Abstract
A multicenter comparison of mitochondrial respiratory chain and complex V enzyme activity tests was performed. The average reproducibility of the enzyme assays is 16% in human muscle samples. In a blinded diagnostic accuracy test in patient fibroblasts and SURF1 knock-out mouse muscle, each lab made the correct diagnosis except for two complex I results. We recommend that enzyme activities be evaluated based on ratios, e.g. with complex IV or citrate synthase activity. In spite of large variations in observed enzyme activities, we show that inter-laboratory comparison of patient sample test results is possible by using normalization against a control sample.
Keywords: Respiratory chain, Diagnosis, Enzyme activity, Mitochondrial disease, Muscle, Fibroblasts
Highlights
► Diagnostic procedures for respiratory chain measurements of 5 labs were compared. ► Mouse SURF1 and control muscle, human control muscle and fibroblasts were examined. ► In almost all cases, a correct diagnosis was made. ► Sample preparation methods affect the results of the enzyme activity measurements. ► Although raw data shows inter-lab variation, normalized data give similar results.
1. Introduction
The incidence of mitochondrial disorders is estimated to be at least 1 in 5000 (Schaefer et al., 2004; Skladal et al., 2003). The clinical phenotypes associated with mitochondrial disorders are extremely diverse, varying from an early onset multi-systemic disease with rapid deterioration and death at a young age, to a very mild exercise intolerance presenting at a high age (Haas et al., 2007; Zeviani and Di Donato, 2004). This broad clinical spectrum complicates the diagnosis of a mitochondrial disease. Laboratory tests performed on tissue samples, in particular muscle, can provide valuable diagnostic information on the functioning of individual components of the mitochondrial energy generating system. Usually, these tests consist of enzyme activity measurements of the mitochondrial oxidative phosphorylation system. In addition, some diagnostic laboratories perform assays to examine the total mitochondrial energy generating system, including mitochondrial oxygen consumption, substrate oxidation, or ATP production measurements (Janssen et al., 2006; Rustin et al., 1994; Will et al., 2006), although these assays are not possible in frozen biopsy samples. Reaching a diagnosis usually requires that the outcome of these laboratory tests is evaluated in the context of the clinical presentation, metabolic investigations, histological findings, and molecular genetic tests (Taylor et al., 2004). Although a diagnosis is seldom reached on the basis of a single diagnostic test, the biochemical evaluation of a muscle biopsy is generally considered to be the “golden standard” in the diagnosis of a mitochondrial disorder. In addition to muscle, useful diagnostic information can be obtained from other tissues and cell types as well. Some mitochondrial disorders are not expressed in muscle, and require a biopsy of other tissues in order to detect the mitochondrial defect; a liver biopsy in case of an MPV17 defect is a good example (Spinazzola et al., 2006). In addition, skin fibroblast analysis is often performed. Fibroblasts have the added value of providing i) important biochemical clues for the identification of a genetic defect, ii) a model system for more in-depth diagnostic analyses, and iii) useful information which may be used to decide whether prenatal diagnostics on the basis of enzyme activity measurements can be performed in families with enzyme deficiencies, where a genetic defect in the mtDNA has been excluded (van den Heuvel et al., 2004).
A direct comparison of results of enzyme activity measurements performed in different diagnostic labs is hampered by the fact that most labs use their own assay conditions and control ranges (Thorburn and Smeitink, 2001). It has been shown before that respiratory chain enzyme activities measured by different labs can show large variations (Gellerich et al., 2004; Medja et al., 2009). Whether the results obtained with these apparently different methods could also lead to different conclusions, has never been tested in patient samples to date, although a recent quality control study using Caenorhabditis elegans mitochondrial samples indicates that this might indeed be the case (Chen et al., 2011). The aim of this study was to compare diagnostic methods in the laboratories of 5 diagnostic centers in Europe: Hôpital Necker-Enfants Malades (Paris, France), C. Besta Institute of Neurology (Milan, Italy), Erasmus Medical Center (Rotterdam, The Netherlands), Newcastle Mitochondrial NSCT Diagnostic Laboratory and Wellcome Trust Centre for Mitochondrial Research (Newcastle upon Tyne, UK), and Nijmegen Center for Mitochondrial Disorders (Nijmegen, The Netherlands). The comparison included a detailed examination of sample preparation methods, enzyme activity assays, and data analysis, as well as results from assays performed on a set of patient-derived and control muscle and fibroblast samples. The analysis of the OXPHOS system is invariably included in the examination of patients with suspected mitochondrial disease, while the measurement of enzymes such as pyruvate dehydrogenase is only usually performed in cases with a specific clinical or biochemical indication. For this reason, our study only focused on the biochemical analysis of the OXPHOS system.
2. Materials and methods
2.1. Enzyme activity measurement protocols
Five laboratories participated in this study. A detailed overview of the enzyme activity assays is given in the Supplementary data. The protocols used for the spectrophotometric respiratory chain enzyme activity measurements that were compared in this study are based on the same assay principles, with the exception of the assays for complexes I and II. For these two enzymes, the labs participating in this study used two different types of assays. The NADH-cytochrome c oxidoreductase assay measures complex I–complex III, and under normal conditions complex I is rate-limiting in this assay. Some labs use this assay in parallel with a NADH–CoQ oxidoreductase, in which only complex I is measured. This latter assay is generally regarded to be more difficult to perform, in particular in samples with relatively low mitochondrial mass, such as fibroblasts. Similarly, for complex II both a succinate:cytochrome c oxidoreductase assay (complex II + III) and a succinate:CoQ oxidoreductase assay were used. In this assay, complex II is rate limiting. The combined assays of complex I + III and II + III can also be used to detect primary CoQ deficiencies. Addition of a CoQ analogue to the reaction mixture results in normalization of the activity that is reduced in CoQ deficient samples (Lopez et al., 2006). For all assays that were used in this study, the reaction mixtures show variation in buffer conditions, substrate concentrations, and even temperature, although the assay principles are the same. The enzyme measurements to determine the effect of the buffer in which mitochondrial extracts were resuspended on the outcome of the enzyme activity measurements in fibroblasts were performed following the methods of lab 4 with minor modifications (Rodenburg, 2011).
Five laboratories participated in this study. A detailed overview of the enzyme activity assays is given in the Supplementary data. The protocols used for the spectrophotometric respiratory chain enzyme activity measurements that were compared in this study are based on the same assay principles, with the exception of the assays for complexes I and II. For these two enzymes, the labs participating in this study used two different types of assays. The NADH-cytochrome c oxidoreductase assay measures complex I–complex III, and under normal conditions complex I is rate-limiting in this assay. Some labs use this assay in parallel with a NADH–CoQ oxidoreductase, in which only complex I is measured. This latter assay is generally regarded to be more difficult to perform, in particular in samples with relatively low mitochondrial mass, such as fibroblasts. Similarly, for complex II both a succinate:cytochrome c oxidoreductase assay (complex II + III) and a succinate:CoQ oxidoreductase assay were used. In this assay, complex II is rate limiting. The combined assays of complex I + III and II + III can also be used to detect primary CoQ deficiencies. Addition of a CoQ analogue to the reaction mixture results in normalization of the activity that is reduced in CoQ deficient samples (Lopez et al., 2006). For all assays that were used in this study, the reaction mixtures show variation in buffer conditions, substrate concentrations, and even temperature, although the assay principles are the same. The enzyme measurements to determine the effect of the buffer in which mitochondrial extracts were resuspended on the outcome of the enzyme activity measurements in fibroblasts were performed following the methods of lab 4 with minor modifications (Rodenburg, 2011).
