Abstract
In the past decade, there have been fundamental advances in our understanding of genetic factors that contribute to the inflammatory bowel diseases (IBDs) Crohn’s disease and ulcerative colitis. The latest international collaborative studies have brought the number of IBD susceptibility gene loci to 163. However, genetic factors account for only a portion of overall disease variance, indicating a need to better explore gene-environment interactions in the development of IBD. Epigenetic factors can mediate interactions between the environment and the genome; their study could provide new insight into the pathogenesis of IBD. We review recent progress in identification of genetic factors associated with IBD and discuss epigenetic mechanisms that could affect development and progression of IBD.
Keywords: Epigenetics, Crohn’s Disease, Ulcerative Colitis, DNA Methylation
Abbreviations used in this paper: CD, Crohn’s disease; CpG, cytosine-guanine dinucleotides; DNMT, DNA methyltransferase; EWAS, epigenome-wide methylation association studies; GWAS, genome-wide association studies; HAT, histone acetyl transferase; HDAC, histone deacetylase; HDACi, histone deacetylatase inhibitors; IBD, inflammatory bowel disease; IL, interleukin; miR, microRNA; mRNA, messenger RNA; NF-κB, nuclear factor κB; PBMC, peripheral blood mononuclear cell; SNP, single nucleotide polymorphism; Th, T-helper; TNF, tumor necrosis factor; UC, ulcerative colitis
The inflammatory bowel diseases (IBDs) Crohn’s disease (CD) and ulcerative colitis (UC) are an important health problem, with an incidence among European adults of 12.7 and 24.3 per 100,000 person-years, respectively, and a prevalence of 0.5% to 1.0%.1 Moreover, the incidence of IBD is increasing among adults and children and in the developed and developing world.1–5 In the United Kingdom, IBDs cost the National Health Service approximately £720 million (approximately $1.1 billion) per annum.6,7
The pathogenesis of IBD is believed to involve an aberrant immune response to intestinal microbiota in genetically susceptible individuals.8 Genetic studies have provided many candidate loci in the past decade, and the innate and acquired immune responses have been implicated in pathogenesis. However, identified genetic factors account for only a modest proportion of the disease variance: 13.6% for CD and 7.5% for UC.9 These figures highlight the need for critical evaluation of genetic discoveries to date and indicate the importance of environmental factors in the pathogenesis of IBD; in addition, the intriguing possibility arises that epigenetics may partially account for the “hidden heritability” in IBD.
We review recent genetic discoveries in IBD and introduce readers to epigenetic factors that could be involved in pathogenesis.
Genetics in IBD: A Generation of Progress
In the past 25 years, there has been intense interest in identifying genetic and more recently epigenetic changes that relate to the pathogenesis of IBD. Few other complex diseases have been the subject of such extensive genetic and epigenetic research.
National consortia and subsequently large international collaborative research groups, such as the International IBD Genetics Consortium, have led the way in performing large-scale appraisals of the genome of patients with IBD (http://www.ibdgenetics.org/). The assumption-free approach of genome-wide association studies (GWAS) has helped to support established etiologic roles of the innate and acquired immune system in IBD and identified interesting new mechanisms such as autophagy.10
Findings from the past 25 years of genetic discovery in IBD have been put into context by the latest meta-analysis of the GWAS and ImmunoChip data.9 The ImmunoChip (Illumina, Inc, San Diego, CA) was developed following GWAS of IBD and other immune diseases; it contains 200,000 single nucleotide polymorphisms (SNPs) relevant to IBD and other immune-mediated diseases. The aims of the ImmunoChip experiments are to replicate and fine map the known IBD susceptibility loci and to identify common links with other immune disorders. The meta-analysis comprised more than 75,000 cases and controls and more than 1.23 million SNPs from several centers worldwide. It identified a further 64 loci, bringing the total number of IBD-associated loci to 163; this is significantly more than for any other complex disease.9
The Genetic Architecture of IBD
Early GWAS identified IBD loci common and unique to CD and UC.11 The latest data show the increasing proportion of loci common to both diseases, with relatively fewer CD- or UC-specific loci.9 Of the 163 identified loci, 110 are associated with both diseases, 30 are CD specific, and 23 are UC specific.9 Studies of gene loci shared by UC and CD may provide insight into their common pathogenic mechanisms. The T-helper (Th)17 and interleukin (IL)-12/IL-23 pathway is well established in the pathogenesis of IBD, with susceptibility gene loci IL23R, IL12B, JAK2, and STAT3 identified in both UC and CD.12,13 Variants in IL12B, which encodes the p40 subunit of IL-12 and IL-23, have been associated with IBD and other immune disorders.
Defects in the function of IL-10, an immunosuppressive cytokine, have also been associated with CD and UC.14,15 A severe, childhood-onset, CD-like form of enterocolitis is associated with rare mutations in IL10R. However, this disorder could be a separate entity from idiopathic IBD.16,17 Other susceptibility genes that regulate immune function include CARD9, IL1R2, REL, SMAD3, and PRDM1.12 Interestingly, the well-established CD risk variants of NOD2 and PTPN22 appear to protect against UC.
CD-Specific Susceptibility Loci and Pathways
CD has a greater genetic component than that of UC, and several CD-specific susceptibility loci have been delineated. The latest genetic data increasingly highlight the relationship between the host innate immune system and the intestinal microbiota in CD. GWAS have indicated that intracellular bacterial processing by autophagy is an important pathogenic mechanism. Importantly, the association between CD and NOD2 has been consistently replicated at the genome-wide significance level18; NOD2 has been mechanistically linked with autophagy.19,20 Cigarette smoking, a strong environmental factor in risk of CD, might affect NOD2 function.21 Furthermore, the product of the CD susceptibility gene ATG16L1 is recruited to the plasma membrane by NOD2, where it initiates bacterial internalization by autophagosomes.15,18,20
Another gene involved in autophagy-induced bacterial killing is IRGM. CD-associated polymorphisms in IRGM lead to reduced protein expression. A different SNP of IRGM protects against Mycobacterium tuberculosis.22,23 The most recent data from ImmunoChip studies indicated an overlap between IBD loci and complex mycobacterial disease loci9; 7 CD susceptibility genes overlap with leprosy susceptibility genes, and 6 mycobacterium susceptibility genes overlap with IBD loci. However, for several of these diseases, the genetic associations have opposite effects.9 Genes involved in the host response to mycobacteria that were previously associated with CD include CARD9 and LTA.11,15 Other CD-specific loci identified related to the immune system include PTPN22, IL2RA, IL27, TNFSF11, and VAMP3.15,18
UC-Specific Susceptibility Loci and Pathways
Although UC susceptibility loci have primarily included genes that regulate intestinal epithelial barrier function, there is recent evidence that HLA variants are involved in the development of UC.9 HLA-DQA1 was the locus most strongly associated with UC (odds ratio, 1.44),with no corresponding increased risk in CD.9 The HLA class II genes are tremendously diverse and control antigen presentation to T cells; they have been implicated in other immune diseases.
Hepatocyte nuclear factor 4A (HNF4A) regulates expression of cell junction proteins in the intestinal epithelial barrier; variants have been associated with UC and with colorectal cancer, a complication of chronic inflammation in patients with IBD.24 Rare SNPs at the HNF4A gene locus, not implicated in UC, are associated with maturity-onset diabetes, inherited in an autosomal dominant fashion.25 Other UC-associated genes that affect epithelial barrier function include CHD1, which encodes E-cadherin, and LAMB1, which encodes the lamina β subunit 1. Many UC risk alleles encode cytokines and inflammatory mediators, including tumor necrosis factor (TNF) receptor superfamily members (TNFRSF14, TNFRSF9), ILs, and IL receptors (IL1R2, IL8Ra/RB, IL7R).12
Relationships With Other Diseases
Jostins et al reported that 70% of IBD loci overlap with loci associated with other complex immune diseases, such as IL23R variants associated with psoriasis and ankylosing spondylitis.26–29 However, these polymorphisms sometimes have opposite effects in different diseases. For example, a variant of PTPN22 protects against CD but is a risk factor for type 1 diabetes and rheumatoid arthritis.30 Extraintestinal manifestations of IBD also share common loci, which may explain their co-occurrence. For example, variants of REL, IL2, and CARD9 are associated with UC and primary sclerosing cholangitis.11,31
Current Agenda for Genetic Studies of IBD
Many of the IBD loci identified so far have not been accurately characterized or fine mapped, and the candidate genes commonly used to describe them are only putative. Moreover, the biological functions of their products, and their complex interactions, in most cases require delineation. Studies are under way to use the greater detail afforded by the ImmunoChip data to fine map loci, and functional studies are needed. Further work is required to determine how specific variants affect levels of messenger RNA (mRNA) and consequently protein, which could provide further insight into mechanisms of pathogenesis. This is likely to take considerable time; NOD2 was identified more than 10 years ago, and there is still uncertainty about its function.29
GWAS have excelled in identifying moderate-risk genetic variants with at least 5% prevalence in the population. Novel approaches are needed to discover lower-prevalence variants with higher effect size. Whole-exome sequencing, which covers only coding areas of the genome, costs less than whole-genome sequencing and tends to afford higher-depth coverage and therefore greater certainty about novel discoveries. It has been successfully used to identify single mutations in very early-onset IBD32 and is perhaps most likely to produce results in individuals with a strong family history or early age of disease onset. However, many polymorphisms that affect disease susceptibility are located in noncoding areas of the genome; the ENCODE project has highlighted the importance of noncoding regions in disease risk.33 Exome sequencing and whole-genome sequencing are each under way, with large-scale endeavors at the Sanger Centre likely to report results in mid-2013 (http://www.ibdresearch.co.uk/).
