Long chain n-3 polyunsaturated fatty acids (n-3 PUFA) derived from oily fish, eicosapentaenoic acid (20:5n-3, EPA) and particularly docosahexaenoic acid (22:6n-3, DHA), can influence cardiac myocyte electrophysiology by modulating ion channels in animal models and in vitro studies [1]. Prospective cohort studies have found a relatively consistent association between the intake of fish and decreased risk of coronary heart disease death. The GISSI trials showed that an additional intake of 0.85 g n-3 PUFA decreased coronary heart disease mortality, possibly due to an antiarrhythmic action or augmentation of autonomic tone [2–4]. More recent evidence suggests that n-3 PUFA supplementation has little effect on all-cause mortality, cardiac death, sudden death or myocardial infarction when all trials are considered in totality [5]. Measuring heart rate variability (HRV) is an indirect, non-invasive approach to assessing cardiac autonomic function [6]. Low HRV is associated with mortality after a myocardial infarction [6–9] and risk of cardiac events in the general population [10]. A meta-analysis concluded that short-term fish oil supplementation may favorably affect certain frequency domain parameters of HRV [11]. The present study examines the effects of low doses of n-3 PUFA on HRV, in healthy non-smoking men and women at moderate risk of cardiovascular disease supplemented for 1 y. Here, effects on HRV during sleep-time are investigated, thereby reducing influences from physical activity and emotional/psychological fluctuations.
The MARINA trial was a single-center dietary intervention study conducted at King's College London, UK between April 2008 and October 2010. Detailed methods and primary outcomes have been reported previously [12]. Ethical approval was obtained from the relevant research ethics committees in the UK (NREC 08/H0802/3) and written informed consent was given by participants. The study was performed in accordance with the principles outlined in the Declaration of Helsinki, and has been registered as a clinical trial at www.controlled-trials.com, unique identifier ISRCTN66664610. Non-smokers aged 45–70 years attended a screening visit and those with a medical history of cardiovascular disease (including atrial fibrillation), overall risk of cardiovascular disease in excess of 20% over the next 10 years (www.qrisk.org), cancer (excluding basal cell carcinoma) in the past five years, type 1 diabetes mellitus, uncontrolled type 2 diabetes (fasting plasma glucose > 7 mmol/L), chronic renal, liver or inflammatory bowel disease, history of substance abuse or alcoholism, pregnancy, weight change of > 3 kg in preceding 2 months, and body mass index < 20 and > 35 kg/m2 were excluded.
A randomized, placebo-controlled, double-blind parallel design tested the effects of three dose levels of n-3 PUFA versus olive oil placebo (BP specification) for 12 months. The doses chosen were selected to be equivalent to eating none (placebo), 1, 2 or 4 portions of oily fish a week and provided 0, 0.45, 0.9 and 1.8 g of n-3 PUFA/d as a purified triacylglycerol with an EPA:DHA ratio of 1.5:1 (Croda Chemicals Europe Ltd, Hull, Yorkshire, UK). Participants were randomly allocated to treatment using minimization to balance age, gender and ethnicity between treatment groups. The treatment associated with the capsule codes were concealed from all study participants, investigators and associated clinical staff.