2.2. Fibroblast sample preparation
Experiments on patient fibroblast sample were performed in accordance with the ethical standards as formulated in the Helsinki Declaration of 1975 (revised 1983). A set of 16 fibroblast cell lines were shipped to each of the participating labs in a blinded manner. This part of the study was coordinated by lab 4. Each center contributed to the study with a number of cell lines. The cell lines are described in Table 2. Each lab cultured the cell lines and subsequently prepared mitochondrial extracts using their routine procedures, with the exception of lab 5 that did not participate in this part of the study because diagnostic fibroblast analysis is not operational in this lab. Furthermore, it should be noted that lab 3 participated although this lab does not offer diagnostic testing of fibroblasts. In each lab, cells were grown at 37 °C in a humidified 5% CO2 atmosphere, and were trypsinized once or twice a week, and medium was replaced at least once a week. The following culture media were used. Lab 1: RPMI 1640 supplemented with glutamax (446 mg/l), 10% (V/V) fetal calf serum, 100 μg/ml streptomycin, 100 IU/ml penicillin, 200 μM uridine and 2.5 mM sodium pyruvate. Lab 2: Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g/l glucose, 10% (V/V) fetal calf serum, 1 mM sodium pyruvate, 50 μg/ml uridine, 200 U/ml Penicillin G, 200 mg/ml streptomycin, and 4 mM glutamine. Lab 3: DMEM containing 4.5 g/l glucose, 1 mM sodium pyruvate, 10% (V/V) FCS, 100 μg/ml streptomycin, 100 IU/ml penicillin, and 0.2% (w/V) uridine. Lab 4: medium 199 supplemented with 10% (V/V) FCS, 100 μg/ml streptomycin, and 100 IU/ml penicillin. Preparation of cell extracts from fibroblasts for enzyme activity measurement was performed as follows. Lab 1: a small aliquot of pellet (fewer than 1 million cells; 0.5–0.7 mg protein) was deep-frozen and subsequently thawed using 1 ml of ice-cold solution (medium A) consisting of 0.25 M sucrose, 20 mM Tris (pH 7.2), 40 mM KCl, 2 mM EGTA, and 1 mg/ml bovine serum albumin, 0.01% digitonin (w/V), and 10% Percoll (V/V). After 10 min incubation at ice temperature, cells were centrifuged (5 min × 5000 g), the supernatant was discarded, and the pellet was washed (5 min × 5000 rpm) with 1 ml of medium A devoid of digitonin and Percoll. The pellet was re-suspended in 30 μl of medium A and used for enzyme assays. Lab 2: a cell pellet of approx. 5 million cells was resuspended in 2 ml Buffer B (250 mM sucrose, 20 mM MOPS KOH pH 7.4). 2 ml of 0.2 mg/ml digitonin in buffer B was added. After incubating on ice for 5 minutes, samples were centrifuged at 5000 ×g for 3 min, and pellets were resuspended in 3 ml of 1 mM sodium EDTA in buffer B. After incubating on ice for 5 min, samples were centrifuged at 10,000 ×g for 3 min. The pellets were resuspended in 1 ml 10 mM potassium phosphate buffer pH 7.4, and snap frozen in liquid nitrogen and thawed at 37 °C three times before enzyme measurements. Lab 3: to a freshly prepared cell pellet of approx. 5 million cells, 500 μl sucrose–HEPES–EDTA buffer was added. The cell suspension was homogenized by pipetting up-and-down 10 times using a 1 ml Eppendorf pipette. The homogenates were stored deep frozen in small aliquots. This material was used directly in the enzyme assays. Lab 4: a cell pellet of approx. 20 million cells was resuspended in 2.9 ml 10 mM Tris-HCl, pH 7.6 and homogenized using a Potter–Elvehjem tube, after which 600 μl 1.5 M sucrose was added. This mixture was centrifuged for 10 min at 600 g. The supernatant was subsequently again centrifuged at 10,000 g for 10 min. The mitochondrial pellet was resuspended in 670 μl 10 mM Tris-HCl, pH 7.6. This was used in the enzyme assays.
Table 2.
Key characteristics of the patient-derived fibroblast cell lines included in this study.
| Sample ID | Enzyme deficiency | Genetic defect |
|---|---|---|
| F1 | None | – |
| F2 | Complex IV | COX10 |
| F3 | Complex V | MT-ATP6/8 |
| F4 | None | – |
| F5 | None | – |
| F7 | None | – |
| F8 | Complex II | SDHA |
| F10 | None | – |
| FA-P1 | Complex I | NDUFS4 |
| FA-P2 | Complex I + complex IV | Depletion |
| 99RD0204 | Complex V | MT-ATP6 |
| 00RD0395 | Complex I | MT-ND5 |
| 04RD0504 | Complex IV | SCO2 |
| I | Complex IV | SURF1 |
| Ib | Complex IV | SURF1 |
| III | Complex I + complex IV | TUFM |
2.3. Muscle samples
For muscle sample analysis, it was not possible to include the samples from patients with established (molecular genetic) mitochondrial defects because of lack of sample availability. Therefore, it was decided to include 3 control muscle samples to evaluate the reproducibility of the enzyme assays. The control muscle tissue (musculus erector spinae) was collected from patients who underwent a surgery to remove redundant muscle tissue. These patients were not suspected to have a mitochondrial disease. This part of the study was coordinated by lab 3. The deep frozen samples were sent in duplicate in a blinded manner, both as a biopsy as well as a muscle homogenate. A 5% (w/V) muscle homogenate was prepared following the protocol of lab 3 as given below. In addition, skeletal muscle samples from two wild type and two SURF1 knock-out mice (Agostino et al., 2003) were sent in a blinded manner for identification of possible enzyme deficiencies. This part of the study was coordinated by lab 2.
2.4. Muscle sample preparation
The protocols for muscle homogenization were as follows:
-
Lab 1
The muscle samples were homogenized in 500 μl of 20 mM Tris, pH 7.2, 0.25 M sucrose, 40 mM KCl, 2 mM EGTA, 1 mg/ml BSA by 5 strokes in a 500 μl ground glass potter. The suspension was centrifuged at 2000 g for 8 min and the pellet discarded and enzyme activities were determined on supernatant.
-
Lab 2
30 mg of frozen muscle were resuspended in 15 vol. (w/V) of 10 mM potassium phosphate buffer pH 7.4, and homogenized with glass/glass potter on ice (about 10 times). The suspension was centrifuged at 800 g for 10 min at 4 °C. The supernatant was used for enzyme activity determinations after 1 cycle of freeze and thawing, and was kept on ice during the assays.
-
Lab 3
Muscle samples were homogenized using a motor driven Potter Elvehjem glass-Teflon homogenizer at a speed of 1300 rpm in 0.25 M sucrose, 10 mM HEPES, 1 mM EDTA, pH 7.4 on melting ice at 0 °C. After homogenization the homogenate was snap frozen in liquid nitrogen and stored below − 70 °C.