Other researchers are looking for associations between specific genotypes and disease phenotypes. To date, NOD2 mutations have been associated with stricturing ileal CD, and DRB1*0103 has been associated with severe extensive UC.34–36 The International IBD Genetics Consortium recently presented preliminary data from the core phenotyping project, which used ImmunoChip data to identify genetic factors that correspond to disease phenotypes. The International IBD Genetics Consortium confirmed the associations of variants of NOD2 and HLA with CD and UC, respectively, as well as associated HLA variants with location of CD and reported the effects of NOD2 and HLA genotypes on age of disease onset.37
Most IBD genetic analyses have been performed in the white populations of Northern Europe and America. More recently, there has been a push to expand this work to other ethnic populations.38 IBD-associated variants of NOD2, for example, are less prevalent in black populations, and CD-associated mutations have not been detected in Asian or Sub-Saharan African populations.39,40
Pharmacogenomics, the study of how genomic factors affect the efficacy, tolerability, and side effects of a therapeutic agent, remains high on the research agenda. Patients are evaluated for thiopurine S-methyltransferase (encoded by TPMT) genotype and phenotyping before initiation of thiopurine therapy is recommended by the US Food and Drug Administration.41,42 There are ongoing attempts to predict patients’ response to other agents based on genetic factors. Studies supported by the Serious Adverse Events Consortium aim to predict mesalamine-induced nephrotoxicity (http://www.ibdresearch.co.uk/5asa/) and serious complications of anti-TNF therapies and thiopurines (http://www.ibdresearch.co.uk/pred4/).
A main goal of IBD research is to develop disease-specific therapeutics. Many researchers are developing reagents to alter activities of genes and pathways identified through GWAS, and the IL-12/IL-23 signaling pathway is one promising target. Ustekinumab, a monoclonal antibody that binds to the shared p40 subunit encoded by IL12B, has undergone phase 2b induction and maintenance trials in patients with CD.43 Apilimod mesylate, briakinumab (ABT-874), and SCH-900222, also target components of the IL-12/IL-23 signaling pathway, and are currently under evaluation.44
From the Environment to Genetics via Epigenetics
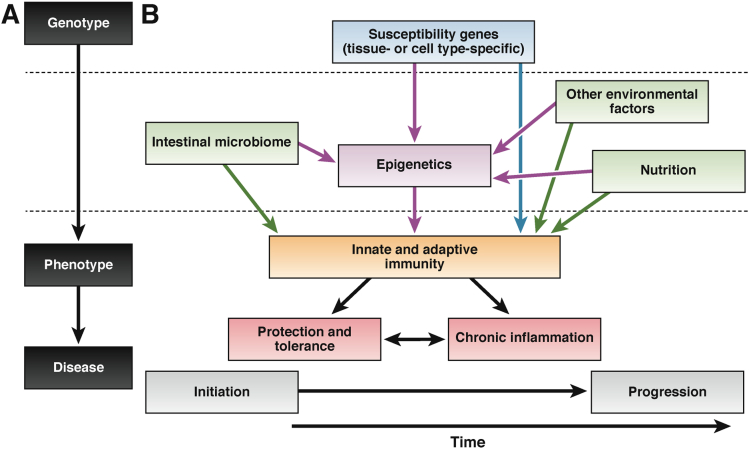
The challenge remains to measure patients’ duration, intensity, and frequency of exposure to the many environmental factors that potentially could contribute to IBD, making the environmental impact on disease difficult to disentangle.45 Epigenetic factors could mediate gene-environment interactions involved in pathogenesis. Epigenetic programming begins at fertilization and continues throughout life. Studies of Agouti mice and the offspring of post–World War II Dutch famine survivors revealed how the environment can affect epigenetic factors. Dietary intake during pregnancy was shown to affect the epigenetic reprogramming step in offspring during early development, an effect that persisted for up to 2 generations.46–50 Moreover, there is evidence for acquired epigenetic changes with aging caused by a range of environmental factors.51 Epigenetics could therefore play a central role in the pathogenesis of IBD and other diseases, affecting interactions among genetic and environmental factors such as the intestinal microbiome (Figure 1).
Figure 1.
Roles for epigenetics in pathogenesis. Epigenetics could mediate between the genetic environment and environmental factors to help determine the phenotype of IBD. The classic paradigm of genotype leading to phenotype and disease (A) has been expanded to embrace key etiologic factors in IBD (B). Epigenetics (purple) may interact with both genetic factors (blue) and environmental factors (green) in affecting the immune system (orange). The subsequent immune response has consequences on whether insults are tolerated or chronic inflammation is initiated and propagated (red).
Adapted with permission from Macmillan Publishers Ltd: Nature Immunology Renz et al, copyright 2011.138
In IBD research, several key developments in molecular studies have led us from genetics to explore epigenetics. GWAS have identified key epigenetic regulatory enzymes such as DNA methyltransferase (DNMT) 3a and more recently DNMT3b as CD susceptibility genes.9,15 Dendritic cells that express CD-associated variants of NOD2 fail to up-regulate microRNA (miR) clusters that regulate Th1 and Th17 cell–mediated immune responses.52 Epigenetic mechanisms have also been shown to regulate the immune system. For example, differentiation of Th2 cells requires epigenetic silencing of the IFNG locus.53
What Is Epigenetics?
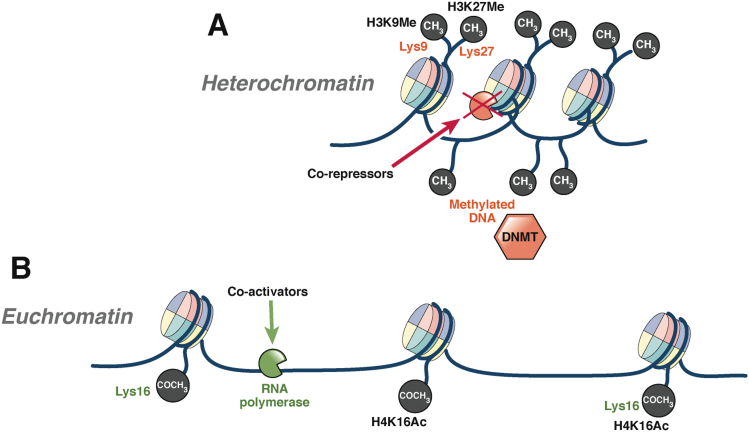
Epigenetics may be defined as mitotically heritable changes in gene function not explained by changes in the DNA sequence. Gene expression can be altered by changes to the structure and function of chromatin (Figure 2). The main epigenetic mechanisms include DNA methylation, histone modification, RNA interference, and the positioning of nucleosomes (which will not covered in depth in this review).
Figure 2.
The structure and function of chromatin. The structure of chromatin helps determine whether genes are transcribed or not. Chromatin comprises DNA strands that entwine an octamer of histone proteins, comprising histone subtypes H2a, H2b (2× dimer), H3, and H4 (1× tetramer).139 In a simple model, chromatin exists as heterochromatin or euchromatin. (A) Heterochromatin is a condensed form of chromatin that does not allow access of transcription factors such as RNA polymerase and therefore prevents gene transcription. In its condensed form, heterochromatin is linked with various corepressors, methylated DNA, low levels of histone acetylation, and methylation of key lysine residues (H3 lysine 9 [H3K9Me] and 27 [H3K27Me]). (B) Euchromatin is a relaxed form of chromatin that allows access to DNA and RNA polymerases and transcription to occur. Euchromatin is associated with coactivators and specific histone acetylation (acetylation on lysine 16 of histone 4 [H4K16Ac]) that together form a large protein complex called an enhanceosome. This enhanceosome then recruits RNA polymerase to perform transcription.68,140
The epigenome can be regarded as both stable and plastic. The epigenome can be regarded as stable as epigenetic marks are passed onto daughter cells during mitosis.54 However, stochastic and environmental factors can cause dynamic changes to the epigenome over time.55 During mitosis, the level of fidelity of epigenomic replication is much lower than that of the genetic sequence (error rate of 1 × 106 for the DNA sequence compared with 1 × 103 for DNA modifications), leading to an accumulation of epigenetic changes over time.45,56 Similarly, several environmental factors produce epimutations (epigenetic changes associated with disease); factors relevant to IBD include smoking, the microbiota, and diet.
Epigenetic marks are reset during meiosis; the epigenome is established early in embryogenesis after undergoing several reprogramming steps, during which the epigenome is most subject to modification.54,57 Given that the epigenome is reset during meiosis, it was believed that epigenetic marks were not passed between generations.
Although environmental exposures in utero can lead to epigenetic changes that persist for up to 2 generations, there is increasing interest in true epigenetic inheritance, which lasts multiple generations.58–60 Transgenerational epigenetic inheritance has attracted both excitement and skepticism in the scientific community and pertains to epigenetic marks resistant to the major reprogramming steps.45,59 The most compelling evidence for transgenerational epigenetic inheritance comes from studies of plants; DNA methylation-mediated silencing of the Lcyc promoter causes variations in floral symmetry in Linaria vulgaris (toadflax) that are stably inherited over many generations.61 Although additional examples have been reported from studies of plants, insects, and mammals, the concept is still met with some reservation.62 Incomplete erasure of epigenetic mutations across generations could contribute to familial predisposition to diseases such as IBD.45
DNA Methylation
DNA methylation is the most widely studied epigenetic modification; in this process, a methyl group is covalently added to cytosines that are part of cytosine-guanine dinucleotides (CpG). Full methylation occurs when cytosine residues on both DNA strands are methylated. CpG dinucleotides are generally sparse in the genome (∼1%) but are relatively concentrated in specific regions called “CpG islands.” CpG islands are defined as a 200-base sequence containing greater than 50% CpG dinucleotides at an observed to statistically expected ratio of 0.6.63 The areas where most tissue-specific methylation appears to border CpG islands have been termed “CpG shores.”64
Transcriptionally repressive activity generally occurs where a gene has methylation of CpG islands in promoter areas and is an important mechanism of gene silencing.65 DNA methylation may lead to transcriptional repression by hindering access of transcription factors to promoter regions, although many researchers believe the reverse is true: that gene silencing subsequently leads to DNA methylation.66,67
DNA is methylated by enzymes called the DNMTs. There are 5 members of the DNMT family: DNMT1 (maintenance of methylation), DNMT2 (involved in RNA methylation), DNMT3a, DNMT3b, and DNMT3L (involved in new methylation). There is evidence that these DNMTs interact and that other epigenetic mechanisms can recruit DNMTs to specific gene loci.63
DNA methylation passes from the mother to the daughter cells during mitosis; DNMT1 mediates full methylation of hemimethylated CpG sites during DNA replication. DNA methylation is part of normal genetic imprinting (hypermethylation of one parental allele leads to monoallelic expression) and inactivation of the X autosome in females. Inherited deficiency of DNMT3B leads to immunodeficiency, centromeric instability, and facial anomalies (ICF) syndrome, whereas complete lack of DNMT enzymes leads to embryonic lethality.65
Histone Modification
Histones can undergo a range of complex modifications; a complete discussion is beyond the scope of this review. Histones have N-terminal amino acid tails that protrude and can be modified by acetylation, methylation, ubiquitination, and phosphorylation.68 Different posttranscriptional modifications to histone ends are believed to recruit different coactivators or corepressors, which determines whether chromatin is in its relaxed or condensed form.69
Histone acetylation is the most well-described posttranslational modification and is regulated by the levels and activity of histone acetyl transferase (HAT) and histone deacetylase (HDAC).70 In a simple model, chromatin is transcriptionally active when lysines on histones H3 and H4 are acetylated. Although it is not exactly clear how acetylated histones affect transcription, they might change the structure of chromatin (acetylation of lysine neutralizes the positive electrostatic charge of the histone, facilitating the opening of chromatin) and thereby reveal binding sites for important coactivators.68 Overexpression or increased activity of HDACs can lead to hypoacetylation and gene silencing.
