., Richardson D.C. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Rooting the tree of life by transition analyses. Biol. Direct. 2006;1:19. doi: 10.1186/1745-6150-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J.W. Avoiding dangerous missense: thermophiles display especially low mutation rates. PLoS Genet. 2009;5:e1000520. doi: 10.1371/journal.pgen.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Forterre P. The two ages of the RNA world, and the transition to the DNA world: a story of viruses and cells. Biochimie. 2005;87:793–803. doi: 10.1016/j.biochi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Friedman R., Drake J.W., Hughes A.L. Genome-wide patterns of nucleotide substitution reveal stringent functional constraints on the protein sequences of thermophiles. Genetics. 2004;167:1507–1512. doi: 10.1534/genetics.104.026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glansdorff N., Xu Y., Labedan B. The last universal common ancestor: emergence, constitution and genetic legacy of an elusive forerunner. Biol. Direct. 2008;3:29. doi: 10.1186/1745-6150-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacene H., Rafa F., Chebhouni N., Boutaiba S., Bhatnagar T., Barratti J.C., Ollivier B. Biodiversity of prokaryotic microflora in E1 Golea Salt lake, Algerian Sahara. J. Arid Environ. 2004;58:273–284. [Google Scholar]
- Happonen L.J., Redder P., Peng X., Reigstad L.J., Prangishvili D., Butcher S.J. Familial relationships in hyperthermo- and acidophilic archaeal viruses. J. Virol. 2010;84:4747–4754. doi: 10.1128/JVI.02156-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatherley D., Graham S.C., Turner J., Harlos K., Stuart D.I., Barclay A.N. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol. Cell. 2008;31:266–277. doi: 10.1016/j.molcel.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Jäälinoja H.T., Roine E., Laurinmäki P., Kivelä H.M., Bamford D.H., Butcher S.J. Structure and host-cell interaction of SH1, a membrane-containing, halophilic euryarchaeal virus. Proc. Natl. Acad. Sci. USA. 2008;105:8008–8013. doi: 10.1073/pnas.0801758105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaatinen S.T., Happonen L.J., Laurinmäki P., Butcher S.J., Bamford D.H. Biochemical and structural characterisation of membrane-containing icosahedral dsDNA bacteriophages infecting thermophilic Thermus thermophilus. Virology. 2008;379:10–19. doi: 10.1016/j.virol.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Jalasvuori M., Jaatinen S.T., Laurinavicius S., Ahola-Iivarinen E., Kalkkinen N., Bamford D.H., Bamford J.K. The closest relatives of icosahedral viruses of thermophilic bacteria are among viruses and plasmids of the halophilic archaea. J. Virol. 2009;83:9388–9397. doi: 10.1128/JVI.00869-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalasvuori M., Pawlowski A., Bamford J.K.H. A unique group of virus-related, genome-integrating elements found solely in the bacterial family Thermaceae and the archaeal family Halobacteriaceae. J. Bacteriol. 2010;192:3231–3234. doi: 10.1128/JB.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 1993;26:795–800. [Google Scholar]
- Khayat R., Tang L., Larson E.T., Lawrence C.M., Young M., Johnson J.E. Structure of an archaeal virus capsid protein reveals a common ancestry to eukaryotic and bacterial viruses. Proc. Natl. Acad. Sci. USA. 2005;102:18944–18949. doi: 10.1073/pnas.0506383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivelä H.M., Roine E., Kukkaro P., Laurinavicius S., Somerharju P., Bamford D.H. Quantitative dissociation of archaeal virus SH1 reveals distinct capsid proteins and a lipid core. Virology. 2006;356:4–11. doi: 10.1016/j.virol.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Koonin E.V., Senkevich T.G., Dolja V.V. The ancient Virus World and evolution of cells. Biol. Direct. 2006;1:29. doi: 10.1186/1745-6150-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E. Crystal contacts as nature’s docking solutions. J. Comput. Chem. 2010;31:133–143. doi: 10.1002/jcc.21303. [DOI] [PubMed] [Google Scholar]
- Krissinel E., Henrick K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Krupovic M., Bamford D.H. Virus evolution: how far does the double beta-barrel viral lineage extend? Nat. Rev. Microbiol. 2008;6:941–948. doi: 10.1038/nrmicro2033. [DOI] [PubMed] [Google Scholar]
- McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt E.A., Painter J. TLSMD web server for the generation of multi-group TLS models. J. Appl. Crystallogr. 2006;39:109–111. [Google Scholar]