-
Lab 4
The muscle samples were minced with a Sorvall TC2 tissue chopper. The minced sample was taken up in 9 vol. (w/V) ice cold SETH buffer (0.25 mol/l sucrose, 2 mmol/l EDTA, 10 mmol/l Tris, 5 × 104 IU/l heparin, pH 7.4) and homogenized using a glass Potter Elvehjem homogenizer. The suspension was centrifuged at 600 g for 10 min. The 600 g supernatant was used for enzyme activity measurements, with the exception of complex V, which was measured in a mitochondrial fraction prepared by centrifuging the 600 g supernatant for 10 min at 14,000 g, after which the pellet was washed once with SETH buffer and finally taken up in SETH buffer containing 5 g/l BSA.
-
Lab 5
Muscle samples were thawed, weighed and rinsed in medium A (120 mM KCl, 20 mM HEPES, 2 mM MgCl2, 1 mM EGTA, 5 mg/ml BSA, pH 7.4). The samples were chopped finely, and homogenized on ice using a T25 Ultraturrax homogenizer (9500 rpm for 5 s) in a total volume of 1 ml of medium A. The crude homogenate was centrifuged at 600 g for 10 min at 4 °C to remove nuclear debris. The supernatant obtained was respun (600 g for 10 min at 4 °C) and all enzyme measurements were performed on this second supernatant.
3. Results
3.1. Reproducibility
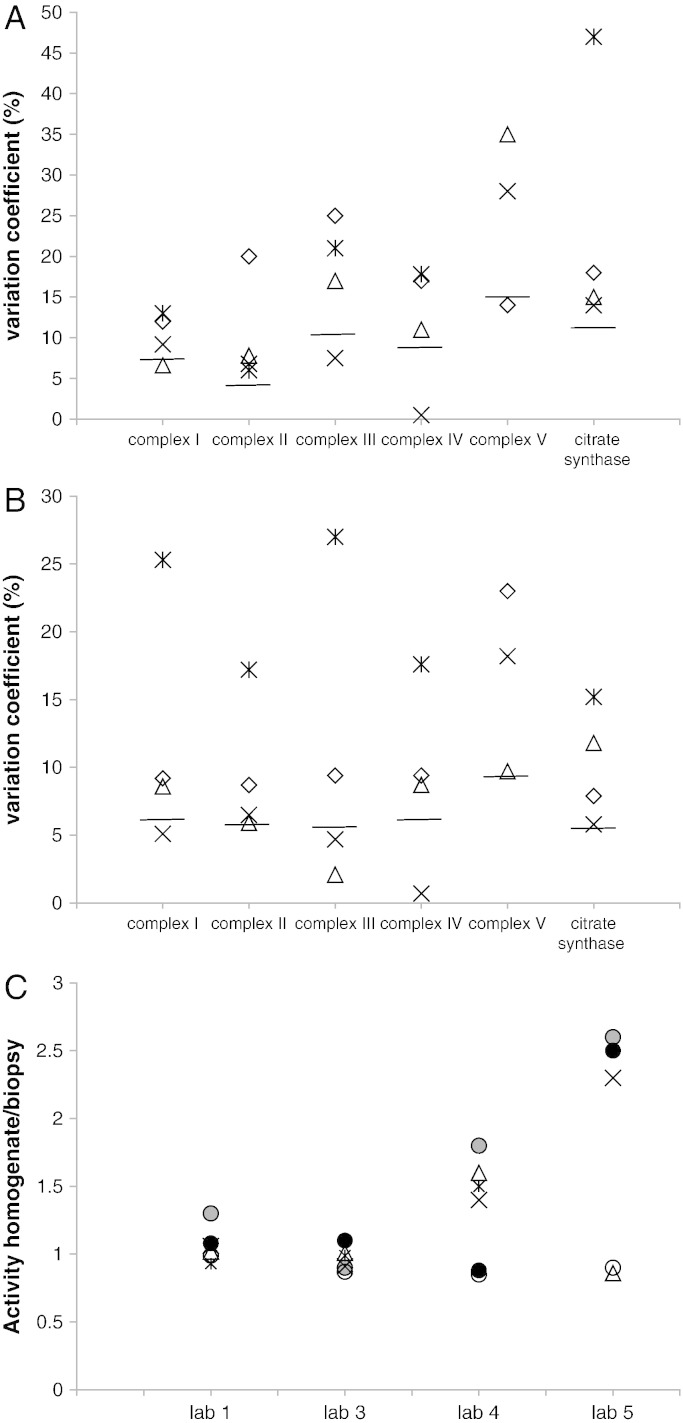
The reproducibility of enzyme measurements and sample preparation was determined by analyzing muscle samples and muscle homogenates in duplicate. Before analysis, results were sent back to the lab that controlled this part of the study; it was not known by the other labs that samples were sent in duplicate. The coefficients of variation (CV) were on average 16% for muscle biopsies (Fig. 1A) and 11% for muscle homogenates (Fig. 1B). The duplicate measurements of complex V were slightly less reproducible. This is in agreement with the notion that complex V analysis in frozen tissue samples is considered to be less reliable (Kirby et al., 2007). It should be noted that in the daily practice, poor duplicate measurements are rejected and measurements are repeated. Whereas in labs 1–4 the %CVs for measurements in homogenates were lower than those in muscle tissue, for lab 5 the %CVs were similar for both types of samples. The analysis of the effect of the homogenization procedure was tested by directly comparing the activity measured in the muscle biopsy with the activity in the corresponding muscle homogenate. Results show that lab 4 and in particular lab 5 found relatively high activities in the homogenates of all respiratory chain complexes (Fig. 1C). Possibly, homogenization of the muscle sample using an ultraturrax homogenizer led to a lowering of complex activities. In most other laboratories only the activity of complex I was higher in the homogenate, in lab 5 the activity found in the homogenate was more than twice the activity in the muscle biopsy. An explanation for this could be that in this laboratory, muscle homogenization was done in buffer without sucrose. No differences were found regarding the activity of citrate synthase. The range of the oxidative phosphorylation (OXPHOS) enzymes and citrate synthase activities found in the muscle biopsies normalized to the mean control level of each lab shows that the %CV is low for complex II and relatively high for complexes IV and V (Table 1). The variation in the data presented here appears to be much better than the results published in a previous study in which bovine skeletal muscle was examined by different labs (Gellerich et al., 2004).
Fig. 1.
Reproducibility of complex and citrate synthase activity measurements in control muscle.
The results obtained with biopsies (1A) or muscle homogenates (1B), and comparison of enzyme activities measured in muscle biopsies and muscle homogenates (1C) are shown. The %CVs were calculated as the SD / mean activity of the biopsy or muscle homogenate. Activities were expressed as mU/U citrate synthase for the OXPHOS complex activities and mU/mg protein for citrate synthase. The median of the %CV for each enzyme assay is indicated by a line in A and B. The ratios given in C were calculated by dividing the activities measured in the muscle homogenate by those measured in the corresponding muscle biopsy. A and B: Diamond: lab 1; triangle: lab 3; cross: lab 4; asterisk: lab 5. C: gray circle: complex I, black circle: complex II, triangle: complex III, cross: complex IV, asterisk: complex V, and white circle: citrate synthase.
