Abstract
There are several well-known challenges in the management of overactive bladder (OAB). This brief review discusses four of these: shortcomings of current OAB classification, incomplete understanding of pathophysiology, undertreatment and managing patient expectations.
The challenge of OAB classification
One of the key variables one needs to consider in any discussion of the management of overactive bladder (OAB) is the definition of the term itself. The current definition is “urgency, with or without urge incontinence, usually with frequency and nocturia,” in the absence of an underlying metabolic or pathologic condition.1 This definition was arrived at by consensus as a result of deliberations by the Standardisation Sub-Committee of the International Continence Society. Since its publication, however, some have raised objections to the suitability of the definition. The “absence of underlying condition” clause, for example, excludes many individuals (e.g., those with urinary tract infection, carcinoma in situ, adjacent inflammation) from being diagnosed with OAB, despite the fact that they might be clinically similar to those who do receive a diagnosis and may benefit from the same treatments.
Should expert bodies reconvene to discuss changing the definition of OAB, they might perhaps consider a new classification system in which patients are divided into subgroups based on distinguishing characteristics. The two broad categories in such a system would be neurogenic and non-neurogenic, with the latter group being further subdivided into those due to an identifiable cause (which is presumably reversible) and idiopathic cases.
One characteristic that may not be relevant for classification is “dry” versus “wet” OAB. Research suggests that the pathophysiology of these two types of OAB is probably the same, and treatment strategies do not differ for the two groups.2
It is, however, important to identify which patients experience pain with urinary symptoms (painful bladder syndrome [PBS]), as the pathophysiology of their condition likely differs from OAB patients who do not experience pain. So while there may be an overlap between patients with OAB and PBS (Fig. 1), they should be considered separate entities and treated as such.2,3
Fig. 1.
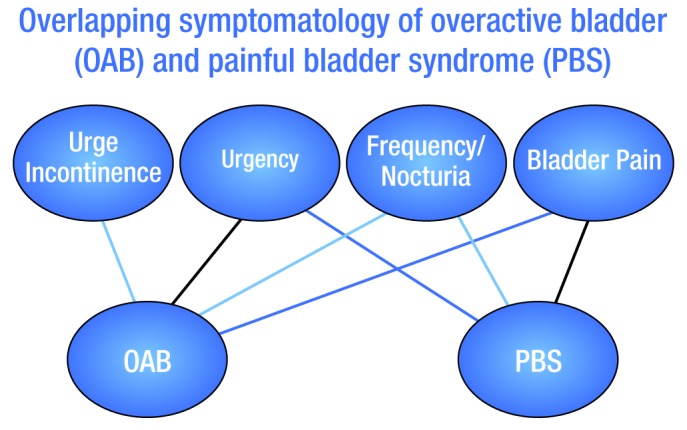
Overlapping symptomatology of overactive bladder (OAB) and painful bladder syndrome (PBS).
To what extent the pathophysiology of OAB is linked to that of other lower urinary tract problems (e.g., bladder outlet obstruction [BOO] prolapse, stress urinary incontinence [SUI]) is not clear. However, there is typically an improvement in OAB symptoms after correction of significant prolapse, SUI and/or BOO in a situation where they coexist with OAB.
Current understanding of OAB pathophysiology
There are various theories that have been put forth in an attempt to explain the pathophysiologic mechanisms underlying OAB. While no single set of data or hypothesis explains all involuntary contractions or occurrences of urgency/frequency, four concepts seem to be valid: 1) patients with OAB have faulty central inhibition, which leads to enhancement of excitatory neurotransmission in the micturition reflex pathway (neurogenic); 2) there is partial denervation of smooth muscle, which leads to co-ordinated myogenic contractions and increased bladder pressure (myogenic); 3) there is a “leaking” of acetylcholine from parasympathetic nerves during filling/storage, which leads to afferent activation (neurogenic-myogenic); and 4) abnormal signals originating in the urothelium are influenced by generation and release of local mediators (e.g., acetylcholine, nitric oxide, urothelial-derived inhibitory factor).
The relative contribution of each of these hypothetical pathways in the development of OAB is still not known. However, recognition that each patient may have a distinct set of pathophysiologic contributors may help to explain the variable response to treatments used for OAB. The myogenic pathway and non-muscarinic modes of sensory activation and efferent neurotransmission, for example, would not be expected to be responsive to treatment with antimuscarinic medication.
Further investigation into the pathophysiology of OAB will be essential to help guide the development of efficacious and safe new therapies using traditional or novel pathways.
The challenge of undertreatment
Treatment strategies include the combined use of behavioural modification and oral drug therapy. For patients with significant coexisting pelvic floor abnormalities (e.g., prolapse, SUI) and/or BOO, these problems also need to be addressed as part of routine treatment. These interventions can help reduce the substantial impact of OAB symptoms on patient quality of life. However, while these strategies are well known, research in OAB indicates that there is a substantial gap between the number of individuals who could benefit from OAB therapy and the number of individuals who are actively taking these treatments. One of the key problems is the very low persistence rates with OAB medications;4,5 the proportion of patients still taking anticholinergic medication after one year ranges from 14% to 35%.4
The challenge of patient expectations
The ultimate goal of OAB therapy is symptom resolution. However, this may not be possible for many patients. Setting realistic treatment goals is a critical part of managing the physician-patient relationship and increases the chances that the patient will persist with therapy. A realistic goal for most patients is symptom improvement.
Conclusions
OAB is a condition associated with substantial deleterious effects on patient quality of life. Although there are treatments available that may help alleviate the symptoms and improve quality of life, there are several barriers that have hampered these efforts to date. Revision of the current definition may lead to more patients being treated with potentially helpful intervention; furthering our understanding of the pathophysiology will help better direct treatment strategies and research efforts; and setting realistic goals is critical to helping ensure patients stay on treatment.
Footnotes
Competing interests: This article is part of a CUAJ supplement sponsored by Astellas Pharma Canada, Inc.
References
- 1.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/S0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Wein AJ, Rackley RR. Overactive bladder: a better understanding of pathophysiology, diagnosis and management. J Urol. 2006;175:S5–10. doi: 10.1016/S0022-5347(05)00313-7. [DOI] [PubMed] [Google Scholar]
- 3.Abrams P, Hanno P, Wein A. Overactive bladder and painful bladder syndrome: there need not be confusion. Neurourol Urodyn. 2005;24:149–50. doi: 10.1002/nau.20082. [DOI] [PubMed] [Google Scholar]
- 4.Wagg A, Compion G, Fahey A, et al. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int. 2012;110:1767–74. doi: 10.1111/j.1464-410X.2012.11023.x. [DOI] [PubMed] [Google Scholar]
- 5.Gopal M, Haynes K, Bellamy SL, et al. Discontinuation rates of anticholinergic medications used for the treatment of lower urinary tract symptoms. Obstet Gynecol. 2008;112:1311–8. doi: 10.1097/AOG.0b013e31818e8aa4. [DOI] [PubMed] [Google Scholar]