There are 18 subtypes of HDAC in mammalian cells. Classes I and II are simple hydrolases, whereas class III HDACs require cofactors. HDAC enzymes can be inhibited by a range of natural (eg, lactate, butyrate) and synthetic compounds.71
RNA Interference
miRs are single-stranded noncoding RNAs typically 22 nucleotides in length that are highly conserved throughout evolution.72 miRs with members of the Argonaut (Ago) family form the RNA interference-silencing complex. This complex regulates translation by binding to 3′ regions of untranslated mRNAs by directly inhibiting mRNA translation or by causing mRNA degradation (depending on the degree of complementarity between the miR and the mRNA target).73
After their description in the mid-1990s, a large number of miRs have been described (>1600 in humans, see http://mirbase.org). miRs are transcribed by RNA polymerase II into hairpin structures called pre-miR. Pre-miR is processed in the nucleus (by enzyme Drosha) and then the cytoplasm by the Dicer enzyme.72 After processing, each miR may show complementarity with many different mRNAs, and each mRNA may be targeted by many different miRs.74,75 miRs regulate gene expression and thereby numerous biological processes, including cell proliferation, differentiation, and death. Altered miR expression has been associated with many diseases.
Potential Pitfalls
A major hurdle in interpreting results from epigenetic studies is to determine causality, that is, whether a particular epigenetic profile is cause or consequence of disease. Furthermore, many of these studies provide only a snapshot of the epigenetic profile after the disease process has been established, rather than describing a temporal relationship between an epigenetic alteration and subsequent disease development. The epigenetic profile changes over time, and apparent associations may be a consequence of the disease itself or other environmental factors such as therapy.76,77 Conditional correlation models have attempted to determine how DNA methylation and genetic factors interact to cause diseases.78
Epigenetic marks are tissue and cell type specific, and therefore selection of a disease-relevant tissue type is crucial. In IBD research, it has been a major challenge to identify disease-relevant cell types. Currently, interest is focused on immune cells in the blood (such as CD4+ and CD8+ T cells) and the intestine (intraepithelial lymphocytes).
Results from studies of whole tissues, such as whole blood or colon biopsy samples, are difficult to interpret because of the heterogeneity among cell types, each with their own epigenetic signature. Early epigenetic studies of IBD were mostly performed with whole tissues, making results difficult to interpret; epigenetic features of individual cells can be masked by those of heterogeneous cell groups. Some researchers have used statistical methods to adjust for differing cell proportions.78,79 Studies are in progress to address this issue.
Epigenetics and IBD
DNA Methylation
Initial DNA methylation studies largely focused on the predisposition to cancer in IBD. DNA methylation changes in colonic epithelial cells that normally occur with aging are accelerated in IBD because of higher cell turnover in inflammation.80 Increased age-related DNA methylation, observed in colon cells of patients with colitis, could lead to genetic instability and development of cancer.80 Increased DNA methylation has been shown in dysplastic and the surrounding nondysplastic colon tissues from patients with UC compared with control subjects or patients with UC who do not have dysplasia.80 Four of 15 loci associated with development of cancer (CDH1, GDNF, HPP1, and MYOD1) were differentially methylated in surgical resection samples from patients with active UC compared with those with quiescent mucosa.81 CDH1 encodes the cell adhesion molecule E-cadherin, which is associated with IBD-associated cancer. Hypermethylation of the CDH1 promoter region has been shown in dysplastic and cancerous gut tissue of patients with UC compared with nondysplastic samples.24,82,83
The increasing interest in the role of DNA methylation in the pathogenesis of IBD has coincided with advances in platform-based DNA methylation array technologies, which have superseded candidate gene methylation profiling techniques.
Initial IBD epigenome-wide methylation association studies (EWAS) used platform-based arrays to analyze peripheral blood samples (Table 1). Nimmo et al analyzed the methylation profile of peripheral blood from women and children with CD.79 Whole-blood DNA was analyzed using the Illumina 27K chip (Illumina, Inc). Fifty genes showed significantly different levels of methylation between patients with IBD and controls, including some involved in immune system activation (MAPK, RPIK3, and IL21R). Ontology analysis highlighted several pathways associated with IBD, including immune system processes, immune response, and host response to bacteria, whereas canonical pathway analysis indicated the involvement of Th17 cell pathways.79
Table 1.
DNA Methylation Studies in IBD in Peripheral Blood and Intestinal Biopsy Specimens
| Authors | Subjects | Study design | Samples | Techniques | Highlighted differentially methylated loci | Number of loci showing differential DNA methylation |
|---|---|---|---|---|---|---|
| Peripheral blood DNA methylation studies | ||||||
| Harris et al, 201284 | Discordant monozygotic twins (4 CD, 7 UC), childhood IBD control (14 CD, 8 UC) | Training set discordant monozygotic twins, testing set childhood IBD control | Peripheral leukocytes PBMCs | Methylation-specific amplification array 450K Illumina BeadChip Bisulfite pyrosequencing |
TEPP | 1 |
| Lin et al, 201285 | 18 patients with IBD (9 CD, 9 UC) | Case control | Epstein–Barr virus—transformed B cells | Illumina GoldenGate Restriction length polymorphisms |
Bcl3, PPARG, STAT3, OSM, STAT5, IL12RB, SOX1, COL18A1 | 49 |
| Nimmo et al, 201279 | 21 ileal CD 19 controls |
Case control, testing set on childhood IBD | Whole blood | Illumina 27K BeadChip | MAPK13, FASLG, PRF1, S100A13, RIPK3, and IL-21R | 50 |
| Intestinal biopsy DNA methylation studies | ||||||
| Cooke et al, 201287 | 8 with active UC, 8 with quiescent UC, 8 with active CD, 8 with quiescent CD, 8 without IBD | Case control Active versus quiescent Pyrosequencing validation Quantitative reverse-transcriptase polymerase chain reaction mRNA quantification |
Rectal biopsy specimens (whole tissue and separated epithelial cells) | Illumina 27K BeadChip | THRAP2, FANCC, TNFSF4, TNFSF12, FUT7, CARD9, ICAM3, and IL8RB | >500 |
| Hasler et al, 201286 | 20 UC discordant monozygotic twins 135 unrelated subjects |
3-layer EWAS: 1. Training set 2. Methylation variable positions 3. Differentially methylated regions |
Intestinal biopsy specimens (whole tissue) | 1. Affymetrix array 2. Illumina 27K BeadChip 3. MeDip 385K |
CFI, SPINK4, and THY1/CD90 | 61 |
| Lin et al 2012151 | 9 CD, 17 UC and 26 nondisease |
Case control Training set (14 vs 14) and testing set (12 vs 12) |
Intestinal tissue from surgery (whole tissue) | Illumina GoldenGate Restriction length polymorphisms |
BGN, SERPINA, TNFSF1A, AATK, GABRA5, MAPK10, and STAT5A | 7 |
Another study showed the tissue-specific nature of epigenetic marks. No significant differences in DNA methylation were observed between children with IBD and controls based on methylation-specific amplification microarray analysis of peripheral blood. However, they found that peripheral blood mononuclear cells (PBMCs) from patients with IBD showed hypermethylation at the TEPP locus, which encodes testes, prostate, and placenta-expressed protein and is of uncertain relevance in IBD.84 A study that analyzed DNA methylation in Epstein–Barr virus—transformed B cells from 18 patients with IBD versus nonaffected siblings identified 49 differentially methylated CpG sites. More than half of the differentially methylated loci contained genes that regulate immune functions, including several (BCL3, STAT3, OSM, STAT5) involved in the IL-12 and IL-23 pathways.85
DNA methylation has also been studied in colonic tissue (Table 1). An EWAS of intestinal biopsy samples from 20 monozygotic twins discordant for UC identified 61 differentially methylated loci, with several containing genes that regulate inflammation (CFI, SPINKK4, THY1/CD90). This study had an interesting design in that after the loci were identified in the analysis of discordant monozygotic twins (to exclude differences in genetic factors), they were validated in an independent cohort.86
To overcome the heterogeneity of cell types in tissues, a methylation-wide profiling study of whole rectal biopsy specimens from patients with active and quiescent UC and CD was validated using isolated epithelial cells from rectal biopsy specimens.87 Many differentially methylated genes were identified in whole tissue, encoding proteins including DOK2 (involved in IL-4–mediated cell proliferation), Tap1 (a major histocompatibility complex class I transport molecule), and members of the TNF family (TNFSF4 and TNFSF12). ULK1 was methylated only in patients with CD; its product has a role in autophagy. Genes identified as being differentially methylated in this study, replicated findings from other EWAS,79 and have also been identified as susceptibility genes in GWAS,12,24 including CDH1, ICAM3, IL8RA, and CARD9.
Histone Modification
Histone modification is a complex process and the least studied epigenetic mechanism in IBD research. Although histone modifications have been shown to regulate genes that control inflammation,88 much of our understanding of histone modifications in the context of IBD has come from experimental and therapeutic trials of histone deacetylase inhibitors (HDACi).
