Abstract
Tropical infectious diseases diagnosis and surveillance are often hampered by difficulties of sample collection and transportation. Filter paper potentially provides a useful medium to help overcome such problems. We reviewed the literature on the use of filter paper, focusing on the evaluation of nucleic acid and serological assays for diagnosis of infectious diseases using dried blood spots (DBS) compared with recognized gold standards. We reviewed 296 eligible studies and included 101 studies evaluating DBS and 192 studies on other aspects of filter paper use. We also discuss the use of filter paper with other body fluids and for tropical veterinary medicine. In general, DBS perform with sensitivities and specificities similar or only slightly inferior to gold standard sample types. However, important problems were revealed with the uncritical use of DBS, inappropriate statistical analysis, and lack of standardized methodology. DBS have great potential to empower healthcare workers by making laboratory-based diagnostic tests more readily accessible, but additional and more rigorous research is needed.
Introduction
When performing diagnostic or epidemiological surveys, particularly in remote areas in resource-poor settings, the facilities for processing blood and maintaining frozen samples frequently do not exist. This finding is especially true for neglected tropical diseases, because they are frequently in populations remote from sophisticated diagnostic facilities. Dried blood spots (DBS) provide a potentially useful and inexpensive means of overcoming these difficulties. Samples, such as finger-prick blood, are easily and quickly collected onto filter paper and shipped at room temperature (even by post). However, blood sample volumes on filter paper are inevitably small, and therefore, rigorous assay validation must be performed to achieve optimum sensitivity and specificity.
Filter paper was first used as a scientific tool in 1815 by the Swedish chemist Jöns Berzelius. In the 1940s, Heatley described the use of filter paper for incorporating antimicrobial solutions in Oxford, giving rise to antibiotic susceptibility disc testing.1 To overcome the difficulties in collecting blood for standard diagnostic tests under field conditions in Cuba, Chediak2 developed a method of identifying syphilis from blood dried on a glass slide in 1932. However, it was Zimmermann3 at the start of World War II in Germany who adapted the method by Chediak2 by drying finger- or ear-prick blood on strips of filter paper to diagnose syphilis using the microscopic agglutination test. In 1950, Joe4 in Leiden, The Netherlands received feces dried onto filter paper by post from Indonesia and was able to detect Shigella, and in 1961, Anderson and others5 published methods for detecting Schistosoma antibodies in DBS sent from endemic areas up to 3 months after collection. Robert Guthrie is widely credited as being the first to use blood dried on filter paper (so-called Guthrie cards) to diagnose phenylketonuria in neonates in 1963.6 Since then filter paper has become a commonly used method of storing and transporting diverse specimen types from humans, animals, and plants. Almost all types of human body fluids (from blood to saliva and feces to breast milk) have been stored on filter paper for a diverse range of biochemical assays (e.g., newborn screening), screening for genetic mutations, determination of metabolites by mass spectrometry, therapeutic drug monitoring, and detection of nucleic acids, antigens, and serological markers for infectious disease diagnosis. The recent call for the use of DBS in diagnostics platforms for the integrated mapping, monitoring, and surveillance of seven neglected tropical diseases and the World Health Organization (WHO/Joint United Nations Programme on HIV/AIDS (UNAIDS) Treatment 2.0 initiative to achieve and sustain universal access to treatment highlights the need for review of the methodology of DBS preparation, storage, and elution to ensure best practice.7
Some aspects of the use of DBS in infectious diseases have been reviewed,8–17 such as for epidemiological studies,15 human immunodeficiency virus (HIV) detection and monitoring,9–12 virology17 and drug assays.18 However, there are no recent clinically orientated overviews of the use of DBS for the diagnosis and surveillance of infectious disease.
