Abstract
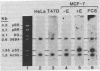
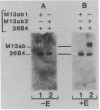
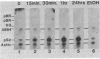
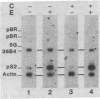
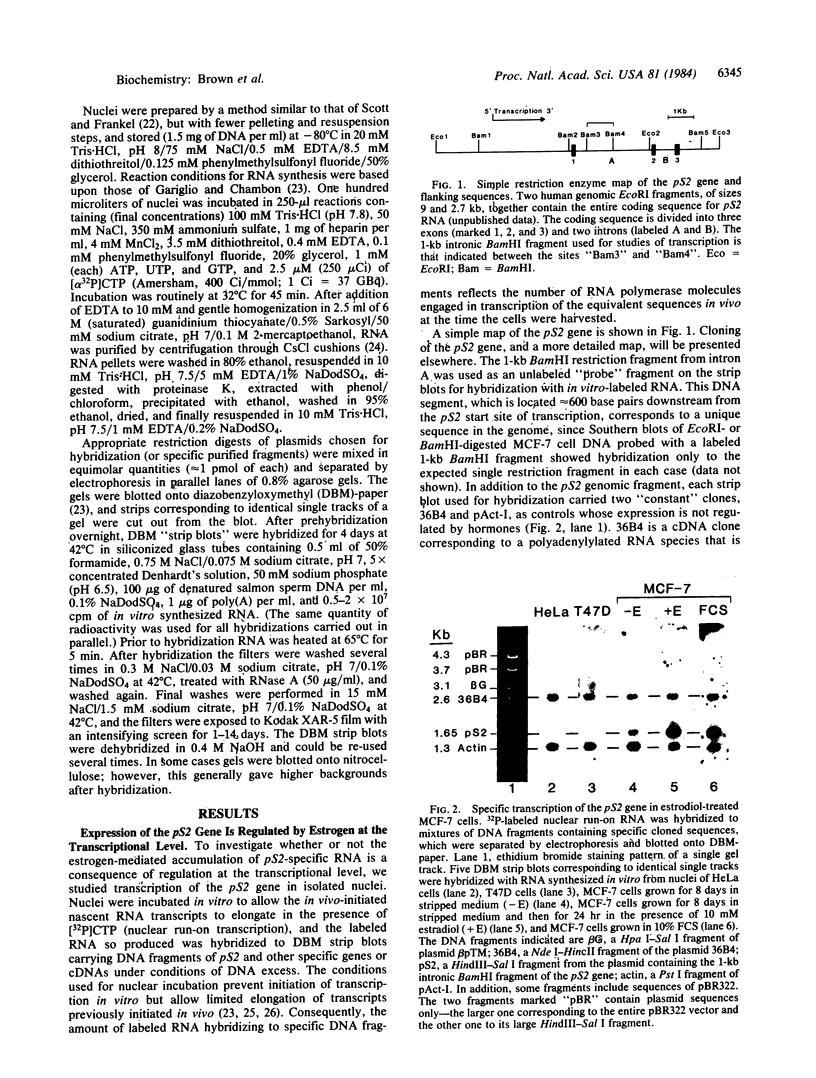
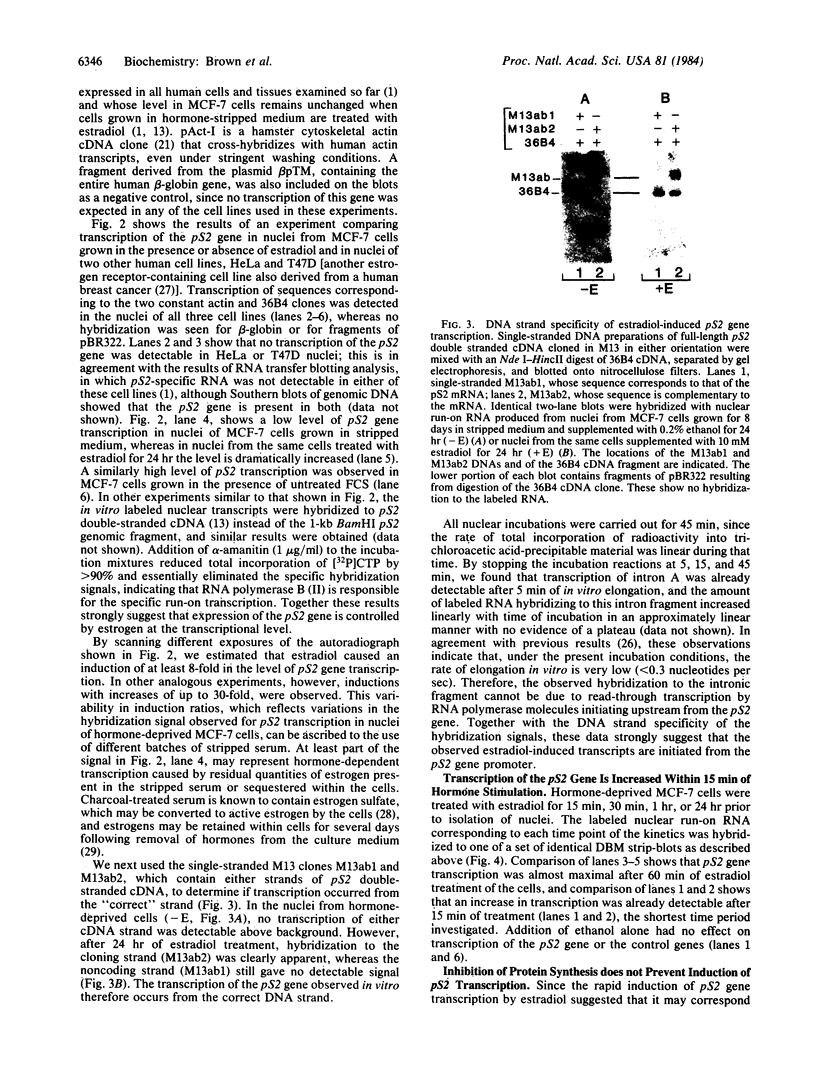
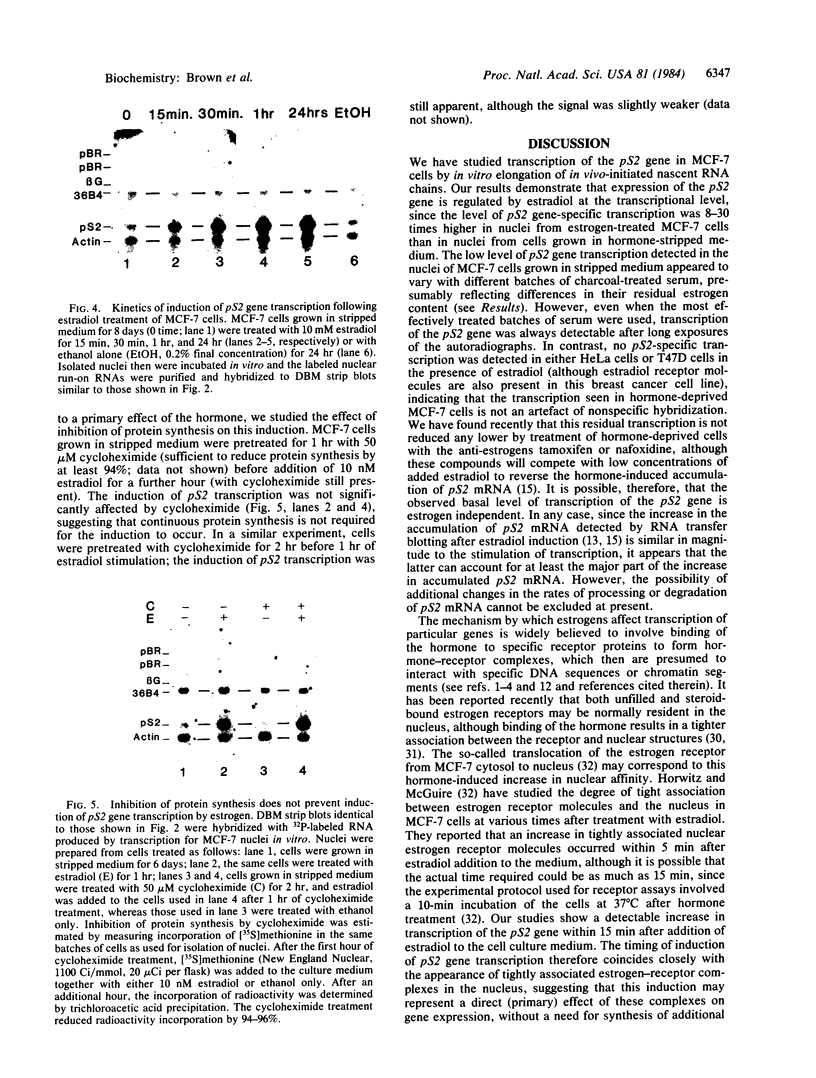
We have shown previously that an increase in the level of accumulated pS2 mRNA is first detectable in MCF-7 cells after 3 hr of estradiol treatment. Using in vitro nuclear run-on transcription with nuclei prepared from MCF-7 cells grown in the presence of estradiol or in estradiol-stripped medium, we demonstrate here that expression of the pS2 gene is controlled by estrogen at the transcriptional level. Induction of transcription is a very early event that is already apparent within 15 min after addition of estradiol to the culture medium. In addition, pretreatment of the cells with the protein synthesis inhibitor cycloheximide does not prevent induction of pS2 gene transcription, indicating that it corresponds to a primary effect of estrogen. The pS2 gene in MCF-7 cells represents a unique example of a human gene whose transcription is directly controlled by estrogen.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock M. L., Shapiro D. J. Estrogen regulates the absolute rate of transcription of the Xenopus laevis vitellogenin genes. J Biol Chem. 1983 May 10;258(9):5449–5455. [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- Brooks S. C., Locke E. R., Soule H. D. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973 Sep 10;248(17):6251–6253. [PubMed] [Google Scholar]
- Chambon P., Ramuz M., Doly J. Relation between soluble DNA-dependent RNA polymerase and "aggregate" RNA polymerase. Biochem Biophys Res Commun. 1965 Oct 26;21(2):156–161. doi: 10.1016/0006-291x(65)90102-6. [DOI] [PubMed] [Google Scholar]
- Coezy E., Borgna J. L., Rochefort H. Tamoxifen and metabolites in MCF7 cells: correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res. 1982 Jan;42(1):317–323. [PubMed] [Google Scholar]