Patterns of histone acetylation in colon tissues from rats with colitis, induced by administration of dextran sulfate sodium and 2,4-trinitrobenzene sulfonic acid, and biopsy specimens from patients with CD have been described. Inflamed tissue and Peyer’s patches from rats with colitis and patients were found to have increased acetylation of H4 (at lysines residues 8 and 12).89
Several mechanisms have been proposed to link histone modification with inflammation, involving the innate immune response to microbiota. Butyrate, an endogenous metabolite formed during fermentation of dietary fibers by the intestinal microbiota, is an HDAC inhibitor. Butyrate increases expression of NOD2 by increasing histone acetylation in its promoter region.90 Histone acetylation has a role in production of intestinal alkaline phosphatase, an endogenous protein responsible for detoxification of bacterial lipopolysaccharide. Sodium butyrate increased intestinal alkaline phosphatase production via its ability to inhibit HDAC.91 Toll-like receptor 4 regulates intestinal homeostasis by preventing excessive inflammatory responses to commensal bacteria and could be regulated by histone deacetylation.92 Expression of a gingival antimicrobial protein, β-defensin 2, is also increased by histone acetylation.93
HDAC inhibitors can be classified according to structural class. These classes include carboxylates (sodium butyrate and valproate), hydroxamic acids (trichostatin A, SAHA, KBH-A42, and ITF2357), benzamides (entinostat or MS-275 and mocetinostat or MGCD-0103), and cyclic peptides (α-apicidin and depsipeptides).94 HDAC inhibitors have primarily been investigated in cancer research but also have anti-inflammatory effects.95 It is worth noting that the enzymes that affect histone acetylation status (HAT, HDAC) do not act exclusively on histones but affect acetylation of a range of proteins, including p53, STAT3, and HIF1α.96 Therefore, HDAC inhibitors act not only through epigenetic mechanisms but also on multiple histone-independent targets, including the transcription factor nuclear factor κB (NF-κB) pathway, cytoskeletal proteins, and cell cycle and apoptosis regulators.97,98
Several anti-inflammatory mechanisms of HDACi have been proposed. The expression of T-regulatory cells, which mediate immune tolerance and abrogate excessive inflammation, is linked to Foxp3 gene expression. Administration of HDACi in mice leads to increased T-regulatory cell differentiation and suppression of bowel inflammation, potentially as a result of acetylation of lysines in the forkhead domain of Foxp3.99 Another mechanism could involve their effects on acetylation of proteins in the NF-κB pathway.96,100 In models of murine colitis, the HDACi ITF2357 increases acetylation of histone 3 and reduces activation of NF-κB.101 HDAC2 is associated with corticosteroid responsiveness, affecting NF-κB regulation of gene expression.102
Butyrate enemas have been used to treat patients with colitis, although inhibition of HDAC may not be their predominant mechanism of action. Butyrate has several effects on the gastrointestinal tract, including maintenance of barrier function and a homeostatic reduction in epithelial cell production of IL-8.103–105 Butyrate reduces the disease activity index of patients as well as nuclear translocation of NF-κB in lamina propria macrophages.106 Other HDACi have also been shown to ameliorate dextran sulfate sodium–induced colitis in mice.101,104
RNA Interference
Studies in animals have shown that intestinal miRs regulate gut homeostasis. Mice deficient in intestinal Dicer1, an miR-processing enzyme, have disorganized intestinal epithelial crypts with increased goblet cells, rapid jejunal epithelial migration, and accelerated apoptosis. Additionally, mice deficient in intestinal Dicer1 have increased inflammation and neutrophil and lymphocyte migration, and reduced epithelial barrier function, compared with mice not deficient in Dicer1.107
A number of studies have investigated differences in miRs between patients with and without IBD (Table 2). Changes in miRs in human IBD were first described in 2008.108 In sigmoid biopsy specimens from patients with active UC, levels of 8 miRs were significantly increased and 3 were decreased compared with samples from patients without UC. miR-192, normally expressed in colonic epithelial cells, was significantly reduced in tissues of patients with active UC.108 miR-192 reduces expression of macrophage inhibitory peptide 2α, a CXC chemokine expressed by epithelial cells; its levels are increased in colon tissues of patients with UC.108
Table 2.
MicroRNA Studies in IBD in Peripheral Blood and Intestinal Biopsy Specimens
| Authors | Subjects | Samples | Techniques | Increased microRNA expression | Decreased microRNA expression |
|---|---|---|---|---|---|
| Peripheral blood microRNA studies | |||||
| Duttagupta et al, 2012129 | 20 active UC vs 20 healthy controls | Peripheral blood | qRT-PCR | miR-188-5p, -378, -422a, -500, -501-5p, -769-5p, and -874 | |
| Paraskevi et al, 2012130 | 128 CD vs 162 healthy controls | Peripheral blood | qRT-PCR | miR-16, -23a, -29a, 106a, -107, -126, -191, -199a-5p, -200c, 362-3p, and 532-3p | |
| 88 active UC vs 162 healthy controls | miR-16, -21, -28-5p, -151-5p, -155, and 199a-5p | ||||
| Wu et al, 2011114 | 14 active CD vs 13 healthy controls | Peripheral blood | Microarray and qRT-PCR | miR-199a-5p, -340, -363-3p, -532-3p, and miRplus-E1271 | miR-149* and miRplus-F1065 |
| 5 quiescent CD vs 13 healthy controls | miR-340* | miR149* | |||
| 13 active UC vs 13 healthy controls | miR-28-5p, -151-5p, -103-2*, -199a-5p, -340*, -362-3p, -532-3p, and miRplus-E1271 | miR-505* | |||
| 10 active UC vs 10 active CD | miR-28-5p, 103-2*, 149*, 151-5p, -340, -532-3p, and miRplus-E1153 | miR-505* | |||
| Zahm et al, 2011115 | 46 active CD vs 32 healthy controls | Serum | LDA qRT-PCR | miR-16, -20a, -21, -30e, -93, -106a, -140, -192, -195, -484, and let-7b | |
| Colonic biopsy microRNA studies | |||||
| Bian et al, 2011109 | 5 active UC vs 4 healthy controls | Colonic biopsy specimens | qRT-PCR | miR-150 | |
| Brest et al, 2011113 | 83 active CD vs 67 healthy controls | Colonic biopsy specimens | qRT-PCR and in situ hybridization | miR 196 | |
| Fasseu et al, 2010111 | 8 active UC vs 8 healthy controls | Colon biopsy specimens | qRT-PCR | miR-7, -31, -135b, 223, 29a, 29b, -126, -127-3p, and -324-3p | miR-188-5p, -215, -320a, and -346 |
| 8 quiescent UC vs 8 healthy controls | miR-196a, -29a, 29b, -126, -127-3b, and -324-3p | miR-188-5p, -215, -320a, and 346 | |||
| 8 active CD vs 8 healthy controls | miR-9, -21, -22, -26a, -29a, 29c, 30b, -31, -34c-5p, -106a, -126, -126*, -127-3p, -130a, -133b, -146a, -146b-3p, -150 ,155, -181c, -196a, -324-3p, -375 | ||||
| 8 quiescent CD vs 8 healthy controls | miR-9*, -21, -22, -26a, 29b, 29c, 30a*, -30b, -30c -31, -34c-5p, 106a, -126, -127-3p, -133b, -146a, 146b-3p, -150, -155, -196a -223, and -324-3p | ||||
| 8 quiescent UC vs 8 quiescent CD | miR-150, 196b, -199a-3p, -199-5p, -223, and 320a | ||||
| Nguyen et al, 2010152 | 8 active CD vs 6 healthy controls | Colonic biopsy specimens | qRT-PCR | miR-7 | |
| Olaru et al, 2011153 | IBD-associated dysplasia vs active IBD | Colonic biopsy specimens | Microarray and qRT-PCR | miR-31, 31*, -96, -135b, -141, -183, -192, -192*, -194, -194*, -200a, -200a*, -200b, -200b*, -200c, -203, -215, -224, -375, -424*, -429, and -552 | miR -122, -139-5p, -142-3p, -146b-5p, -155, -223, -490-3p, 501-5p, -892b, and -1288 |
| Pekow et al, 2012154 | 8 active UC vs 8 healthy controls | Colonic biopsy specimens | qRT-PCR | miR-143 and -145 | |
| Takagi et al, 2010110 | 12 active UC vs 12 healthy controls | Sigmoid colon biopsy | Microarray and qRT-PCR | miR-21 and -155 | |
| Wu et al, 2008108 | 15 active UC vs 15 healthy controls | Sigmoid colon biopsy | Microarray and qRT-PCR | miR-16, -21, 23a, 24, 29a, 126, 195, and left-7f | miR-192, 375, and 422b |
| Wu et al, 2010112 | 5 active colonic CD vs 13 healthy controls | Sigmoid colon biopsy specimens | Microarray and qRT-PCR | miR-23b, -106a, and -191 | miR-19b and -629 |
| 6 active small bowel CD vs 13 healthy controls | Terminal ileal biopsy specimens | Microarray and qRT-PCR | miR-16, -21, -223, and 594 | ||
qRT-PCR, quantitative reverse-transcriptase polymerase chain reaction.
miR-150 is up-regulated in mice with dextran sulfate sodium–induced colitis in colon tissues from patients with UC; its levels correlate inversely with those of its target c-Myb, which has a role in apoptosis.109 Up-regulation of miR-21, which promotes inflammation, has been reported in several studies of patients with active UC and CD colitis (but not ileitis), along with miR-155.110–112 miR-196 is overexpressed in the inflamed epithelium of patients with CD and may reduce IRGM-mediated autophagy.113
Distinct miR signatures have been identified in peripheral blood samples from patients with IBD compared with controls and in patients with CD compared with those with UC.114 Several miRs have been found to be significantly up-regulated or down-regulated in 2 or more studies, including miRs-16, -21, -28-5p, -149, -151-5p, -199-a, and -532-3p.114–116 Eleven miRs were also found to be differentially expressed between serum samples from pediatric patients with CD and healthy children.115
Further adequately powered studies are required to identify IBD-associated miR profiles in intestinal tissues and serum, plasma, and separated blood cells. Specific miR profiles might be able to predict IBD susceptibility, progression, and response to therapy. Moreover, identifying the targets of these miRs will provide additional insight into the pathogenesis of IBD.