Table 1.
Results of enzyme activity measurements in frozen muscle samples.
| Sample ID | Enzyme activity ratios | Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 5 |
|---|---|---|---|---|---|---|
| Muscle 1 + 4 | CI/CS | 175% | 121% | 116% | 128% | 169% |
| CII/CS | 149% | 154% | 107% | 155% | 131% | |
| CIII/CS | 138% | 107% | 124% | 71% | 222% | |
| CIV/CS | 75% | 25% | 62% | 44% | 14% | |
| CV/CS | 86% | 115% | 74% | 147% | n.d. | |
| CS | 82% | 42% | 90% | 44% | n.d. | |
| Muscle 2 + 6 | CI/CS | 96% | 20% | 82% | 70% | 106% |
| CII/CS | 107% | 88% | 104% | 111% | 116% | |
| CIII/CS | 149% | 81% | 137% | 89% | 149% | |
| CIV/CS | 42% | 25% | 38% | 33% | 18% | |
| CV/CS | 275% | 262% | 50% | 36% | n.d. | |
| CS | 44% | 38% | 57% | 38% | n.d. | |
| Muscle 3 + 5 | CI/CS | 173% | 66% | 128% | 102% | 99% |
| CII/CS | 138% | 103% | 96% | 110% | 92% | |
| CIII/CS | 159% | 87% | 113% | 76% | 150% | |
| CIV/CS | 67% | 34% | 61% | 47% | 15% | |
| CV/CS | 149% | 133% | 66% | 107% | n.d. | |
| CS | 71% | 53% | 70% | 58% | n.d. |
The enzyme activities were expressed relative to the average of activities measured in control muscle, which was set to 100%. At the time of measurement, it was not known by the participating labs that the muscle samples were in fact duplicates. The numbering of the muscle samples is according to how they were sent to the different centers.
3.2. Diagnostic accuracy: mouse muscle samples
To compare the diagnostic accuracy of the laboratory procedures operational in the labs participating in this study, it was not possible to use human patient muscle samples. As an alternative, 4 mouse skeletal muscle samples were analyzed in a blinded manner. It was known beforehand that there were two samples of control mice and two samples from knock-out mice. However, it was not known which enzyme was deficient. As not all labs have control values for mouse skeletal muscle available, identification of possible enzyme deficiencies was based on a comparison between results obtained in different muscle samples. The results show large variety in absolute activities measured in the different labs (Fig. 2A), which appear to be both lab and enzyme dependent. Nevertheless, on the basis of these activities, each lab was able to correctly identify two complex IV deficiencies in the set of 4 samples. After the identity of the samples was disclosed, relative enzyme activities could be derived from the absolute data by dividing the results of individual samples by the average of the results obtained in control samples (Fig. 2B). From this representation of the data, it appears that the relative activities measured in the different labs are very similar, and from these data the enzyme deficiencies are easily identifiable (Fig. 2B).
Fig. 2.
Results of the analysis of wild type and SURF1 knock-out mouse muscle analysis.
Two wild type (WT) and two SURF1 knock-out (KO) samples were analyzed in a blinded manner and results were expressed on citrate synthase base (A). The relative activities were calculated by dividing the activities measured in individual samples by the average of activities measured in wild type mouse muscle (B). Diamond: lab 1; square: lab 2; triangle: lab 3; cross: lab 4; and asterisk: lab 5.
3.3. Diagnostic accuracy: patient fibroblast samples
For a comparison of diagnostic accuracy of human sample analysis, a panel of fibroblast cell lines from a total of 16 patients and controls were tested in a blinded manner. The patients had a previously established enzyme deficiency due to a known molecular genetic defect, except for one patient, who had mtDNA depletion without an identified genetic defect (Table 2). The rationale for using patient fibroblasts instead of patient muscle for this part of the study, is described in the Discussion section. The fibroblast cell lines were cultured using the standard cell culture procedures in use in the different centers, and also sample preparation and enzyme activity measurements were performed using the methods in use for diagnostic purposes in the participating labs. The results of the enzyme activity measurements are given in Table 3. For the interpretation of the results, the activities were compared to the different control values used in each lab, and conclusions were drawn as if the sample was sent in for normal diagnostic examination. The conclusions, summarized in Table 4, showed that most samples were correctly identified as control or deficiency, with a few exceptions. Lab 1 missed one complex I deficiency, and one combined complex I + IV deficiency was wrongly identified as an isolated complex IV deficiency. Lab 3 labeled one control sample incorrectly as complex I deficient.
Table 3.
Results of enzyme activity measurements in fibroblast cell lines. The numbering of the cell lines is given in Table 2.
| Complex I |
Complex II |
Complex III |
Complex IV |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 1 | Lab 2 | Lab 3 | Lab 4 | |
| F1 | 57.0 | 17.7 | 4.0 | 38.8 | 99.0 | 16.8 | 14.5 | 134.6 | 841.0 | 133.6 | 10.5 | 333.0 | 411.0 | 126.1 | 28.4 | 197.0 |