There are important problems with uncritical use of DBS, inappropriate statistical analysis, and lack of standardization of terminology and methodology. We, therefore, reviewed the literature on the use of filter papers and focused on evaluation of DBS assays compared with recognized gold standards for the diagnosis and/or surveillance of infectious diseases for both nucleic acid amplification tests (NAATs) and serological assays. Statistical analysis of the studies included in this review was not performed, because most of the papers cited used different assays, settings, and reference methods, suggesting that a meta-analysis would not provide meaningful information. We discuss key issues in the preparation, processing, and storage of DBS and briefly review the use of filter paper with samples other than blood. Filter paper specimens are also used for veterinary health, with some overlap with human health. We, therefore, briefly summarize this parallel work, particularly for livestock diseases with significant economic impact. We highlight key difficulties encountered in using DBS, discuss the heterogeneity in terminology and methodology used, and suggest improvements in these areas (Box 1).
Box 1.
| We searched the electronic databases MEDLINE and Embase for studies published between 1980 and December 13, 2011. Publications that evaluated the use of DBS as alternatives for gold standard samples for human infectious disease diagnosis were included. We excluded in-house assays for HIV, Hepatitis B and C, cytomegalovirus, measles, and rubella because of the existence of well-recognized commercially available assays for these pathogens. Details of excluded in-house assays are provided in Supplemental Table E. During the selection process, studies examining the practical aspects and implications of using DBS compared with non-filter paper samples were included. Additionally, a non-exhaustive selection of studies on the use of filter paper for samples other than whole blood and animal pathogens was also identified. We used the following search terms: dried blood, blood spot,* DBS, dried serum, serum spot,* filter paper, filter card,* filter disc,* filter disk,* blotting paper, Guthrie card, Whatman paper, Isocode stix, FTA*. We made use of corresponding Medical Subject Heading (MeSH) terms for the above keywords. Non-infectious neonatal diseases and non-English publications were excluded. A full review protocol is provided in Supplemental Appendix 1. |
Results
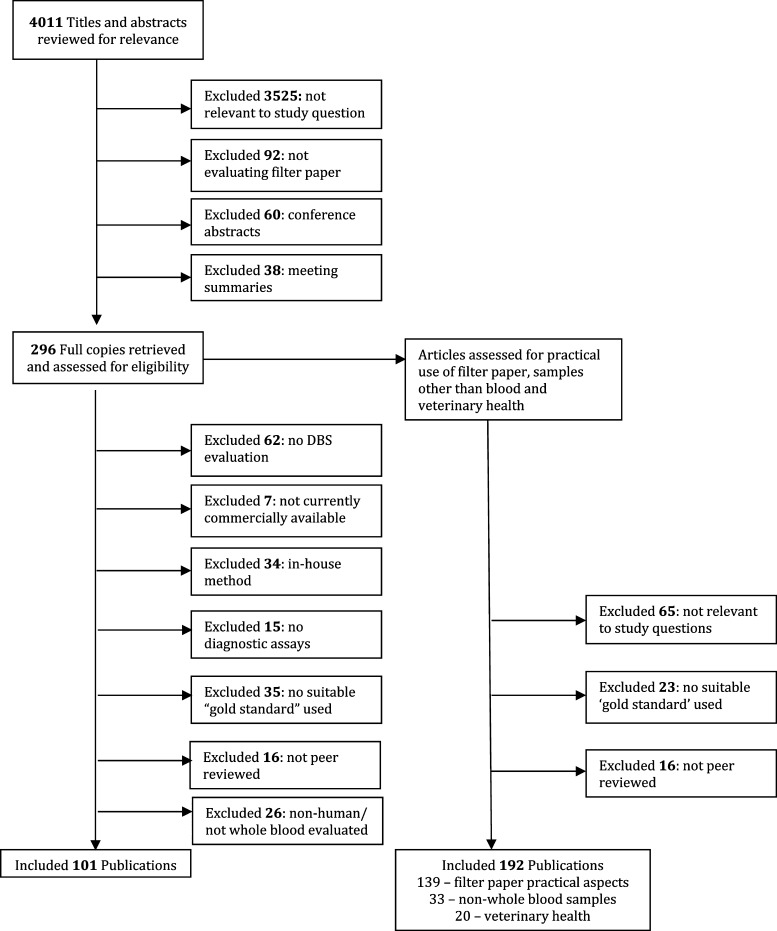
Figure 1 depicts the process of study selection for inclusion in the review. In total, 4,011 potential references were identified, of which 101 references evaluated DBS against a recognized gold standard and 192 references assessed the practical aspects of filter paper use, non-whole blood samples, and veterinary health.