- Navaza J., Lepault J., Rey F.A., Alvarez-Rúa C., Borge J. On the fitting of model electron densities into EM reconstructions: a reciprocal-space formulation. Acta Crystallogr. D Biol. Crystallogr. 2002;58:1820–1825. doi: 10.1107/s0907444902013707. [DOI] [PubMed] [Google Scholar]
- Oren A. Molecular ecology of extremely halophilic Archaea and Bacteria. FEMS Microbiol. Ecol. 2002;39:1–7. doi: 10.1111/j.1574-6941.2002.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Perrakis A., Morris R., Lamzin V.S. Automated protein model building combined with iterative structure refinement. Nat. Struct. Biol. 1999;6:458–463. doi: 10.1038/8263. [DOI] [PubMed] [Google Scholar]
- Prangishvili D. Evolutionary insights from studies on viruses of hyperthermophilic archaea. Res. Microbiol. 2003;154:289–294. doi: 10.1016/S0923-2508(03)00073-1. [DOI] [PubMed] [Google Scholar]
- Prangishvili D., Garrett R.A. Viruses of hyperthermophilic Crenarchaea. Trends Microbiol. 2005;13:535–542. doi: 10.1016/j.tim.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Pritham E.J., Putliwala T., Feschotte C. Mavericks, a novel class of giant transposable elements widespread in eukaryotes and related to DNA viruses. Gene. 2007;390:3–17. doi: 10.1016/j.gene.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Rice G., Stedman K., Snyder J., Wiedenheft B., Willits D., Brumfield S., McDermott T., Young M.J. Viruses from extreme thermal environments. Proc. Natl. Acad. Sci. USA. 2001;98:13341–13345. doi: 10.1073/pnas.231170198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissanen I., Pawlowski A., Harlos K., Grimes J.M., Stuart D.I., Bamford J.K. Crystallization and preliminary crystallographic analysis of the major capsid proteins VP16 and VP17 of bacteriophage P23-77. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012;68:580–583. doi: 10.1107/S1744309112010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T.R., Sheldrick G.M. Substructure solution with SHELXD. Acta Crystallogr. D Biol. Crystallogr. 2002;58:1772–1779. doi: 10.1107/s0907444902011678. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Yokobori S., Ohkuri T., Yokogawa T., Nishikawa K., Yamagishi A. Extremely thermophilic translation system in the common ancestor commonote: ancestral mutants of Glycyl-tRNA synthetase from the extreme thermophile Thermus thermophilus. J. Mol. Biol. 2007;369:1060–1069. doi: 10.1016/j.jmb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Sime-Ngando T., Lucas S., Robin A., Tucker K.P., Colombet J., Bettarel Y., Desmond E., Gribaldo S., Forterre P., Breitbart M., Prangishvili D. Diversity of virus-host systems in hypersaline Lake Retba, Senegal. Environ. Microbiol. 2011;13:1956–1972. doi: 10.1111/j.1462-2920.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- Skirnisdottir S., Hreggvidsson G.O., Hjörleifsdottir S., Marteinsson V.T., Petursdottir S.K., Holst O., Kristjansson J.K. Influence of sulfide and temperature on species composition and community structure of hot spring microbial mats. Appl. Environ. Microbiol. 2000;66:2835–2841. doi: 10.1128/aem.66.7.2835-2841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen A.F., Yu C., Evans E.J., Stuart D.I., Davis S.J., Gilbert R.J. Domain metastability: a molecular basis for immunoglobulin deposition? J. Mol. Biol. 2010;399:207–213. doi: 10.1016/j.jmb.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D.I., Levine M., Muirhead H., Stammers D.K. Crystal structure of cat muscle pyruvate kinase at a resolution of 2.6 A. J. Mol. Biol. 1979;134:109–142. doi: 10.1016/0022-2836(79)90416-9. [DOI] [PubMed] [Google Scholar]
- Winter G. xia2: an expert system for macromolecular crystallography data reduction. J. Appl. Crystallogr. 2010;43:186–190. [Google Scholar]
- Woese C.R. On the evolution of cells. Proc. Natl. Acad. Sci. USA. 2002;99:8742–8747. doi: 10.1073/pnas.132266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wommack K.E., Colwell R.R. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000;64:69–114. doi: 10.1128/mmbr.64.1.69-114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonrhein C., Blanc E., Roversi P., Bricogne G. Automated structure solution with autoSHARP. Methods Mol. Biol. 2007;364:215–230. doi: 10.1385/1-59745-266-1:215. [DOI] [PubMed] [Google Scholar]
- Yu M.X., Slater M.R., Ackermann H.W. Isolation and characterization of Thermus bacteriophages. Arch. Virol. 2006;151:663–679. doi: 10.1007/s00705-005-0667-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.