On the day prior to each study visit, participants were requested to abstain from strenuous exercise up to the visit and to follow standardized dietary advice. Participants reported to the St Thomas' Hospital clinical research facility between 08:00 h and 10:00 h and an Actiheart monitor was fitted (CamNtech Ltd, Cambridge, UK) [13,14] to be worn for 24 h. Following isolation of data during sleep-time, determined by participant records and confirmed by activity data derived from the in-built accelerometer, the data was analyzed using Actiheart 4 software (version 4.0.91) and Kubios HRV software (version 2.0, available online at http://kubios.uef.fi/, Biosignal Analysis and Medical Imaging Group, Department of Applied Physics, University of Eastern Finland, Finland) and checked for quality. Time domain parameters included standard deviation of normal-to-normal (NN) intervals (SDNN), standard deviation of the average NN intervals in 5 min segments of the whole recording (SDANN), square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD), the percentage of adjacent NN intervals that differed by more than 50% (pNN50), and triangular index (Ti), the integral of the density distribution (the number of all NN intervals) divided by the maximum of the density distribution. Frequency domain parameters included high frequency (HF), low frequency (LF), and very low frequency (VLF) power, and the ratio of the LF and HF frequency band powers (LF/HF). Some of the measures represent overall variability: including SDNN and Ti [6]. Estimates of short-term components of HRV include RMSSD, pNN50, and HF (0.15 to 0.4 Hz). SDANN and VLF (0.003 to 0.04 Hz) reflect longer-term components, probably a combination of sympathetic and parasympathetic activities. LF power is thought to reflect sympathetic modulation of HR [6].
Heart rate variability was defined as a secondary outcome variable of this clinical trial, therefore power calculations for sample size were based on primary endpoints of the trial. Average values on treatment (mean of 6 and 12 months) data were analyzed on an intention to treat basis using analysis of covariance (ANCOVA) with linear trend test, based on a weighted combination (0:1:2:4) of the treatment effects expected in the 4 groups using Stata 11 software (StataCorp LP). Analyses were adjusted for baseline values as well as for age, gender, ethnicity and body mass index. Standard distributional checks were made, and analyses were made following log or other transformation. Following log transformation, geometric means are presented.
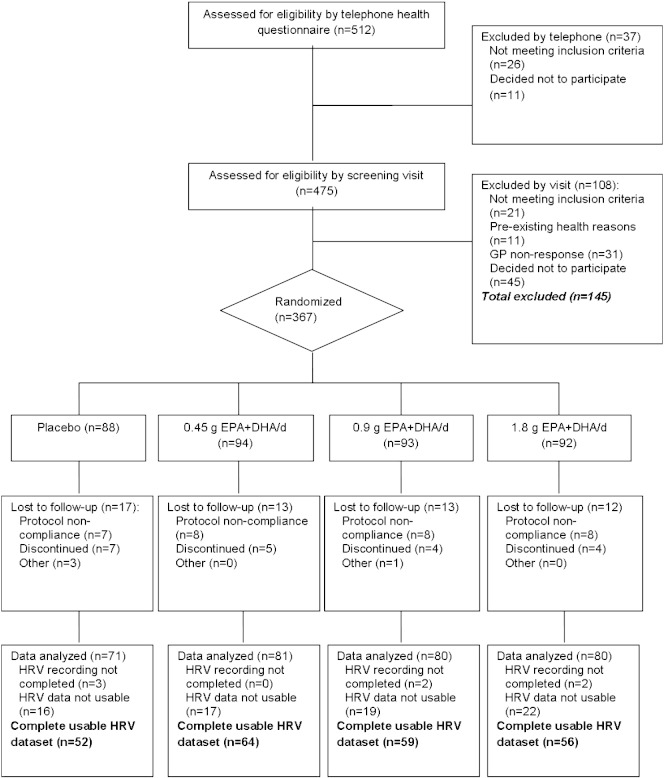
Of 367 participants randomly assigned to treatment, 305 participants completed the HRV recordings (Fig. 1). After processing and removal of poor quality data, 231 participants remained with usable sleep-time data for all three visits (Table 1). Capsule counts indicated that 209 of the participants consumed ≥ 90% of the capsules provided. The proportions of EPA and DHA in erythrocyte lipids increased in a dose-dependent manner indicating long-term compliance to the intervention [12]. There were significant increases in SDANN, Ti and VLF power with n-3 PUFA supplementation, but no evidence for a dose-related response (Table 2). There were also significant treatment effects for frequency domain parameter, LF/HF, indicating an increase in sympathetic activity relative to parasympathetic activity. There were no statistically significant effects of treatment for heart rate (data not shown), nor remaining time and frequency domain parameters.