Figure 1.
Selection of reports included in the analysis.
HIV 1 and 2 and HTLV 1.
Efforts to make HIV testing more accessible in rural areas in developing countries, where > 90% of new HIV infections occur, are critical for controlling the disease.19 DBS have the potential to provide simple, robust, and affordable options to collect whole blood for screening, quality control of point-of-care tests, HIV viral load measurements, and drug resistance testing in environments where traditional venous blood collection/transport cannot be performed.9,11,12,20 Twenty-four studies examined the use of DBS for detection of HIV compared with serum or plasma; 12 studies evaluated serological assays, and 12 studies evaluated NAATs (Supplemental Table A).
Serological assays using DBS samples were evaluated in 13 diverse countries, thereby probably representing all HIV-1 subtypes, using third generation enzyme-linked immunosorbent assays (ELISAs) that detect antibodies, fourth generation ELISAs that detect antibodies and antigens, and specific antigen tests (p24). The p24 antigen tests are used as an alternative to NAATs to detect infection in infants (Table 1). Only one study examined detection of HIV-2 using DBS against serum, reporting sensitivity and specificity of 87.5% and 100%, respectively.21
Table 1.
Summary of studies evaluating serological and NAAT diagnosis of HIV comparing DBS with whole blood (DNA) and serum/plasma (RNA)
| Assay type | HIV-1 detection | No. of studies | Sensitivity (%) | Specificity (%) | Refs. |
|---|---|---|---|---|---|
| Serology | Ab/Ag | 7 | 100 | 98.7–100 | 21, 145, 150–154 |
| Serology | Ag (p24) | 5 | 84–98.8 | 98–100 | 146, 147, 155–157 |
| Serology | Western blot | 1 | 92 | 100 | 145 |
| NAAT | DNA | 6 | 97–100 | 99.6–100 | 20, 22–26 |
| NAAT | RNA | 6 | 99.2–100 | 95.6–100 | 22, 28, 29, 158–160 |
| NAAT | DNA and RNA | 3 | 99.7–100 | 100 | 24, 26, 27 |
DBS have been evaluated for the detection of HIV-1 with diverse NAATs in 11 countries. Although HIV is an RNA virus, proviral HIV-1 DNA detection is commonly used for infant diagnosis. Six studies evaluated the Roche Amplicor and Roche Cobas Taqman (Basel, Switzerland) assays on DBS, giving sensitivities and specificities between 97% and 100% and between 99.6% and 100%, respectively.20,22–26
Most HIV viral load assays use quantitative reverse transcriptase polymerase chain reaction (PCR), which requires large quantities of plasma (100–600 μL) to transcribe RNA into DNA before amplification. Other than extracellular HIV-1 RNA amplified from plasma samples, DBS contain whole blood and therefore, intracellular HIV-1 RNA and HIV-1 proviral DNA. As a result, when HIV-1 viral load assays are used with DBS, both HIV-1 RNA and HIV-1 DNA will be amplified, making it potentially more sensitive than HIV-1 DNA plasma assays. This finding has implications for early detection of HIV but also, potential overestimation of viral load.
Three studies evaluated the Roche and Abbott (Abbott Park, North Chicago, IL) NAATs to detect HIV-1 RNA and DNA in DBS versus whole blood.26–28 The bioMerieux (Craponne, France) HIV-1 RNA assay cannot amplify HIV-1 DNA. False positive results by quantitative NAATs are a concern when used for qualitative purposes, but these assays remain a promising alternative for infant diagnosis.20,29 Indeed, the WHO recommends testing infants for HIV DNA, HIV RNA, or the ultrasensitive p24 antigen on plasma or DBS samples given that the sensitivity and specificity of DBS are > 98%.30 Two papers examined the possibility of detecting human T-lymphotropic virus type I (HTLV-1) serologically or by in-house NAATs.31,32 Both studies showed good performance compared with plasma but had relatively small sample sizes.31,32
Hepatitis viruses.