- Cox R. F. Quantitation of elongating form A and B RNA polymerases in chick oviduct nuclei and effects of estradiol. Cell. 1976 Mar;7(3):455–465. doi: 10.1016/0092-8674(76)90176-8. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Dodemont H. J., Soriano P., Quax W. J., Ramaekers F., Lenstra J. A., Groenen M. A., Bernardi G., Bloemendal H. The genes coding for the cytoskeletal proteins actin and vimentin in warm-blooded vertebrates. EMBO J. 1982;1(2):167–171. doi: 10.1002/j.1460-2075.1982.tb01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariglio P., Bellard M., Chambon P. Clustering of RNA polymerase B molecules in the 5' moiety of the adult beta-globin gene of hen erythrocytes. Nucleic Acids Res. 1981 Jun 11;9(11):2589–2598. doi: 10.1093/nar/9.11.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward M. A., Brock M. L., Shapiro D. J. Activation of vitellogenin gene transcription is a direct response to estrogen in Xenopus laevis liver. Nucleic Acids Res. 1982 Dec 20;10(24):8273–8284. doi: 10.1093/nar/10.24.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Nuclear mechanisms of estrogen action. Effects of estradiol and anti-estrogens on estrogen receptors and nuclear receptor processing. J Biol Chem. 1978 Nov 25;253(22):8185–8191. [PubMed] [Google Scholar]
- Jakowlew S. B., Breathnach R., Jeltsch J. M., Masiakowski P., Chambon P. Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984 Mar 26;12(6):2861–2878. doi: 10.1093/nar/12.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. V., Greene G. L., Closs L. E., DeSombre E. R., Nadji M. Receptors reconsidered: a 20-year perspective. Recent Prog Horm Res. 1982;38:1–40. doi: 10.1016/b978-0-12-571138-8.50006-8. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Seldran M., Geiser M. Preferential binding of estrogen-receptor complex to a region containing the estrogen-dependent hypomethylation site preceding the chicken vitellogenin II gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):429–433. doi: 10.1073/pnas.81.