Fig. 1.
Consort diagram of the flow of participants throughout the study and HRV data analysis.
Table 1.
Details of the study participants at run-in by randomly assigned treatment group.
| Placebo (n = 52) | 0.45 g/d (n = 64) | 0.9 g/d (n = 59) | 1.8 g/d (n = 56) | P valuec | |
|---|---|---|---|---|---|
| Age (years)a | 56 (54,58) | 55 (53,57) | 55 (54,57) | 54 (52,55) | 0.455 |
| Sexb | |||||
| Male | 21 (40.4%) | 28 (43.8%) | 22 (37.3%) | 23 (41.1%) | 0.911 |
| Female | 31 (59.6%) | 36 (66.2%) | 37 (62.7%) | 33 (58.9%) | |
| Ethnicityb | |||||
| White | 44 (84.6%) | 50 (78.1%) | 49 (83.1%) | 48 (85.7%) | 0.377 |
| Black | 2 (3.8%) | 2 (3.1%) | 0 (0.0%) | 3 (5.4%) | |
| Asian | 2 (3.8%) | 5 (7.8%) | 5 (8.5%) | 0 (0.0%) | |
| Far Eastern | 3 (5.8%) | 4 (6.3%) | 4 (6.8%) | 1 (1.8%) | |
| Other | 1 (1.9%) | 3 (4.7%) | 1 (1.7%) | 4 (7.1%) | |
| BMI (kg/m2)a | |||||
| Women | 26 (24,27) | 25 (24,27) | 26 (25,28) | 25 (24,26) | 0.387 |
| Men | 26 (25,28) | 26 (25,27) | 26 (25,28) | 25 (24,27) | 0.686 |
| SBP (mm Hg)a | 121 (116,125) | 121 (117,124) | 123 (120,127) | 119 (115,123) | 0.513 |
| DBP (mm Hg)a | 76 (73,78) | 76 (74,79) | 78 (76,81) | 76 (73,78) | 0.293 |
| Glucose (mmol/L)a | 5.4 (5.3,5.6) | 5.5 (5.3,5.8) | 5.4 (5.2,5.5) | 5.3 (5.1,5.4) | 0.160 |
| TC:HDLa | 3.7 (3.4,3.9) | 3.7 (3.5,4.0) | 3.4 (3.2,3.6) | 3.5 (3.2,3.8) | 0.127 |
| Erythrocyte EPA (%) | 1.29 (1.17,1.40) | 1.26 (1.14,1.38) | 1.32 (1.18,1.46) | 1.27 (1.14,1.40) | 0.917 |
| Erythrocyte DHA (%) | 6.51 (6.08,6.94) | 6.50 (6.16,6.84) | 6.27 (5.88,6.65) | 6.35 (5.94,6.76) | 0.771 |
SBP, seated systolic blood pressure; DBP, seated diastolic blood pressure; TC:HDL, total cholesterol to high density lipoprotein cholesterol ratio; Asian = South Asian, SE Asian & Middle Eastern.
Mean, 95% confidence intervals in parentheses.
n (%).
P values are given for comparisons between treatment groups by one-way ANOVA for age, BMI, BP, fasting glucose, TC:HDL ratio and erythrocyte membrane n-3 PUFA, and by chi-square test for distributions of sexes and ethnic groups between treatment groups.
Table 2.
Interbeat interval and time and frequency domain heart rate variability indices at baseline and following treatment with increasing doses of (n-3) long chain polyunsaturated fatty acids versus placebo. Treatment effects are corrected for age, sex, ethnicity, BMI, and the pre-treatment value of the measure.