Interaction Between Genetics and Epigenetics in Complex Disease
An intriguing field of investigation is the relationship between genetic and epigenetic factors. There is evidence of colocalization of differentially methylated CpGs at predisposing SNPs identified at GWAS. In our own EWAS of CD, we showed enrichment of methylation changes within 50 kilobases from GWAS-identified susceptibility loci, including IL-19, IL-27, TNF, and NOD2.79 In a recent large methylation study of patients with rheumatoid arthritis, in 5 of 9 MHC genes, a specific genotype was associated with differential methylation.78
This phenomena has also been observed in studies of patients with type 2 diabetes mellitus, where a specific allele, rs8050136, within the obesity and diabetes susceptibility gene FTO is associated with increased DNA methylation.117 However, Toperoff et al associated a different SNP, rs1121980, with hypomethylation of FTO.118
It is not clear how these SNPs affect methylation of the gene. They could increase the numbers of CpG dinucleotides or alter the access of the methylation machinery to the gene.117 Allele- or haplotype-specific methylation occurs more commonly with cis-acting polymorphisms.119 A potential cofounder of the Illumina BeadArray, used in most DNA methylation studies, is that certain probes contain SNPs or repetitive elements that can affect methylation analysis.120
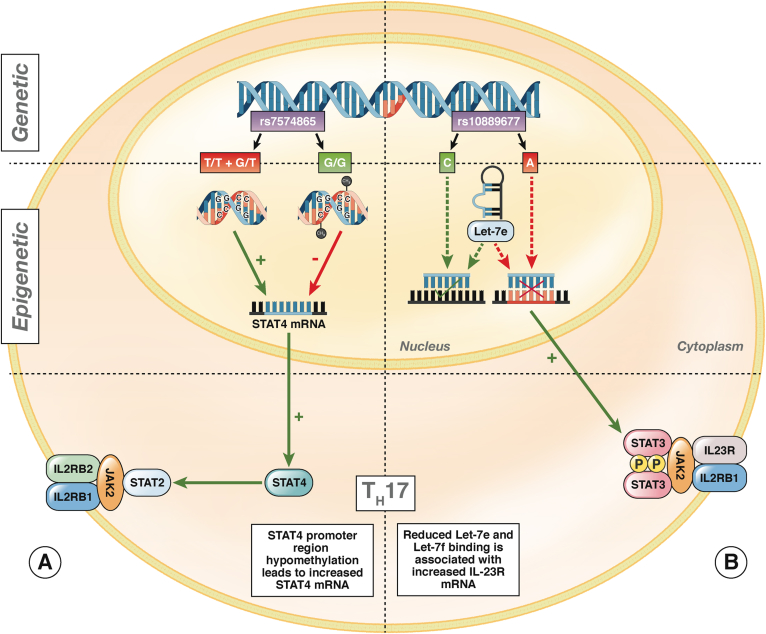
Variants in STAT4 have also been reported to alter its methylation (Figure 3A). Additional evidence of haplotype-specific methylation has been shown in the promoter regions of IL8RA and IL8RB. Rectal biopsy specimens from patients with IBD were shown to have increased methylation of the CpG island closest to the transcriptional start site of IL8RA: the proposed binding site of transcription factor PU.1 (SPI-1).87 The risk allele rs11676348 alters a CpG, is located between IL8RA and IL8RB coding sequences, and contains a binding site for the transcription factor STAT3.87 Although not specifically probed itself by the Illumina 27K, differential methylation was observed on either side of rs11676348.87
Figure 3.
The relationship between genetic polymorphisms and epigenetic factors. Epigenetic features of T cells in patients with IBD affect Th1 and Th17 cell differentiation. (A) STAT4 is associated with several immune diseases, acting as a transcription factor for IL-12 and IL-23 that leads to Th1 and Th17 cell differentiation.141 An SNP in STAT4, rs7574865, is associated with several immune disorders, including IBD, rheumatoid arthritis, type 1 diabetes, and lupus.142–146 The rs7574865 risk variants (T/T + G/T) are associated with promoter region hypomethylation in colon tissues and PBMCs of patients with IBD. STAT4 promoter hypomethylation was associated with increases in STAT4 mRNA and could promote the Th1 phenotype and interferon γ production.147 In T cells from patients with asthma, STAT4 expression is also regulated by DNA methylation at promoter regions. Interestingly, STAT4 expression was markedly increased after treatment with a DNMT inhibitor.148 (B) An IBD-associated SNP in IL23-R, rs10889677, is associated with increased levels of IL-23R mRNA and protein. This could result from reduced binding of microRNAs Let-7e and Let-7f at the regulatory 3′ untranslated region of the rs10889677 risk variant (A) compared with cells from patients without IBD (C).149 Reduced binding of Let-7e and Let-7f to rs10889677 is associated with increased levels of IL23R mRNA and protein, potentially leading to sustained activation of Th17 cells and the chronic inflammation associated with IBD.149
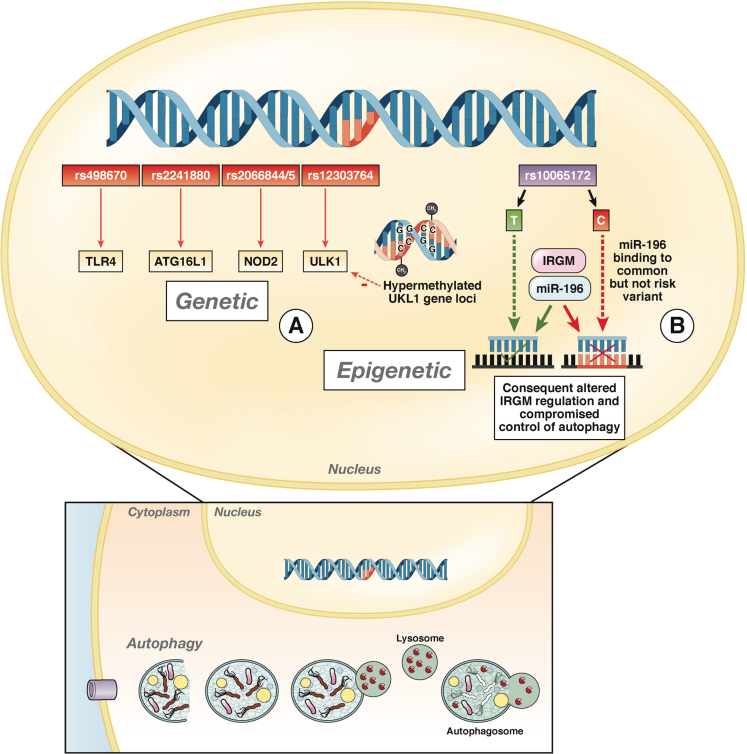
Similarly, SNPs can affect the complementarity of miR binding. IBD-associated variants of IL23R, which have a role in IL-12 and IL-23 signaling, may show altered binding with miRs Let-7e and Let-7f, leading to altered expression of IL23R and inappropriate Th17 activation (Figure 3B). IRGM mediates innate immune defense against intracellular organisms, including Mycobacterium tuberculosis.23 Variants of IRGM alter the binding site for miR-196 (Figure 4B).113
Figure 4.
Loci identified in GWAS indicating roles for the innate immune response to the microbiota and autophagy in the pathogenesis of CD. Several CD risk alleles were identified in GWAS in genes that control autophagy, including TLR4, ATG16L1, IRGM, and ULK1. (A) The ULK1 locus has been associated with susceptibility to CD; it encodes a serine-threonine kinase involved in the autophagy response to starvation. ULK1 is hypermethylated in cells from patients with CD compared with controls.87 (B) IRGM encodes a gene that regulates the innate response to intracellular organisms, including Mycobacterium tuberculosis. The CD risk allele rs10065172 is associated with a deletion upstream of IRGM.150 This SNP had been termed noncausative due to an absence in alteration of protein sequence or splice sites. However, the risk variant has an altered binding site for microRNA-196. Individuals with this SNP down-regulate IGRM. The consequence is a functional reduction of autophagy and processing of the adhesive invasive Escherichia coli, which has been associated with CD.113
Another study evaluated the risk of UC conferred by 3 common allelic variants of 3 pre-miRs (miR-146a, -196a, and -499). Three SNPs (rs11614913, rs2910164, and rs3746444) were genotyped in 170 patients with UC and 403 control patients. The AG heterozygous genotype of rs3746444, encoding miR-499, was significantly associated with an increased risk of UC (odds ratio, 1.51). The same genotype was also associated with older age of onset, left-sided colitis, hospitalization, and dependence on corticosteroids.121
Clinical Implications
A number of potential clinical applications of epigenetics in diagnostics and therapeutics are receiving attention. The diagnostic applications of epigenetics include the use of biomarkers to confirm diagnosis, stratify disease course and response to chemotherapy, and predict development of cancer.122 Particularly pertinent for IBD, methylation changes in SFRP2, measured in fecal DNA samples, have been used to identify patients with colorectal cancer with approximately 75% sensitivity and specificity.123 Biomarkers have been found in a range of body fluids, including sputum, urine, and saliva for lung, bladder, and head and neck cancers, respectively.124–126 DNA methylation is a quantitative trait and therefore an attractive biomarker. A panel of relevant hypomethylated or hypermethylated CpGs might someday be used to distinguish between UC and CD, enable disease stratification, and predict treatment response.
The tissue-specific nature of epigenetic changes becomes especially relevant when considering their use as biomarkers. In oncology, tumor-specific aberrant DNA methylation might be used to identify patients with cancer.127 DNA methylation might also be useful in identifying patients with UC who are most likely to develop cancer. DNA methylation was increased in dysplastic and nondysplastic tissues from patients with colitis-associated cancer compared with those without cancer.81 More recently, changes in neoplasia-associated DNA methylation in SLIT2 and TMEFF2 were found in mucosal biopsy specimens and fecal DNA from patients with IBD who are at high risk for developing dysplasia or cancer.128
Disease-specific DNA methylation might be difficult to detect because of the heterogeneity of cell types in whole tissue samples and only become evident when isolated cell types are analyzed.84 The additional resources required to separate cells may limit the applicability of DNA methylation as a biomarker, although early studies of whole blood have produced promising results for biomarker development.