Eight studies evaluated the use of DBS for the diagnosis of hepatitis viruses (Supplemental Table B). Three studies evaluated DBS hepatitis C (HCV) serology against serum or plasma, finding high sensitivity and specificity (> 98%).33–35 Two studies investigated DBS for hepatitis A (HAV) serology and reported sensitivities > 90% and specificity approaching 100%.36,37 DBS were also used successfully to detect the humoral response to HAV vaccination.37 Only two studies have examined the use of DBS samples for hepatitis B (HBV) serology, yielding different performances for three serological HBV assay types, with sensitivities ranging from 78% (for anti-HBs) to 97% (for HBs-Ag).38,39 The inclusion of combined HCV, HBV, and HIV diagnoses on one DBS could be a potentially cost-effective way to expand screening in resource-poor and remote populations.
The detection of HCV and hepatitis E virus by NAATs seems promising, but more evaluations are needed before conclusions can be drawn. More evaluation of the optimal storage DBS conditions for HCV NAAT is required, because studies have given conflicting results.35,40
Flaviviruses.
Capture or sandwich ELISAs are used to serologically diagnose acute dengue (immunoglobulin M [IgM] and IgG antibodies and nonstructural protein 1 [NS1] antigen) and in surveillance and outbreak investigations. Five studies comparing dengue antibody ELISAs using DBS and serum reported high sensitivities (> 86%) and specificities (> 89%)41–45 (Table 2). One study reported poor correlation of DBS with serum results,44 but the statistical analysis was inappropriate.46 Antibody titers determined from DBS were more variable and lower than those titers from sera, suggesting a limited role in the diagnostic confirmation of acute dengue. All studies concluded that DBS IgG determination could be used successfully for seroprevalence studies.
Table 2.
Summary of studies evaluating DBS for Flavivirus and chikungunya diagnosis
| Disease, assay type and country | Ref. | Number of samples/filter paper type | Test | Sensitivity (%) | Specificity (%) | Notes |
|---|---|---|---|---|---|---|
| Dengue serology | ||||||
| Puerto Rico | 41 | NR/unspecified filter paper | In-house IgM and IgG ELISA | 97 IgM; 96 IgG | 97 IgM; 91 IgG | IgM results are for weak positives (OD = 0.2–0.35). |
| Vietnam | 44 | 781 patients/Whatman 903 | Dengue fever IgM and IgG ELISA (Focus Diagnostics) | NR | NR | DBS correlated poorly with serum, particularly for acute 1° and acute 2° dengue infection. However, correlation was inappropriate for analysis.46 Limited role of IgM from DBS for diagnostic confirmation of dengue cases. IgG was useful for seroprevalence studies. No effect of 1 month storage on results. |
| Cuba | 45 | 189 patients/ Whatman 2992 | In-house ultramicro-ELISA | 92.1 | 98.6 | |
| French Guiana | 43 | 130 patients/ Whatman paper | In-house ELISA IgM | 89 | 94 | IgM stable at room temperature for 1 month and at 4°C for > 2 months. |
| Nicaragua | 42 | 169 patients/ Whatman No.3 | In-house ELISA IgM, IgA, and IgG | 96 IgM; 93 IgA; 86 IgG | 89 IgM; 89 IgA; 92 IgG | Detecting IgM or IgA is useful for acute dengue diagnosis. IgG is optimal for dengue incidence surveillance. Danger of cross-reactivity of IgG with other flaviviruses. |
| Dengue NAAT | ||||||
| Cuba | 47 | 52 samples/ Nobuto paper | In-house PCR | 93 | 100 | Samples prepared with blood spiked with dengue virus. Lower limit of detection for dengue serotype 2 than 3. RNA stable at 37°C for 1 year. Risk of viral infectivity from paper for 48 hours at room temperature. |
| French Guiana | 45 | 130 patients/ Whatman paper | In-house PCR | 90.7 | 82.9 | Serotyping also performed. Sensitivity and specificity were highest during the first 4 days of infection, falling rapidly thereafter. However, virus still detectable in 27% up to day 12 in capillary but not venous samples. |
| Japanese B encephalitis virus serology | ||||||
| Thailand | 161 | 243 patients/ Nobuto paper | In-house ELISA and in-house HI | 72 and 26/38 and 33 during epidemic and non-epidemic periods | NR | ELISA and HI tests were compared with serum. ELISA was more sensitive during epidemic periods. Newer commercially available assays are available but have so far not been evaluated on DBS. |
| Chikungunya serology | ||||||
| La Reunion | 48 | 144 patients/ Whatman 903 | IgG ELISA (National Arbovirus Reference Laboratory, Lyon, France) | 97.9 | 100 | Seroprevalence study. IgM also detected with similar OD thresholds as sera, but no independent quality control performed. |
HI = hemagglutination inhibition; NR = not recorded; OD = optical density.