| F2 | 75.0 | 27.1 | 12.3 | 38.4 | 118.0 | 22.0 | 12.1 | 210.9 | 1235.0 | 168.6 | 24.5 | 456.0 | 160.0 | 42.4 | 9.7 | 71.0 |
| F3 | 55.0 | 12.6 | 12.4 | 47.4 | 88.0 | 10.6 | 4.6 | 191.7 | 780.0 | 97.7 | 13.4 | 485.0 | 419.0 | 94.4 | 38.8 | 240.0 |
| F4 | 59.0 | 11.9 | 13.2 | 27.2 | 92.0 | 8.3 | 8.6 | 150.6 | 696.0 | 64.8 | 39.2 | 346.0 | 424.0 | 70.6 | 53.0 | 193.0 |
| F5 | 58.0 | 15.7 | 7.0 | 26.7 | 105.0 | 12.4 | 7.6 | 141.4 | 640.0 | 83.8 | 12.1 | 250.0 | 452.0 | 79.5 | 20.0 | 178.0 |
| F7 | 62.0 | 22.7 | 12.3 | 30.9 | 103.0 | 12.7 | 4.5 | 171.2 | 793.0 | 66.9 | 20.7 | 411.0 | 496.0 | 83.0 | 33.9 | 216.0 |
| F8 | 51.0 | 17.3 | 7.7 | 39.4 | 33.0 | 4.0 | 3.7 | 42.6 | 574.0 | 71.2 | 11.9 | 310.0 | 335.0 | 82.8 | 16.2 | 176.0 |
| F10 | 55.0 | 20.7 | 4.9 | 49.0 | 91.0 | 14.3 | 6.5 | 247.4 | 532.0 | 75.1 | 22.5 | 547.0 | 357.0 | 93.8 | 34.0 | 280.0 |
| FA-P1 | 23.0 | 2.5 | 0.1 | 6.1 | 186.0 | 17.1 | 4.3 | 111.3 | 1405.0 | 70.1 | 8.9 | 313.0 | 1025.0 | 81.1 | 24.0 | 149.0 |
| FA-P2 | 54.0 | 4.9 | 4.1 | 9.0 | 174.0 | 17.6 | 21.0 | 137.0 | 1084.0 | 65.4 | 30.0 | 247.0 | 274.0 | 32.8 | 20.0 | 21.0 |
| 99RD0204 | 46.0 | 12.5 | 6.2 | 23.0 | 79.0 | 11.4 | 7.9 | 107.8 | 931.0 | 126.6 | 14.0 | 235.0 | 323.0 | 104.7 | 28.4 | 136.0 |
| 00RD0395 | 44.0 | 5.3 | 4.8 | 12.5 | 112.0 | 12.3 | 9.1 | 153.3 | 969.0 | 86.1 | 20.0 | 377.0 | 469.0 | 87.2 | 36.0 | 219.0 |
| 04RD0504 | 38.0 | 16.6 | 7.0 | 16.4 | 43.0 | 18.7 | 11.1 | 101.3 | 667.0 | 63.9 | 28.4 | 241.0 | 112.0 | 34.6 | 8.7 | 74.0 |
| I | 48.0 | n.d. | 17.5 | 23.3 | 107.0 | n.d. | 14.5 | 121.3 | 1852.0 | n.d. | 57.0 | 465.0 | 107.0 | n.d. | 15.0 | 16.0 |
| Ib | 45.0 | n.d. | 12.9 | 17.9 | 73.0 | n.d. | 11.7 | 116.8 | 1090.0 | n.d. | 48.0 | 289.0 | 45.0 | n.d. | 8.2 | 14.0 |
| III | 38.0 | n.d. | 6.3 | 2.3 | 83.0 | n.d. | 20.0 | 95.6 | 1102.0 | n.d. | 43.0 | 188.0 | 158.0 | n.d. | 31.0 | 3.0 |
| Complex V |
Citrate synthase |
Complex II + III |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 1 | Lab 4 | |
| F1 | 159.0 | 98.0 | 11.8 | 168.0 | 146.0 | 116.0 | 49.8 | 177.0 | 97.0 | 49.0 |
| F2 | 237.0 | 132.4 | 14.8 | 207.0 | 240.0 | 151.6 | 68.2 | 285.0 | 109.0 | 85.0 |
| F3 | 36.0 | 36.9 | 6.1 | 12.0 | 181.0 | 93.0 | 50.9 | 275.0 | 97.0 | 64.0 |
| F4 | 118.0 | 67.2 | 12.9 | 170.0 | 128.0 | 59.6 | 60.3 | 185.0 | 60.0 | 59.0 |
| F5 | 130.0 | 76.1 | 15.7 | 124.0 | 134.0 | 75.4 | 36.2 | 159.0 | 72.0 | 49.0 |
| F7 | 130.0 | 55.1 | 15.9 | 192.0 | 155.0 | 57.6 | 38.2 | 208.0 | 92.0 | 60.0 |
| F8 | 143.0 | 111.5 | 15.3 | 178.0 | n.d. | 76.1 | 57.3 | 162.0 | 23.0 | 24.0 |
| F10 | 127.0 | 89.4 | 17.1 | 255.0 | n.d. | 75.8 | 51.2 | 278.0 | 63.0 | 92.0 |
| FA-P1 | 470.0 | 60.1 | 15.0 | 66.0 | 311.0 | 68.8 | 42.4 | 146.0 | 294.0 | 38.0 |
| FA-P2 | 189.0 | 69.0 | 17.0 | 78.0 | 329.0 | 79.9 | 84.0 | 185.0 | 254.0 | 50.0 |
| 99RD0204 | 54.0 | 39.7 | 8.2 | 42.0 | 184.0 | 93.0 | 47.0 | 115.0 | 137.0 | 31.0 |
| 00RD0395 | 129.0 | 89.9 | 17.4 | 173.0 | 203.0 | 84.5 | 104.0 | 172.0 | 161.0 | 76.0 |
| 04RD0504 | 105.0 | 77.1 | 18.8 | 158.0 | 125.0 | 76.4 | 87.0 | 198.0 | 85.0 | 35.0 |
| I | 146.0 | n.d. | 19.0 | 99.0 | 337.0 | n.d. | 103.0 | 228.0 | 187.0 | 62.0 |
| Ib | 88.0 | n.d. | 14.0 | 79.0 | 244.0 | n.d. | 64.5 | 170.0 | 165.0 | 41.0 |
| III | 138.0 | n.d. | 22.0 | 131.0 | 291.0 | n.d. | 98.0 | 159.0 | 201.0 | 29.0 |
Activities are in nmoles per min per mg protein for lab 1 and in units per mg protein for labs 2–4.
Table 4.
Conclusions from the enzyme activity measurements performed in the fibroblast cell lines.
| Sample ID | Enzyme deficiency | Lab 1 | Lab 2 | Lab 3 | Lab 4 |
|---|---|---|---|---|---|
| F1 | None | Normal | Normal | Complex I | Normal |
| F2 | Complex IV | Complex IV | Complex IV | Complex IV | Complex IV |
| F3 | Complex V | Complex V | Complex V | Complex V | Complex V |
| F4 | None | Normal | Normal | Normal | Normal |
| F5 | None | Normal | Normal | Normal | Normal |
| F7 | None | Normal | Normal | Normal | Normal |
| F8 | Complex II | Complex II | Complex II | Complex II | Complex II |
| F10 | None | Normal | Normal | Normala | Normal |
| FA-P1 | Complex I | Complex I | Complex I | Complex I | Complex I |
| FA-P2 | Complex I + complex IV | Complex I + complex IV | Complex I + complex IV | Complex I + complex IV | Complex I + complex IV |
| 99RD0204 | Complex V | Borderline complex Vb | Complex V | Complex V | Borderline complex Vb |
| 00RD0395 | Complex I | Normal | Complex I | Complex I | Complex I |
| 04RD0504 | Complex IV | Complex IV | Complex IV | Complex IV | Complex IV |
| I | Complex IV | Complex IV | Complex IV | Complex IV | Complex IV |
| Ib | Complex IV | Complex IV | complex IV | Complex IV | Complex IV |
| III | Complex I + complex IV | Complex IV | Complex I + complex IV | Complex I + complex IV | Complex I + complex IV |
Conclusions are based on the results presented in Table 3. Wrong conclusions are underlined.
Cell line F10 gave a borderline complex I activity that was interpreted by lab 3 as a normal activity.
Cell line 99RD0204 gave a borderline complex V activity that was interpreted by labs 1 and 4 as a reduced complex V activity.