Likewise, miRs have been advocated as possible biomarkers. When analyzing more than 300 miRNAs present in colonic tissue biopsy specimens, distinct profiles were identified in IBD versus control samples (miR-26a, -29b, -126, -127-3p, -324-3p) and between quiescent UC and CD (miR-196b, 199a-3p, -199-5b, -150, -223).111 Distinct IBD profiles of miRs have also been described in peripheral blood, providing appealing, minimally invasive biomarkers. A miR panel from peripheral blood was able to distinguish between patients with UC and CD; levels of 10 miRs were increased and the level of one was decreased in patients with UC compared with CD.108 miRs derived from platelets, microvesicles, and PBMCs have also been measured in attempts to differentiate patients with and without UC. Seven miRs were differentially expressed in patients with UC compared with controls.129 The specificity of miRs for IBD is not known and may limit their applicability as diagnostic biomarkers, but they could be used to monitor disease activity. For example, the proinflammatory miR-21 is increased in peripheral blood of patients with IBD but is also increased in patients with other diseases, including colorectal cancer.130,131
Therapeutics
Further studies of epigenetic factors associated with IBD could lead to new therapeutic strategies, whether they specifically target epigenetic mechanisms or affect the pathways they control. Pharmacologic agents that affect epigenetic processes include HDACi, HAT inhibitors, and DNMT inhibitors. However, these have not been tested in patients with IBD. HDACi were first licensed in the United States for treatment of T-cell lymphoma and are now being evaluated for inflammatory disorders such as rheumatoid arthritis, multiple sclerosis, and juvenile arthritis.132,133 A controlled trial of butyrate, conducted more than 10 years ago, found that a combination of butyrate and mesalamine compounds induced remission significantly more frequently than mesalamine alone in patients with refractory UC.134 These so-called “pan-HDAC inhibitors” lack specificity for individual HDAC enzymes and are consequently accompanied by side effects such as worsening of atherosclerosis and immunosuppression. The ultimate objective will be to develop specific HDACi that target only enzymes involved in intestinal inflammation.96 For example, inhibition of HDAC9 alone could increase the function of FoxP3 T-regulatory cells and help to ameliorate colitis.135
Conclusions
Great strides have been made in understanding the genetic basis for IBD, providing insight into new pathogenic mechanisms and expanding existing ones. Further studies are required to fine map the 163 known IBD susceptibility loci and determine how they contribute to disease risk. Epigenomics is an emerging field that adds an extra layer of complexity to genetic analyses. Epigenetic studies could provide exciting clues into the pathogenesis of IBD but, like genetics, are unlikely to address all outstanding questions. It is more likely that epigenetics analyses will be integrated into larger bioanalytical models alongside other emerging IBD research disciplines, such as transcriptomics, metagenomics, glycomics, glycoproteomics, and metabolomics.
Epigenetic research could provide biomarkers for use in diagnosis of IBD, along with predicting disease progression and response to therapy. Prospective studies supported by the European Union that follow the course of disease in newly diagnosed patients and relate phenotype to biomarker profile could deliver clinically useful results in the next decade to help in personalizing care.136,137
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding N.T.V. is funded through the EU FP7 grant “IBD-BIOM,” and N.A.K. is funded through the Wellcome Trust.
References
- 1.Molodecky N.A., Soon I.S., Rabi D.M. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 2.Henderson P., Hansen R., Cameron F.L. Rising incidence of pediatric inflammatory bowel disease in Scotland. Inflamm Bowel Dis. 2012;18:999–1005. doi: 10.1002/ibd.21797. [DOI] [PubMed] [Google Scholar]
- 3.Benchimol E.I., Guttmann A., Griffiths A.M. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58:1490–1497. doi: 10.1136/gut.2009.188383. [DOI] [PubMed] [Google Scholar]
- 4.Ng S.C., Bernstein C.N., Vatn M.H. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 5.Gunesh S., Thomas G.A., Williams G.T. The incidence of Crohn’s disease in Cardiff over the last 75 years: an update for 1996-2005. Aliment Pharmacol Ther. 2008;27:211–219. doi: 10.1111/j.1365-2036.2007.03576.x. [DOI] [PubMed] [Google Scholar]
- 6.IBD Standards Group UK . 2009. Quality care cervice standards for the healthcare of people who have inflammatory bowel disease.http://www.ibdstandards.org.uk/ibd-standards.asp Available at: Accessed March 1, 2013. [Google Scholar]
- 7.van der Valk M.E., Mangen M.-J.J., Leenders M. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2012 Nov 7 doi: 10.1136/gutjnl-2012-303376. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 9.Jostins L., Ripke S., Weersma R.K. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;490:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Limbergen J., Stevens C., Nimmo E.R. Autophagy: from basic science to clinical application. Mucosal Immunol. 2009;2:315–330. doi: 10.1038/mi.2009.20. [DOI] [PubMed] [Google Scholar]
- 11.Khor B., Gardet A., Xavier R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson C.A., Boucher G., Lees C.W. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 14.Franke A., Balschun T., Karlsen T.H. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 15.Franke A., Mcgovern D.P.B., Barrett J.C. Meta-analysis increases to 71 the tally of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glocker E.-O., Kotlarz D., Boztug K. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glocker E.-O., Frede N., Perro M. Infant colitis—it’s in the genes. Lancet. 2010;376:1272. doi: 10.1016/S0140-6736(10)61008-2. [DOI] [PubMed] [Google Scholar]
- 18.Barrett J.C., Hansoul S., Nicolae D.L. Genome-wide association defines more than thirty distinct susceptibility loci for Crohn’s disease. Nat Genet. 2009;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooney R., Baker J., Brain O. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 20.Travassos L.H., Carneiro L.A.M., Ramjeet M. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 21.Aldhous M.C., Soo K., Stark L.A. Cigarette smoke extract (CSE) delays NOD2 expression and affects NOD2/RIPK2 interactions in intestinal epithelial cells. PloS One. 2011;6:e24715. doi: 10.1371/journal.pone.0024715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho J.H., Brant S.R. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140:1704–1712. doi: 10.1053/j.gastro.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Intemann C.D., Thye T., Niemann S. Autophagy gene variant IRGM -261T contributes to protection from tuberculosis caused by Mycobacterium tuberculosis but not by M. africanum strains. PLoS Pathog. 2009;5:e1000577. doi: 10.1371/journal.ppat.1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett J.C., Lee J.C., Lees C.W. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330–1334. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamagata K., Furuta H., Oda N. Mutations in the hepatocyte nuclear factor-4gene in maturity-onset diabetes of the young (MODY1) Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 26.Cargill M., Schrodi S.J., Chang M. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton P., Clayton D., Cardon L. Association scan of 14,500 nsSNPs in four common diseases identifies variants involved in autoimmunity. Nat Genet. 2009;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho J.H., Gregersen P.K. Genomics and the multifactorial nature of human autoimmune disease. N Engl J Med. 2011;365:1612–1623. doi: 10.1056/NEJMra1100030. [DOI] [PubMed] [Google Scholar]
- 29.Lees C.W., Barrett J.C., Parkes M. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 30.Wang K., Baldassano R., Zhang H. Comparative genetic analysis of inflammatory bowel disease and type 1 diabetes implicates multiple loci with opposite effects. Hum Mol Genet. 2010;19:2059–2067. doi: 10.1093/hmg/ddq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muriboberg K., Melum E., Folseraas T. Three ulcerative colitis susceptibility loci are associated with primary sclerosing cholangitis and indicate a role for IL2, REL and CARD9. Hepatology. 2012;53:1977–1985. doi: 10.1002/hep.24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worthey E.A., Mayer A.N., Syverson G.D. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med. 2011;13:255–262. doi: 10.1097/GIM.0b013e3182088158. [DOI] [PubMed] [Google Scholar]
- 33.Dunham I., Kundaje A., Aldred S.F. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad T., Armuzzi A., Bunce M. The molecular classification of the clinical manifestations of Crohn’s disease. Gastroenterology. 2002;122:854–866. doi: 10.1053/gast.2002.32413. [DOI] [PubMed] [Google Scholar]
- 35.Futami S., Aoyama N., Honsako Y. HLA-DRB1*1502 allele, subtype of DR15, is associated with susceptibility to ulcerative colitis and its progression. Dig Dis Sci. 1995;40:814–818. doi: 10.1007/BF02064985. [DOI] [PubMed] [Google Scholar]
- 36.Lesage S., Zouali H., Cézard J.-P. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet. 2002;70:845–857. doi: 10.1086/339432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lees C.W. Characterization of the ∼40,000 patient cohort of the International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) J Crohn’s Colitis. 2013;7:S4. [Google Scholar]
- 38.Brant S.R. Promises, delivery, and challenges of inflammatory bowel disease risk gene discovery. Clin Gastroenterol Hepatol. 2012;11:22–26. doi: 10.1016/j.cgh.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Dassopoulos T., Nguyen G.C., Talor M.V. NOD2 mutations and anti-Saccharomyces cerevisiae antibodies are risk factors for Crohn’s disease in African Americans. Am J Gastroenterol. 2010;105:378–386. doi: 10.1038/ajg.2009.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue N. Lack of common NOD2 variants in Japanese patients with Crohn’s disease. Gastroenterology. 2002;123:86–91. doi: 10.1053/gast.2002.34155. [DOI] [PubMed] [Google Scholar]
- 41.US Food and Drug Administration Imuran (azathioprine) product information. 2011. Drugs@FDA 1–9.