| Placebo (n = 52) | 0.45 g/d (n = 64) | 0.9 g/d (n = 59) | 1.8 g/d (n = 56) | P valuea | ||
|---|---|---|---|---|---|---|
| IBI (ms) | Baselineb | 924 (862, 990) | 966 (934, 1008) | 903 (836, 976) | 971 (936, 1008) | |
| Rxb | 955 (920, 992) | 965 (934, 996) | 918 (884, 953) | 973 (940, 1008) | ||
| Treatment effectc | 1.000 [Reference] | 1.000 (0.952, 1.049) | 0.962 (0.916, 1.010) | 1.000 (0.951, 1.052) | 0.602 | |
| SDNN (ms) | Baselineb | 95.0 (86.8, 103.9) | 92.8 (85.7, 100.5) | 95.1 (85.7, 105.6) | 92.4 (85.4, 100.0) | |
| Rxb | 87.7 (81.4, 94.6) | 96.1 (89.5, 103.2) | 90.3 (85.1, 95.8) | 94.0 (87.3, 101.3) | ||
| Treatment effectc | 1.000 [Reference] | 1.104 (1.022, 1.193) | 1.034 (0.955, 1.119) | 1.072 (0.988, 1.162) | 0.069 | |
| SDANN (ms) | Baselineb | 76.3 (68.0, 85.6) | 72.9 (64.4, 82.5) | 74.0 (65.2, 83.9) | 65.1 (59.2, 71.4) | |
| Rxb | 67.2 (61.0, 74.0) | 74.4 (67.9, 81.6) | 74.6 (68.8, 80.9) | 74.2 (66.7, 82.4) | ||
| Treatment effectc | 1.000 [Reference] | 1.117 (0.992, 1.258) | 1.123 (0.995, 1.268) | 1.141 (1.007, 1.293) | 0.020 | |
| RMSSD (ms) | Baselineb | 33.8 (30.4, 37.5) | 36.0 (32.0, 40.5) | 35.4 (31.6, 40.0) | 37.6 (32.9, 43.0) | |
| Rxb | 36.5 (32.7, 40.6) | 36.4 (32.9, 40.3) | 33.6 (30.6, 36.8) | 36.8 (33.2, 40.7) | ||
| Treatment effectc | 1.000 [Reference] | 0.964 (0.871, 1.066) | 0.894 (0.807, 0.991) | 0.947 (0.852, 1.052) | 0.129 | |
| Ti | Baselineb | 21.6 (19.4, 24.1) | 20.9 (19.3, 22.7) | 21.1 (19.4, 23.1) | 21.1 (19.2, 23.2) | |
| Rxb | 20.0 (18.5, 21.6) | 22.3 (20.9, 23.8) | 21.3 (19.9, 22.7) | 22.0 (20.4, 23.8) | ||
| Treatment effectc | 1.000 [Reference] | 1.127 (1.037, 1.224) | 1.075 (0.988, 1.169) | 1.098 (1.008, 1.196) | 0.014 | |
| HF (ms2) | Baseline b | 324 (260, 404) | 330 (261, 417) | 347 (274, 440) | 350 (270, 452) | |
| Rxb | 347 (275, 439) | 337 (271, 419) | 302 (248, 369) | 326 (268, 398) | ||
| Treatment effectc | 1.000 [Reference] | 0.962 (0.784, 1.180) | 0.829 (0.673, 1.022) | 0.902 (0.729, 1.117) | 0.198 | |
| LF (ms2) | Baselineb | 696 (559, 867) | 731 (591, 903) | 767 (630, 934) | 790 (641, 973) | |
| Rxb | 637 (500, 811) | 702 (576, 856) | 675 (564, 809) | 752 (610, 926) | ||
| Treatment effectc | 1.000 [Reference] | 1.053 (0.903, 1.229) | 0.989 (0.845, 1.157) | 1.048 (0.892, 1.231) | 0.650 | |
| LF/HF | Baselineb | 2.15 (1.81, 2.55) | 2.21 (1.87, 2.61) | 2.21 (1.85, 2.64) | 2.26 (1.87, 2.74) | |
| Rxb | 1.83 (1.52, 2.20) | 2.08 (1.76, 2.47) | 2.23 (1.92, 2.61) | 2.30 (1.92, 2.77) | ||
| Treatment effectc | 1.000 [Reference] | 1.097 (0.946, 1.271) | 1.212 (1.042, 1.409) | 1.163 (0.997, 1.357) | 0.020 | |
| VLF (ms2) | Baselineb | 5363 (4493, 6403) | 5021 (4297, 5866) | 4962 (4271, 5765) | 4870 (4198, 5649) | |
| Rxb | 4444 (3844, 5138) | 5492 (4768, 6326) | 4857 (4334, 5443) | 5141 (4447, 5944) | ||
| Treatment effectc | 1.000 [Reference] | 1.271 (1.105, 1.461) | 1.149 (0.997, 1.325) | 1.199 (1.036, 1.387) | 0.005 |
IBI, interbeat interval (also known as RR interval), the time interval between R spikes of the QRS complex; SDNN, standard deviation of all NN intervals (normal-to-normal intervals, similar to R–R, but on normalized IBI data); SDANN, standard deviation of the averaged NN intervals, calculated from 5 min epochs; RMSSD, the square root of the mean of the sum of squares of differences between adjacent NN intervals; Ti, total number of all NN intervals divided by the height of the histogram of all NN intervals with bins of 7.8125 ms. HF, high frequency power, or variation; LF, low frequency power; LF/HF, ratio of LF to HF power; VLF, very low frequency power.