3.4. Normalization
Different methods to normalize enzyme activities were used by the different labs. Most often, normalization to citrate synthase was used. Lab 4 used normalization to both complex IV and citrate synthase. Also lab 1 uses multiple enzyme activity ratios to judge whether an activity was reduced or not. To test the role of the normalization procedure, all fibroblast data were re-examined using different normalization procedures. We focused on complex I, as for this assay one false positive and two false negative results were reported. The complex I activities were normalized for citrate synthase, complex IV and complex II activities. For the latter two, the results for the cell lines that were deficient for complex IV or complex II, respectively, were omitted for obvious reasons. Table 5 shows the results obtained for the 5 control cell lines that were included in this study. The results show that normalization for citrate synthase and complex IV gave similar results for all labs. For normalization on complex II, lab 3 showed different results, which is most probably caused by the fact that lab 3 performed the measurements in a fibroblast homogenate, whereas the other labs performed the measurements in a mitochondria-enriched fraction. This also emphasizes that for measuring in whole cell homogenates, normalization for complex II, and probably also complex IV, is not advisable. For measuring in mitochondria-enriched fractions, normalization of complex I on either citrate synthase, complex IV or complex II does not seem to make a lot of difference, although normalization for complexes IV and II gave slightly narrower ranges. Next, we examined the three cell lines in which complex I results were false-positive or negative. We also included one control cell line that was labeled by lab 3 as normal but borderline for complex I. The results of lab 1 show that the two complex I deficient cell lines clearly had a relatively low complex I activity, although this lab interpreted the results as normal (Table 6). The way in which data were normalized did not seem to make much difference. For the two cell lines indicated by lab 3 as complex I deficient or normal but borderline for complex I (based on activities normalized for citrate synthase) normalization on complex IV or II did not improve the results of this lab, as expected because, as mentioned above, for measurements in fibroblasts homogenates normalization on citrate synthase is preferred.
Table 5.
Summary of the results of enzyme activity measurements performed in control fibroblast.
| Citrate synthase |
Complex IV |
Complex II |
||||
|---|---|---|---|---|---|---|
| Lowest control | Highest control | Lowest control | Highest control | Lowest control | Highest control | |
| Lab 1 | 93% | 109% | 91% | 112% | 93% | 108% |
| Lab 2 | 62% | 161% | 70% | 137% | 76% | 128% |
| Lab 3 | 44% | 177% | 56% | 145% | 22% | 220% |
| Lab 4 | 86% | 128% | 87% | 122% | 87% | 139% |
The results were obtained in the control cell lines included in this study (see Table 1). The enzyme activities were normalized for the average of each enzyme in the different labs. The lowest and highest control values give an impression of the range of enzyme activities in control fibroblasts measured in the participating labs.
Table 6.
Re-evaluation of the enzyme activity measurements.
| Citrate synthase |
Complex IV |
Complex II |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Complex I deficient |
Control |
Complex I deficient |
Control |
Complex I deficient |
|||||||
| F1b | F10b | 00RD0395a | IIIa | F1 | F10 | 00RD0395 | IIIc | F1 | F10 | 00RD0395 | III | |
| Lab 1 | 93% | n.d. | 51% | 31% | 101% | 112% | 68% | n.a. | 97% | 102% | 66% | 77% |
| Lab 2 | 62% | 111% | 26% | 41% | 70% | 110% | 30% | n.a. | 76% | 103% | 31% | n.d. |
| Lab 3 | 44% | 53% | 25% | 38% | 56% | 58% | 53% | n.a. | 22% | 61% | 42% | 25% |
| Lab 4 | 128% | 103% | 42% | 8% | 122% | 109% | 35% | n.a. | 139% | 96% | 39% | 12% |
The data presented here was obtained by normalizing the data given in Table 3 for the average of activities measured in the control cell lines included in this study.
n.d. not determined; n.a. not applicable.
Reported by lab 1 as normal complex I activity.
Reported by lab 3 as possible complex I deficiency.
Cell line III has a complex IV deficiency, therefore normalization to complex IV is not possible.
3.5. Sample preparation
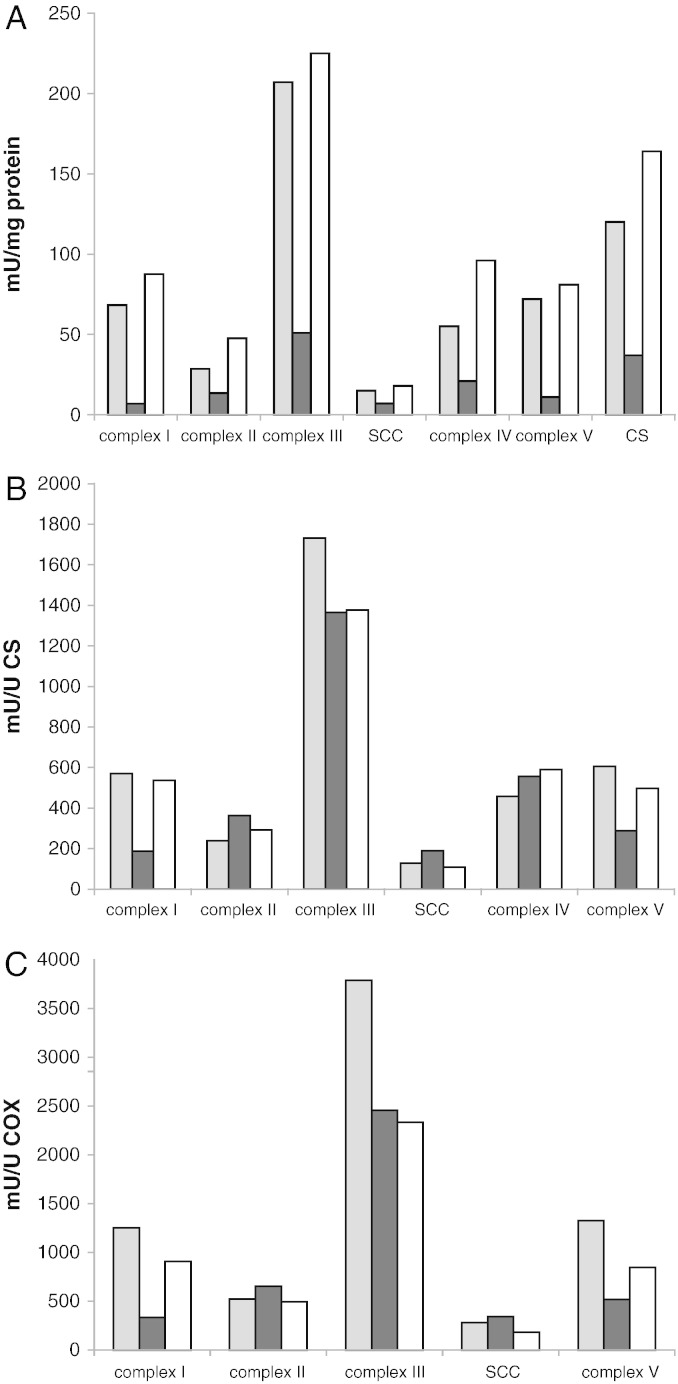
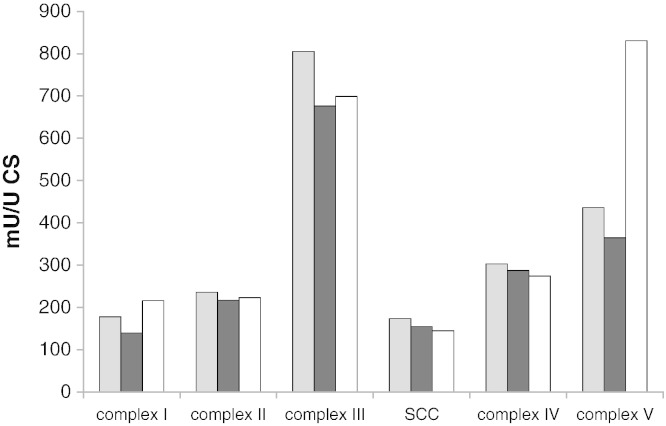
As each of the four labs used different methods to prepare cell extracts for enzyme activity measurements, we tested the effect of the type of extract on the activities measured. For this purpose, extracts were prepared from a randomly picked cell line and measured using the enzyme assays of lab 4. Although the complex II activity of this cell line was quite low, this did not interfere with the purpose of this experiment. For practical reasons, it was not possible to include the procedure of lab 2 in this experiment. The results show that the three extracts tested gave different purities of mitochondria, as judged by the activity of the different enzymes when expressed relative to the protein concentration in the extracts (Fig. 3A). The method of lab 3 gave relatively low activities on protein base, which is probably due to the fact that this method is based on whole cell extracts which resulted in extracts with a 2 to 3 fold higher protein content. When expressed relative to citrate synthase or on complex IV, these differences were much less striking, except for complex I and complex V where we found that the relative activities appeared to correlate with the purity of the mitochondrial extract (Fig. 3B and C). To test whether this is indeed due to the purity of the extract or to a direct effect of the different buffers on enzyme activities, we tested the effect of addition of different buffers to the enzyme assays. Also for this experiment, a randomly picked control cell line was used. The results clearly showed that the buffers had no effect on the enzyme activities measured, with the exception of complex V, which had a higher activity in the buffer of lab 4 compared to that of labs 1 and 3 (Fig. 4). This partly explains the relatively high activity of complex V measured using the method of lab 4 in Fig. 3A. These results indicate that the differences in the absolute activities of complex I observed in this study are most likely due to the way in which mitochondrial extracts were prepared, and not on the buffer in which mitochondria were resuspended.