- 42.Roberts R.L., Barclay M.L. Current relevance of pharmacogenetics in immunomodulation treatment for Crohn’s disease. J Gastroenterol Hepatol. 2012;27:1546–1554. doi: 10.1111/j.1440-1746.2012.07220.x. [DOI] [PubMed] [Google Scholar]
- 43.Sandborn W.J., Gasink C., Gao L.-L. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. doi: 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- 44.Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut. 2012;61:918–932. doi: 10.1136/gutjnl-2011-300904. [DOI] [PubMed] [Google Scholar]
- 45.Petronis A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature. 2010;465:721–727. doi: 10.1038/nature09230. [DOI] [PubMed] [Google Scholar]
- 46.Morgan H.D., Sutherland H.G.E., Martin D.I.K. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 2006;23:314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 47.Schaible T.D., Harris R.A., Dowd S.E. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum Mol Genet. 2011;20:1687–1696. doi: 10.1093/hmg/ddr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waterland R.A., Jirtle R.L. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tobi E.W., Lumey L.H., Talens R.P. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heijmans B.T., Tobi E.W., Lumey L.H. Archive of the prenatal environment. Epigenetics. 2009;4:526–531. doi: 10.4161/epi.4.8.10265. [DOI] [PubMed] [Google Scholar]
- 51.Relton C.L., Davey Smith G. Epigenetic epidemiology of common complex disease: prospects for prediction, prevention, and treatment. PLoS Med. 2010;7:e1000356. doi: 10.1371/journal.pmed.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brain O., Allan P., Pichulik T. NOD2 regulation of micrornas. Gut. 2011;60:A37. A37. [Google Scholar]
- 53.Janson P.C.J., Winerdal M.E., Winqvist O. At the crossroads of T helper lineage commitment—epigenetics points the way. Biochim Biophys Acta. 2009;1790:906–919. doi: 10.1016/j.bbagen.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Faulk C., Dolinoy D.C. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics. 2011;6:791–797. doi: 10.4161/epi.6.7.16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Santis M., Selmi C. The therapeutic potential of epigenetics in autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:92–101. doi: 10.1007/s12016-011-8293-8. [DOI] [PubMed] [Google Scholar]
- 56.Ushijima T., Watanabe N., Okochi E. Fidelity of the methylation pattern and its variation in the genome. Genome Res. 2003;13:868–874. doi: 10.1101/gr.969603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smallwood S.A., Kelsey G. De novo DNA methylation: a germ cell perspective. Trends Genet. 2012;28:33–42. doi: 10.1016/j.tig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Blewitt M.E., Vickaryous N.K., Paldi A. Dynamic reprogramming of DNA methylation at an epigenetically sensitive allele in mice. PLoS Genet. 2006;2:e49. doi: 10.1371/journal.pgen.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grossniklaus U., Kelly B., Ferguson-Smith A.C. Transgenerational epigenetic inheritance: how important is it? Nat Rev Genet. 2013;14:228–235. doi: 10.1038/nrg3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lane N., Dean W., Erhardt S. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- 61.Cubas P., Vincent C., Coen E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature. 1999;401:157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- 62.Youngson N.A., Whitelaw E. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet. 2008;9:233–257. doi: 10.1146/annurev.genom.9.081307.164445. [DOI] [PubMed] [Google Scholar]
- 63.Portela A., Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 64.Doi A., Park I., Wen B. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2010;41:1350–1353. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hughes T., Webb R., Fei Y. DNA methylome in human CD4+ T cells identifies transcriptionally repressive and non-repressive methylation peaks. Genes Immun. 2010;11:554–560. doi: 10.1038/gene.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuroda A., Rauch T.A., Todorov I. Insulin gene expression is regulated by DNA methylation. PloS One. 2009;4:e6953. doi: 10.1371/journal.pone.0006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clark S.J., Melki J. DNA methylation and gene silencing in cancer: which is the guilty party? Oncogene. 2002;21:5380–5387. doi: 10.1038/sj.onc.1205598. [DOI] [PubMed] [Google Scholar]
- 68.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Wang Z., Zang C., Rosenfeld J.A. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shahbazian M.D., Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 71.Latham T., Mackay L., Sproul D. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 2012;40:4794–4803. doi: 10.1093/nar/gks066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Connell R.M., Rao D.S., Chaudhuri A.A. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 73.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 74.Dalal S.R., Kwonc J.H. The role of microRNA in inflammatory bowel disease. Gastroenterol Hepatol. 2010;6:714–722. [PMC free article] [PubMed] [Google Scholar]
- 75.Baek D., Villén J., Shin C. The impact of microRNAs on protein output. Nature. 2009;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rakyan V.K., Down T.A., Balding D.J. Epigenome-wide association studies for common human diseases. Nat Rev Genet. 2011;12:529–541. doi: 10.1038/nrg3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drong A.W., Lindgren C.M., McCarthy M.I. The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther. 2012;92:707–715. doi: 10.1038/clpt.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y., Aryee M.J., Padyukov L. Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis. Nat Biotechnol. 2013;31:142–147. doi: 10.1038/nbt.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nimmo E.R., Prendergast J.G., Aldhous M.C. Genome-wide methylation profiling in Crohn’s disease identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. Inflamm Bowel Dis. 2012;18:889–899. doi: 10.1002/ibd.21912. [DOI] [PubMed] [Google Scholar]
- 80.Issa J.P., Ahuja N., Toyota M. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61:3573–3577. [PubMed] [Google Scholar]
- 81.Saito S., Kato J., Hiraoka S. DNA methylation of colon mucosa in ulcerative colitis patients: correlation with inflammatory status. Inflamm Bowel Dis. 2011;17:1955–1965. doi: 10.1002/ibd.21573. [DOI] [PubMed] [Google Scholar]
- 82.Azarschab P., Porschen R., Gregor M. Epigenetic control of the E-cadherin gene (CDH1) by CpG methylation in colectomy samples of patients with ulcerative colitis. Genes Chromosomes Cancer. 2002;35:121–126. doi: 10.1002/gcc.10101. [DOI] [PubMed] [Google Scholar]
- 83.Wheeler J.M., Kim H.C., Efstathiou J.A. Hypermethylation of the promoter region of the E-cadherin gene (CDH1) in sporadic and ulcerative colitis associated colorectal cancer. Gut. 2001;48:367–371. doi: 10.1136/gut.48.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harris R.A., Nagy-Szakal D., Pedersen N. Genome-wide peripheral blood leukocyte DNA methylation microarrays identified a single association with inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18:2334–2341. doi: 10.1002/ibd.22956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin Z., Hegarty J.P., Yu W. Identification of disease-associated DNA methylation in B cells from Crohn’s disease and ulcerative colitis patients. Dig Dis Sci. 2012;57:3145–3153. doi: 10.1007/s10620-012-2288-z. [DOI] [PubMed] [Google Scholar]
- 86.Häsler R., Feng Z., Bäckdahl L. A functional methylome map of ulcerative colitis. Genome Res. 2012;22:2130–2137. doi: 10.1101/gr.138347.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cooke J., Zhang H., Greger L. Mucosal genome-wide methylation changes in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2128–2137. doi: 10.1002/ibd.22942. [DOI] [PubMed] [Google Scholar]
- 88.Saccani S., Natoli G. Dynamic changes in histone H3 Lys 9 methylation occurring at tightly regulated inducible inflammatory genes. Genes Dev. 2002;16:2219–2224. doi: 10.1101/gad.232502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsaprouni L.G., Ito K., Powell J.J. Differential patterns of histone acetylation in inflammatory bowel diseases. J Inflamm. 2011;8:1. doi: 10.1186/1476-9255-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leung C.-H., Lam W., Ma D.-L. Butyrate mediates nucleotide-binding and oligomerisation domain (NOD) 2-dependent mucosal immune responses against peptidoglycan. Eur J Immunol. 2009;39:3529–3537. doi: 10.1002/eji.200939454. [DOI] [PubMed] [Google Scholar]
- 91.Malo M.S., Biswas S., Abedrapo M.A. The pro-inflammatory cytokines, IL-1beta and TNF-alpha, inhibit intestinal alkaline phosphatase gene expression. DNA Cell Biol. 2006;25:684–695. doi: 10.1089/dna.2006.25.684. [DOI] [PubMed] [Google Scholar]
- 92.Takahashi K., Sugi Y., Hosono A. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J Immunol. 2009;183:6522–6529. doi: 10.4049/jimmunol.0901271. [DOI] [PubMed] [Google Scholar]
- 93.Yin L., Chung W.O. Epigenetic regulation of human β-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol. 2011;4:409–419. doi: 10.1038/mi.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shuttleworth S.J., Bailey S.G., Townsend P.A. Histone Deacetylase inhibitors: new promise in the treatment of immune and inflammatory diseases. Curr Drug Targets. 2010;11:1430–1438. doi: 10.2174/1389450111009011430. [DOI] [PubMed] [Google Scholar]
- 95.Halili M.A., Andrews M.R., Sweet M.J. Histone deacetylase inhibitors in inflammatory disease. Curr Top Med Chem. 2009;9:309–319. doi: 10.2174/156802609788085250. [DOI] [PubMed] [Google Scholar]
- 96.Glauben R., Siegmund B. Inhibition of histone deacetylases in inflammatory bowel diseases. Mol Med. 2011;17:426–433. doi: 10.2119/molmed.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park J.-S., Lee E.-J., Lee J.-C. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int Immunopharmacol. 2007;7:70–77. doi: 10.1016/j.intimp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 98.Xu W.S., Parmigiani R.B. Marks P a. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 99.Tao R., de Zoeten E.F., Ozkaynak E. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 100.Chen L.f., Fischle W., Verdin E. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 101.Glauben R., Batra A., Stroh T. Histone deacetylases: novel targets for prevention of colitis-associated cancer in mice. Gut. 2008;57:613–622. doi: 10.1136/gut.2007.134650. [DOI] [PubMed] [Google Scholar]
- 102.Ito K., Yamamura S., Essilfie-Quaye S. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butzner J.D., Parmar R., Bell C.J. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat. Gut. 1996;38:568–573. doi: 10.1136/gut.38.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Glauben R., Batra A., Fedke I. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. 2006;176:5015–5022. doi: 10.4049/jimmunol.176.8.5015. [DOI] [PubMed] [Google Scholar]