Significance tests investigate a linear trend with dose.
Values are geometric means; 95% CI in parentheses. Data were log-transformed before analysis.
Treatment effect versus placebo (average of change following each treatment divided by change on placebo at 6 and 12 months), based on estimated marginal means (adjusted for covariates: age, sex, ethnicity, BMI, and the value at baseline).
The present one year intervention study confirmed that HRV was increased during sleep-time by intakes of n-3 PUFA achievable through diet, including parameters of longer-phase variability. The relative increase in VLF and SDANN following n-3 PUFA has rarely been observed in clinical trials [15], but is in agreement with observational data from a cohort of older US adults, with VLF appearing to increase at around the level of 1–2 servings per week compared to 1–3 servings per month or less [16]. Subtle longer-phase fluctuations in heart rate can be more easily discerned during sleep, since greater amplitude oscillations in HRV in response to sympathetic input arising from activity and psychological influences are absent. Indeed, during waking periods, SDANN is powerfully determined by physical activity, or probably the metabolic and thermoregulatory changes associated with physical activity [17], and therefore it would be of interest to test whether increases in this longer-phase variability could be detected during a 24 h ambulatory HRV measurement period.
Low VLF power is a strong prognostic indicator of mortality after a myocardial infarction, particularly arrhythmic death [7], and reduced SDANN has been associated with increased mortality following a myocardial infarction [8]. Less is known about the prognostic utility of these parameters in individuals without pre-existing CHD [10]. The VLF band of the power spectrum may represent longer-phase periodic physiological shifts arising from hormonal changes, the renin–angiotensin system and thermoregulation [18].
Limitations of this study include the loss of data from 81 completing participants due to poor quality HRV recordings, and the fact that power calculations were not performed for any of the HRV parameters addressed in the analysis. However, strengths of the study include the use of a dose range of n-3 PUFA consistent with the range found in human diets, the comparatively long duration of dietary intervention, and evidence of good compliance. These data suggest that intakes of n-3 PUFA equivalent to one serving of oily fish a week alter some longer-term parameters of HRV in non-smoking healthy adults at mild to moderately increased risk of cardiovascular disease during sleep-time.
Financial support was received from the Food Standards Agency (UK) and the Department of Health (UK), project code N02041. The authors acknowledge support from the Department of Health (UK) via the National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre award to Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust.
The authors have no financial or commercial interest in any company or organization sponsoring the research. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health, UK.
