Abstract
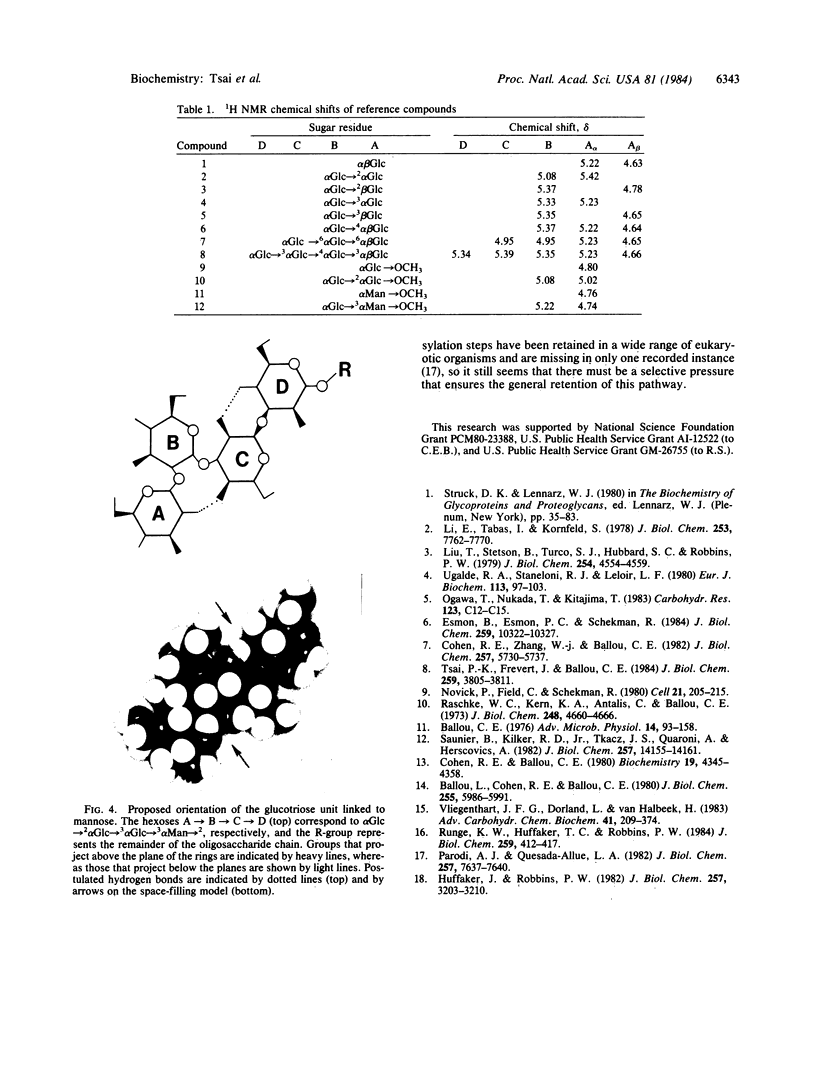
The total cell wall mannoprotein has been isolated from a mutant of Saccharomyces cerevisiae that fails to remove the glucose units of the dolichol-linked precursor after transfer of the oligosaccharide to asparagine units in the protein. The oligosaccharides released from this mannoprotein by endoglucosaminidase H digestion show 1H NMR signals assignable to three alpha-linked glucose units as delta 5.52, 5.27, and 5.17, and a comparison with the chemical shifts of reference compounds shows that these signals are consistent with the structure alpha Glc----2 alpha Glc----3 alpha Man----2. This provides a direct confirmation for the structure previously assigned to the lipid-linked precursor. Analysis of the larger oligosaccharides confirms that the presence of the glucose units does not prevent elongation of the alpha 1----6-linked polymannose backbone or addition of alpha 1----3-linked mannose to the core.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14(11):93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- Ballou L., Cohen R. E., Ballou C. E. Saccharomyces cerevisiae mutants that make mannoproteins with a truncated carbohydrate outer chain. J Biol Chem. 1980 Jun 25;255(12):5986–5991. [PubMed] [Google Scholar]
- Cohen R. E., Ballou C. E. Linkage and sequence analysis of mannose-rich glycoprotein core oligosaccharides by proton nuclear magnetic resonance spectroscopy. Biochemistry. 1980 Sep 2;19(18):4345–4358. doi: 10.1021/bi00559a031. [DOI] [PubMed] [Google Scholar]
- Cohen R. E., Zhang W., Ballou C. E. Effects of mannoprotein mutations on Saccharomyces cerevisiae core oligosaccharide structure. J Biol Chem. 1982 May 25;257(10):5730–5737. [PubMed] [Google Scholar]
- Esmon B., Esmon P. C., Schekman R. Early steps in processing of yeast glycoproteins. J Biol Chem. 1984 Aug 25;259(16):10322–10327. [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Temperature-sensitive yeast mutants deficient in asparagine-linked glycosylation. J Biol Chem. 1982 Mar 25;257(6):3203–3210. [PubMed] [Google Scholar]
- Li E., Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. I. Structure of the lipid-linked oligosaccharide precursor of the complex-type oligosaccharides of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7762–7770. [PubMed] [Google Scholar]
- Liu T., Stetson B., Turco S. J., Hubbard S. C., Robbins P. W. Arrangement of glucose residues in the lipid-linked oligosaccharide precursor of asparaginyl oligosaccharides. J Biol Chem. 1979 Jun 10;254(11):4554–4559. [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Nukada T., Kitajima T. Synthesis of a linear, hexahexosyl unit of a high-mannose type of glycan chain of a glycoprotein. Carbohydr Res. 1983 Nov 11;123(1):C12–C15. doi: 10.1016/0008-6215(83)88399-2. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Quesada-Allue L. A. Protein glycosylation in Trypanosoma cruzi. I. Characterization of dolichol-bound monosaccharides and oligosaccharides synthesized "in vivo". J Biol Chem. 1982 Jul 10;257(13):7637–7640. [PubMed] [Google Scholar]
- Raschke W. C., Kern K. A., Antalis C., Ballou C. E. Genetic control of yeast mannan structure. Isolation and characterization of mannan mutants. J Biol Chem. 1973 Jul 10;248(13):4660–4666. [PubMed] [Google Scholar]
- Runge K. W., Huffaker T. C., Robbins P. W. Two yeast mutations in glucosylation steps of the asparagine glycosylation pathway. J Biol Chem. 1984 Jan 10;259(1):412–417. [PubMed] [Google Scholar]
- Saunier B., Kilker R. D., Jr, Tkacz J. S., Quaroni A., Herscovics A. Inhibition of N-linked complex oligosaccharide formation by 1-deoxynojirimycin, an inhibitor of processing glucosidases. J Biol Chem. 1982 Dec 10;257(23):14155–14161. [PubMed] [Google Scholar]
- Tsai P. K., Frevert J., Ballou C. E. Carbohydrate structure of Saccharomyces cerevisiae mnn9 mannoprotein. J Biol Chem. 1984 Mar 25;259(6):3805–3811. [PubMed] [Google Scholar]
- Ugalde R. A., Staneloni R. J., Leloir L. F. Microsomal glucosidases of rat liver. Partial purification and inhibition by disaccharides. Eur J Biochem. 1980 Dec;113(1):97–103. doi: 10.1111/j.1432-1033.1980.tb06144.x. [DOI] [PubMed] [Google Scholar]