- 105.Plöger S., Stumpff F., Penner G.B. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 106.Lührs H., Gerke T., Müller J.G. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37:458–466. doi: 10.1080/003655202317316105. [DOI] [PubMed] [Google Scholar]
- 107.McKenna L.B., Schug J., Vourekas A. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010;139:1654–1664. doi: 10.1053/j.gastro.2010.07.040. 1664.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu F., Zikusoka M., Trindade A. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635.e24. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 109.Bian Z., Li L., Cui J. Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. J Pathol. 2011;225:544–553. doi: 10.1002/path.2907. [DOI] [PubMed] [Google Scholar]
- 110.Takagi T., Naito Y., Mizushima K. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J Gastroenterol Hepatol. 2010;25(Suppl 1):S129–S133. doi: 10.1111/j.1440-1746.2009.06216.x. [DOI] [PubMed] [Google Scholar]
- 111.Fasseu M., Tréton X., Guichard C. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PloS One. 2010;5:e13160. doi: 10.1371/journal.pone.0013160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu F., Zhang S., Dassopoulos T. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis. 2010;16:1729–1738. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brest P., Lapaquette P., Souidi M. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn’s disease. Nat Genet. 2011;43:242–245. doi: 10.1038/ng.762. [DOI] [PubMed] [Google Scholar]
- 114.Wu F., Guo N.J., Tian H. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2012;17:241–250. doi: 10.1002/ibd.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zahm A.M., Thayu M., Hand N.J. Circulating microRNA is a biomarker of pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2011;53:26–33. doi: 10.1097/MPG.0b013e31822200cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Archanioti P., Gazouli M., Theodoropoulos G. Micro-RNAs as regulators and possible diagnostic bio-markers in inflammatory bowel disease. J Crohn’s Colitis. 2011;5:520–524. doi: 10.1016/j.crohns.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 117.Bell C.G., Finer S., Lindgren C.M. Integrated genetic and epigenetic analysis identifies haplotype-specific methylation in the FTO type 2 diabetes and obesity susceptibility locus. PloS One. 2010;5:e14040. doi: 10.1371/journal.pone.0014040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toperoff G., Aran D., Kark J.D. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet. 2012;21:371–383. doi: 10.1093/hmg/ddr472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tycko B. Allele-specific DNA methylation: beyond imprinting. Hum Mol Genet. 2010;19:R210–R220. doi: 10.1093/hmg/ddq376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Byun H.-M., Siegmund K.D., Pan F. Epigenetic profiling of somatic tissues from human autopsy specimens identifies tissue- and individual-specific DNA methylation patterns. Hum Mol Genet. 2009;18:4808–4817. doi: 10.1093/hmg/ddp445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Okubo M., Tahara T., Shibata T. Association study of common genetic variants in pre-microRNAs in patients with ulcerative colitis. J Clin Immunol. 2011;31:69–73. doi: 10.1007/s10875-010-9461-y. [DOI] [PubMed] [Google Scholar]
- 122.Ushijima T., Nakajima T., Maekita T. DNA methylation as a marker for the past and future. J Gastroenterol. 2006;41:401–407. doi: 10.1007/s00535-006-1846-6. [DOI] [PubMed] [Google Scholar]
- 123.Müller H.M., Oberwalder M., Fiegl H. Methylation changes in faecal DNA: a marker for colorectal cancer screening? Lancet. 2004;363:1283–1285. doi: 10.1016/S0140-6736(04)16002-9. [DOI] [PubMed] [Google Scholar]
- 124.Palmisano W.A., Divine K.K., Saccomanno G. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60:5954–5958. [PubMed] [Google Scholar]
- 125.Dulaimi E. Detection of bladder cancer in urine by a tumor suppressor gene hypermethylation panel. Clin Cancer Res. 2004;10:1887–1893. doi: 10.1158/1078-0432.ccr-03-0127. [DOI] [PubMed] [Google Scholar]
- 126.Rosas S.L., Koch W., da Costa Carvalho M.G. Promoter hypermethylation patterns of p16, O6-methylguanine-DNA-methyltransferase, and death-associated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res. 2001;61:939–942. [PubMed] [Google Scholar]
- 127.Miyamoto K., Ushijima T. Diagnostic and therapeutic applications of epigenetics. Jpn J Clin Oncol. 2005;35:293–301. doi: 10.1093/jjco/hyi088. [DOI] [PubMed] [Google Scholar]
- 128.Azuara D., Rodriguez-Moranta F., de Oca J. Novel methylation panel for the early detection of neoplasia in high-risk ulcerative colitis and Crohn’s colitis patients. Inflamm Bowel Dis. 2013;19:165–173. doi: 10.1002/ibd.22994. [DOI] [PubMed] [Google Scholar]
- 129.Duttagupta R., DiRienzo S., Jiang R. Genome-wide maps of circulating miRNA biomarkers for ulcerative colitis. PloS One. 2012;7:e31241. doi: 10.1371/journal.pone.0031241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paraskevi A., Theodoropoulos G., Papaconstantinou I. Circulating microRNA in inflammatory bowel disease. J Crohn’s Colitis. 2012;6:900–904. doi: 10.1016/j.crohns.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 131.Kanaan Z., Rai S.N., Eichenberger M.R. Plasma miR-21: a potential diagnostic marker of colorectal cancer. Ann Surg. 2012;256:544–551. doi: 10.1097/SLA.0b013e318265bd6f. [DOI] [PubMed] [Google Scholar]
- 132.Smith K.T., Workman J.L. Histone deacetylase inhibitors: anticancer compounds. Int J Biochem Cell Biol. 2009;41:21–25. doi: 10.1016/j.biocel.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 133.Vojinovic J., Damjanov N., D’Urzo C. Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2011;63:1452–1458. doi: 10.1002/art.30238. [DOI] [PubMed] [Google Scholar]
- 134.Vernia P., Annese V., Bresci G. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur J Clin Invest. 2003;33:244–248. doi: 10.1046/j.1365-2362.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- 135.de Zoeten E.F., Wang L., Sai H. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology. 2010;138:583–594. doi: 10.1053/j.gastro.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.European Commission IBD Biom. 2012. http://ec.europa.eu/research/health/medical-research/severe-chronic-diseases/projects/ibd-biom_en.html Available at: Accessed March 1, 2013.
- 137.European Commission IBD Character. 2012. http://ec.europa.eu/research/health/medical-research/severe-chronic-diseases/projects/ibd-character_en.html Available at: Accessed March 1, 2013.
- 138.Renz H., von Mutius E., Brandtzaeg P. Gene-environment interactions in chronic inflammatory disease. Nat Immunol. 2011;12:273–277. doi: 10.1038/ni0411-273. [DOI] [PubMed] [Google Scholar]
- 139.Margueron R., Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet. 2010;11:285–296. doi: 10.1038/nrg2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scarpa M., Stylianou E. Epigenetics: concepts and relevance to IBD pathogenesis. Inflamm Bowel Dis. 2012;18:1982–1996. doi: 10.1002/ibd.22934. [DOI] [PubMed] [Google Scholar]
- 141.Korman B., Kastner D.L., Gregersen P.K. STAT4: genetics, mechanisms, and implications for autoimmunity review for current allergy and asthma reports. Curr Allergy Asthma Rep. 2009;8:398–403. doi: 10.1007/s11882-008-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Remmers E.F., Plenge R.M., Lee A.T. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 2007;357:977–986. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martínez A., Varadé J., Márquez A. Association of the STAT4 gene with increased susceptibility for some immune-mediated diseases. Arthritis Rheum. 2008;58:2598–2602. doi: 10.1002/art.23792. [DOI] [PubMed] [Google Scholar]
- 144.Diaz-Gallo L.M., Palomino-Morales R.J., Gómez-García M. STAT4 gene influences genetic predisposition to ulcerative colitis but not Crohn’s disease in the Spanish population: a replication study. Hum Immunol. 2010;71:515–519. doi: 10.1016/j.humimm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 145.Glas J., Seiderer J., Nagy M. Evidence for STAT4 as a common autoimmune gene: rs7574865 is associated with colonic Crohn’s disease and early disease onset. PloS One. 2010;5:e10373. doi: 10.1371/journal.pone.0010373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Abelson A.-K., Delgado-Vega A.M., Kozyrev S.V. STAT4 associates with systemic lupus erythematosus through two independent effects that correlate with gene expression and act additively with IRF5 to increase risk. Ann Rheum Dis. 2009;68:1746–1753. doi: 10.1136/ard.2008.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim S.W., Kim E.S., Moon C.M. Abnormal genetic and epigenetic changes in signal transducer and activator of transcription 4 in the pathogenesis of inflammatory bowel diseases. Dig Dis Sci. 2012;57:2600–2607. doi: 10.1007/s10620-012-2199-z. [DOI] [PubMed] [Google Scholar]
- 148.Shin H.-J., Park H.-Y., Jeong S.-J. STAT4 expression in human T cells is regulated by DNA methylation but not by promoter polymorphism. J Immunol. 2005;175:7143–7150. doi: 10.4049/jimmunol.175.11.7143. [DOI] [PubMed] [Google Scholar]
- 149.Zwiers A., Kraal L., van de Pouw Kraan T.C.T.M. Cutting edge: a variant of the IL-23R gene associated with inflammatory bowel disease induces loss of microRNA regulation and enhanced protein production. J Immunol. 2012;188:1573–1577. doi: 10.4049/jimmunol.1101494. [DOI] [PubMed] [Google Scholar]
- 150.Parkes M., Barrett J.C., Prescott N. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn disease susceptibility. Nat Genet. 2009;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin Z., Hegarty J.P., Cappel J.A. Identification of disease-associated DNA methylation in intestinal tissues from patients with inflammatory bowel disease. Clin Genet. 2011;80:59–67. doi: 10.1111/j.1399-0004.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 152.Nguyen H.T.T., Dalmasso G., Yan Y. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J Biol Chem. 2010;285:1479–1489. doi: 10.1074/jbc.M109.057141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Olaru A.V., Selaru F.M., Mori Y. Dynamic changes in the expression of MicroRNA-31 during inflammatory bowel disease-associated neoplastic transformation. Inflamm Bowel Dis. 2011;17:221–231. doi: 10.1002/ibd.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pekow J.R., Dougherty U., Mustafi R. miR-143 and miR-145 are downregulated in ulcerative colitis: putative regulators of inflammation and protooncogenes. Inflamm Bowel Dis. 2012;18:94–100. doi: 10.1002/ibd.21742. [DOI] [PMC free article] [PubMed] [Google Scholar]