We would also like to acknowledge CRODA Europe Ltd (Hull, East Yorkshire, UK) for formulating and supplying the oils for the intervention. We are grateful to Professor Malcolm Law, Dr Lee Hooper and Professor Kennedy Cruickshank for agreeing to be on the Data Monitoring Committee. The assistance of Paula Darroch, manager of the St Thomas' Hospital Clinical Research Centre, Fiona Lewis, Robert Gray, Dr Roy Sherwood, and Laura O'Sullivan is gratefully acknowledged. We are particularly grateful to the participants of the study.
References
- 1.Adkins Y., Kelley D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21:781–792. doi: 10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Marchioli R., Barzi F., Bomba E., Chieffo C., Di Gregorio D., Di Mascio R. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 3.Tavazzi L., Maggioni A.P., Marchioli R., Barlera S., Franzosi M.G., Latini R. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D., Wu J.H. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 5.Rizos E.C., Ntzani E.E., Bika E., Kostapanos M.S., Elisaf M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 6.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 7.Bigger J.T., Fleiss J.L., Steinman R.C., Rolnitzky L.M., Kleiger R.E., Rottman J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 8.Vaishnav S., Stevenson R., Marchant B., Lagi K., Ranjadayalan K., Timmis A.D. Relation between heart rate variability early after acute myocardial infarction and long-term mortality. Am J Cardiol. 1994;73:653–657. doi: 10.1016/0002-9149(94)90928-8. [DOI] [PubMed] [Google Scholar]
- 9.Quintana M., Storck N., Lindblad L.E., Lindvall K., Ericson M. Heart rate variability as a means of assessing prognosis after acute myocardial infarction. A 3-year follow-up study. Eur Heart J. 1997;18:789–797. doi: 10.1093/oxfordjournals.eurheartj.a015344. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji H., Larson M.G., Venditti F.J., Jr., Manders E.S., Evans J.C., Feldman C.L. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Stud Circ. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 11.Xin W., Wei W., Li X.Y. Short-term effects of fish-oil supplementation on heart rate variability in humans: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2013;97:926–935. doi: 10.3945/ajcn.112.049833. [DOI] [PubMed] [Google Scholar]
- 12.Sanders T.A., Hall W.L., Maniou Z., Lewis F., Seed P.T., Chowienczyk P.J. Effect of low doses of long-chain n-3 PUFAs on endothelial function and arterial stiffness: a randomized controlled trial. Am J Clin Nutr. 2011;94:973–980. doi: 10.3945/ajcn.111.018036. [DOI] [PubMed] [Google Scholar]
- 13.Brage S., Brage N., Franks P.W., Ekelund U., Wareham N.J. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005;59:561–570. doi: 10.1038/sj.ejcn.1602118. [DOI] [PubMed] [Google Scholar]
- 14.Kristiansen J., Korshoj M., Skotte J.H., Jespersen T., Sogaard K., Mortensen O.S. Comparison of two systems for long-term heart rate variability monitoring in free-living conditions—a pilot study. Biomed Eng Online. 2011;10:27. doi: 10.1186/1475-925X-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carney R.M., Freedland K.E., Stein P.K., Steinmeyer B.C., Harris W.S., Rubin E.H. Effect of omega-3 fatty acids on heart rate variability in depressed patients with coronary heart disease. Psychosom Med. 2010;72:748–754. doi: 10.1097/PSY.0b013e3181eff148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozaffarian D., Stein P.K., Prineas R.J., Siscovick D.S. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117:1130–1137. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 17.Roach D., Wilson W., Ritchie D., Sheldon R. Dissection of long-range heart rate variability: controlled induction of prognostic measures by activity in the laboratory. J Am Coll Cardiol. 2004;43:2271–2277. doi: 10.1016/j.jacc.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 18.Togo F., Kiyono K., Struzik Z.R., Yamamoto Y. Unique very low-frequency heart rate variability during deep sleep in humans. IEEE Trans Biomed Eng. 2006;53:28–34. doi: 10.1109/TBME.2005.859783. [DOI] [PubMed] [Google Scholar]