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keydar I., Chen L., Karby S., Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979 May;15(5):659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Riser M. E., O'Malley B. W. Regulated expression of the chicken ovalbumin gene in a human estrogen-responsive cell line. J Biol Chem. 1983 Oct 25;258(20):12693–12701. [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A. Estradiol regulates the transcription of the prolactin gene. J Biol Chem. 1982 Mar 10;257(5):2133–2136. [PubMed] [Google Scholar]
- McKnight G. S., Hager L., Palmiter R. D. Butyrate and related inhibitors of histone deacetylation block the induction of egg white genes by steroid hormones. Cell. 1980 Nov;22(2 Pt 2):469–477. doi: 10.1016/0092-8674(80)90357-8. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McKnight G. S. The induction of ovalbumin and conalbumin mRNA by estrogen and progesterone in chick oviduct explant cultures. Cell. 1978 Jun;14(2):403–413. doi: 10.1016/0092-8674(78)90125-3. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Frankel F. R. Enrichment of estradiol-receptor complexes in a transcriptionally active fraction of chromatin from MCF-7 cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1291–1295. doi: 10.1073/pnas.77.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Strobl J. S., Monaco M. E., Lippman M. E. The role of intracellular equilibria and the effect of antiestrogens on estrogen-receptor dissociation kinetics from perfused cultures of human breast cancer cells. Endocrinology. 1980 Aug;107(2):450–460. doi: 10.1210/endo-107-2-450. [DOI] [PubMed] [Google Scholar]
- Vic P., Vignon F., Derocq D., Rochefort H. Effect of estradiol on the ultrastructure of the MCF7 human breast cancer cells in culture. Cancer Res. 1982 Feb;42(2):667–673. [PubMed] [Google Scholar]
- Vignon F., Terqui M., Westley B., Derocq D., Rochefort H. Effects of plasma estrogen sulfates in mammary cancer cells. Endocrinology. 1980 Apr;106(4):1079–1086. doi: 10.1210/endo-106-4-1079. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Lieberman M. E., Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984 Feb 23;307(5953):747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]