Fig. 3.
Effect of mitochondrial extract preparation on the outcome of the enzyme activity measurements in fibroblasts.
The extraction procedures were according to the protocols of the participating labs, the enzyme activity measurements were according to the protocols of lab 4. For this purpose, a control cell line was used. Fig. 3A: activities are expressed as mU/mg protein; Fig. 3B: mU/U citrate synthase; Fig. 3C: mU/U complex IV. Gray bars: lab 1; black bars: lab 3; and white bars: lab 4.
Fig. 4.
Effect of the buffer in which mitochondrial extracts were resuspended on the outcome of the enzyme activity measurements in fibroblasts.
The extraction procedure was according to the protocol of lab 4, and the final mitochondrial pellet was resuspended in the final buffer according to the protocols of the different labs. Enzyme activities were determined using the methods described in Methods section 2.2. The activities are given as mU/U CS. Gray bars: lab 1; black bars: lab 3; and white bars: lab 4.
4. Discussion
Two types of samples were used in this study. For the evaluation of the diagnostic accuracy of enzyme activity measurements, mouse muscle and human fibroblasts were used. For the evaluation of the reproducibility, control human muscle tissue (musculus erector spinae) was used. The relatively low citrate synthase activities found in these control muscle samples could be due to the use of the musculus erector spinae. Muscle biopsies from suspected mitochondrial patients are usually taken from other muscles, e.g. M. quadriceps or M. tibialis anterior. Although muscle sample analysis is generally regarded to give the most informative diagnostic results, it was not possible to use human muscle for this part of the study because of a lack of muscle samples from patients with a genetically characterized OXPHOS deficiency, and therefore, samples from complex IV deficient and healthy mice were used. The patient-derived fibroblast cell lines that were included in this study all came from patients in whom the enzyme deficiency was known to be expressed in fibroblasts, therefore possible false-negative results could not be due to a tissue-specific expression of the deficiency. As each lab used their own standard laboratory procedures to evaluate the samples, this study design allowed for an evaluation of the entire process from fibroblast culturing, fibroblast and muscle sample preparation, enzyme activity measurements, and interpretation of the results. The reproducibility test showed that the CV of the enzyme assays was on average 11% for muscle homogenates and 16% for muscle biopsies. The CV of complex V was slightly higher and was on average 18% in homogenates and 28% in muscle biopsies.
As already documented in a previous quality control study (Gellerich et al., 2004), the absolute activities reported by the participating labs appear to show a large degree of variation, both in muscle and in fibroblasts. Our results demonstrate that the method of sample preparation is one of the major causes for this variation. This in agreement with previously published observations (Spinazzi et al., 2011). Other probable causes include the small but numerous differences in the enzyme activity assays, the different equipment used to measure activities, and possibly also the different cell culture media. It would be preferable if standardization of the protocols to measure OXPHOS enzyme activities could be achieved, as this would allow a universal interpretation of diagnostic results. Such standardization will however be difficult to establish for several reasons, including the necessity for each lab to use the same equipment. In addition, control values will have to be established and standardized again, which is a time consuming and expensive effort. More importantly, it will be severely hampered by the limited possibilities to obtain suitable, age-matched control samples for tissues such as muscle and liver. The results of our study indicate that a standardization of diagnostic procedures may not be necessary. In fact, we demonstrate that enzyme activity ratios, in particular ratios between the respiratory chain enzymes are quite similar when normalized to values measured in a set of control samples, irrespective of the many differences in procedures and equipment. This is not surprising, as all enzyme assays used in this study have been validated and are linear for observed activity versus amount of enzyme. In this study, for a small number of fibroblast cell lines, wrong conclusions were drawn about the possible diagnosis. Importantly, the false negative test results were all in the low normal to mildly reduced range. This illustrates the well known difficulties in interpreting enzyme measurement results in order to obtain a mitochondrial diagnosis (Rustin et al., 1991). The poor correlation between residual enzyme activities and clinical phenotype of patients with an OXPHOS deficiency, further emphasizes that a correct interpretation of test results, in particular those with a borderline activity is crucial. However, it is very important to note that the current study did not include the evaluation of enzyme activity results in the context of the clinical phenotype of the patient, which is the normal diagnostic scenario. A diagnosis is usually made by integrating the clinical observations with the results of many different diagnostic tests, including histological/histochemical evaluation, body fluids metabolites analyses, biochemical assays, and molecular genetics investigations (Haas et al., 2008). Thus, although the current study suggests that in 5% or so of cases a diagnosis could be missed; it seems likely that in the day-to-day practice this percentage will be lower. This will especially be the case for large diagnostic centers that have more specialized diagnostic tests available; a suspicious clinical phenotype combined with borderline respiratory chain enzyme activities will be followed by additional laboratory diagnostic testing, e.g. mtDNA and POLG gene sequencing, BN-PAGE analysis of respiratory chain enzyme complexes, and Western blotting in different tissues. It is important to note that the interpretation of enzyme measurements in the context of clinical observations and laboratory data is also highly relevant for patients presenting with a reduced respiratory chain enzyme activity that is secondary to a non-mitochondrial respiratory chain disease (Hui et al., 2006).
5. Conclusions
On the basis of our analysis, it can be concluded that results of biochemical evaluation of samples from patients suspected of having a mitochondrial disorder should be interpreted with caution but always in the context of the patient's clinical phenotype. Evaluation of enzyme activities in relation to the activity of other mitochondrial enzymes, such as citrate synthase, complex IV, and complex II may be helpful in cases that are difficult to interpret. Finally, when sending out samples to other diagnostic centers for confirmation of diagnostic test results, we advise to include a reference sample that can be used to normalize the results. This will allow for a direct comparison of test results from different labs, provided that the labs use similar procedures to prepare mitochondrial extracts.
The following are the supplementary data related to this article.
Overview of the assay conditions used in the participating labs for the measurement of respiratory chain enzymes, complex V, and citrate synthase.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.mito.2012.11.004.
Acknowledgments
This work was supported by the European Community's sixth Framework Programme for Research, Priority 1 “Life sciences, genomics and biotechnology for health”, contract numbers LSHM-CT-2004-005260 (MITOCIRCLE). The authors would like to thank S.J.G. Hoefs, M. Sadeghi Niaraki, P. Smits, B.J.M. Stoltenborg, and E.T.M. Wintjes for their excellent technical assistance. We thank Dr. Carlo Viscomi for providing the muscles from wild-type and SURF1 knock-out mice.
References
- Agostino A., Invernizzi F., Tiveron C., Fagiolari G., Prelle A., Lamantea E., Giavazzi A., Battaglia G., Tatangelo L., Tiranti V., Zeviani M. Constitutive knockout of Surf1 is associated with high embryonic lethality, mitochondrial disease and cytochrome c oxidase deficiency in mice. Hum. Mol. Genet. 2003;12:399–413. doi: 10.1093/hmg/ddg038. [DOI] [PubMed] [Google Scholar]
- Chen X., Thorburn D.R., Wong L.J., Vladutiu G.D., Haas R.H., Le T., Hoppel C., Sedensky M., Morgan P., Hahn S.H. Quality improvement of mitochondrial respiratory chain complex enzyme assays using Caenorhabditis elegans. Genet. Med. 2011;13:794–799. doi: 10.1097/GIM.0b013e31821afca5. [DOI] [PubMed] [Google Scholar]
- Gellerich F.N., Mayr J.A., Reuter S., Sperl W., Zierz S. The problem of interlab variation in methods for mitochondrial disease diagnosis: enzymatic measurement of respiratory chain complexes. Mitochondrion. 2004;4:427–439. doi: 10.1016/j.mito.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Haas R.H., Parikh S., Falk M.J., Saneto R.P., Wolf N.I., Darin N., Cohen B.H. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120:1326–1333. doi: 10.1542/peds.2007-0391. [DOI] [PubMed] [Google Scholar]
- Haas R.H., Parikh S., Falk M.J., Saneto R.P., Wolf N.I., Darin N., Wong L.J., Cohen B.H., Naviaux R.K. The in-depth evaluation of suspected mitochondrial disease. Mol. Genet. Metab. 2008;94:16–37. doi: 10.1016/j.ymgme.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui J., Kirby D.M., Thorburn D.R., Boneh A. Decreased activities of mitochondrial respiratory chain complexes in non-mitochondrial respiratory chain diseases. Dev. Med. Child Neurol. 2006;48:132–136. doi: 10.1017/S0012162206000284. [DOI] [PubMed] [Google Scholar]
- Janssen A.J., Trijbels F.J., Sengers R.C., Wintjes L.T., Ruitenbeek W., Smeitink J.A., Morava E., van Engelen B.G., van den Heuvel L.P., Rodenburg R.J. Measurement of the energy-generating capacity of human muscle mitochondria: diagnostic procedure and application to human pathology. Clin. Chem. 2006;52:860–871. doi: 10.1373/clinchem.2005.062414. [DOI] [PubMed] [Google Scholar]
- Kirby D.M., Thorburn D.R., Turnbull D.M., Taylor R.W. Biochemical assays of respiratory chain complex activity. Methods Cell Biol. 2007;80:93–119. doi: 10.1016/S0091-679X(06)80004-X. [DOI] [PubMed] [Google Scholar]
- Lopez L.C., Schuelke M., Quinzii C.M., Kanki T., Rodenburg R.J., Naini A., Dimauro S., Hirano M. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am. J. Hum. Genet. 2006;79:1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medja F., Allouche S., Frachon P., Jardel C., Malgat M., de Camaret B.M., Slama A., Lunardi J., Mazat J.P., Lombes A. Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion. 2009;9:331–339. doi: 10.1016/j.mito.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Rodenburg R.J. Biochemical diagnosis of mitochondrial disorders. J. Inherit. Metab. Dis. 2011;34:283–292. doi: 10.1007/s10545-010-9081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin P., Chretien D., Bourgeron T., Wucher A., Saudubray J.M., Rotig A., Munnich A. Assessment of the mitochondrial respiratory chain. Lancet. 1991;338:60-60. doi: 10.1016/0140-6736(91)90057-v. [DOI] [PubMed] [Google Scholar]
- Rustin P., Chretien D., Bourgeron T., Gerard B., Rotig A., Saudubray J.M., Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta. 1994;228:35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Schaefer A.M., Taylor R.W., Turnbull D.M., Chinnery P.F. The epidemiology of mitochondrial disorders—past, present and future. Biochim. Biophys. Acta. 2004;1659:115–120. doi: 10.1016/j.bbabio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Skladal D., Halliday J., Thorburn D.R. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain. 2003;126:1905–1912. doi: 10.1093/brain/awg170. [DOI] [PubMed] [Google Scholar]
- Spinazzi M., Casarin A., Pertegato V., Ermani M., Salviati L., Angelini C. Optimization of respiratory chain enzymatic assays in muscle for the diagnosis of mitochondrial disorders. Mitochondrion. 2011;11:893–904. doi: 10.1016/j.mito.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Spinazzola A., Viscomi C., Fernandez-Vizarra E., Carrara F., D'Adamo P., Calvo S., Marsano R.M., Donnini C., Weiher H., Strisciuglio P., Parini R., Sarzi E., Chan A., Dimauro S., Rotig A., Gasparini P., Ferrero I., Mootha V.K., Tiranti V., Zeviani M. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat. Genet. 2006;38:570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- Taylor R.W., Schaefer A.M., Barron M.J., McFarland R., Turnbull D.M. The diagnosis of mitochondrial muscle disease. Neuromuscul. Disord. 2004;14:237–245. doi: 10.1016/j.nmd.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Thorburn D.R., Smeitink J. Diagnosis of mitochondrial disorders: clinical and biochemical approach. J. Inherit. Metab. Dis. 2001;24:312–316. doi: 10.1023/a:1010347808082. [DOI] [PubMed] [Google Scholar]
- van den Heuvel L.P., Smeitink J.A., Rodenburg R.J. Biochemical examination of fibroblasts in the diagnosis and research of oxidative phosphorylation (OXPHOS) defects. Mitochondrion. 2004;4:395–401. doi: 10.1016/j.mito.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Will Y., Hynes J., Ogurtsov V.I., Papkovsky D.B. Analysis of mitochondrial function using phosphorescent oxygen-sensitive probes. Nat. Protoc. 2006;1:2563–2572. doi: 10.1038/nprot.2006.351. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Di Donato S. Mitochondrial disorders. Brain. 2004;127:2153–2172. doi: 10.1093/brain/awh259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of the assay conditions used in the participating labs for the measurement of respiratory chain enzymes, complex V, and citrate synthase.