Dengue nucleic acid detection from DBS was also highly sensitive (> 90.7%) compared with serum. The 100% specificity reported by Prado and others47 may reflect the nature of the samples, which were prepared by spiking whole blood with dengue virus. Consistent with the period of highest viremia, sensitivity was highest on day 1 of infection and fell rapidly by day 4. Matheus and others43 found that dengue RNA could still be detected in dried capillary blood samples from a small number of patients 12 days after infection, whereas corresponding venous samples were negative. Dengue RNA on DBS could be detected after storage at 37°C for 1 year.47 It is important to note that the virus may remain viable and confers an infective risk during at least the first 48 hours after spotting on untreated filter paper.47
Other viruses.
In a seroprevalence study of chikungunya virus, IgG was successfully detected in DBS with 97.9% sensitivity compared with serum.48 Although IgM was not fully evaluated on DBS, it seemed to give similar results to those from sera.48
Three studies evaluated measles antibody (IgM or IgG) detection using DBS.49–51 Uzicanin and others51 showed that the sensitivity of DBS compared with serum increased for IgM from 95.7% for samples collected from days 1 to 6 of the illness to 100% when samples were collected 1 week after the appearance of the rash.51
We found only one study evaluating the use of DBS for Epstein–Barr virus (EBV) serology. Interestingly, this study compared venous and capillary blood spotted on two different filter paper types (Whatman 903 and No. 3) for ELISA (EBNA1 plus VCA-p18) and found similar sensitivities of 75–80% and specificities of 97–100% compared with plasma.52 For the detection of cytomegalovirus (CMV), a serological assay and an NAAT test were evaluated between plasma and DBS. The NAAT was 100% sensitive and specific, whereas the serological assay had lower sensitivity and specificity (both were > 93%) (Supplemental Table C).53,54 At 4°C DBS storage, measles antibody and EBV IgA and IgG were stable for at least 24 weeks.49,52
Malaria.
For the diagnosis and speciation of malaria, we found no evaluations of commercially available DBS assays using PCR in peer-reviewed journals. Two studies compared PCR on DBS against liquid whole blood and found a lower sensitivity, particularly for samples with low parasitaemia55,56 (Table 3). DBS PCR compared with microscopy achieves comparable performance or in some studies, is more sensitive.57 However, DBS PCR has a lower sensitivity than PCR on whole blood. Because both DBS PCR and microscopy may miss low-level parasitemia that whole-blood PCR detects, DBS PCR seems to have a higher specificity than whole-blood PCR. This result is because of the imperfect nature of the gold standard of microscopy.56,58 Based on 10 papers included in this review, malaria detection using the nested PCR on DBS by Snounou and others59 seemed to be a suitable alternative to microscopy. DBS are also commonly used for detection of malaria resistance molecular markers.60
Table 3.
Summary of studies evaluating DBS for malaria (malaria NAAT assays)
| Country | Ref. | Sample size/filter paper | Assay | Pf | Po | Pv | Pm | Unknown | Sensitivity (%) | Specificity (%) | Reference test | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thailand | 162 | 56 samples/ Whatman 903 | In-house | ✓ | 94.6 | NR | Thin/thick blood smear | |||||
| Malaysia | 163 | 166 patients/ Whatman 3MM | In-house (adjusted) | ✓ | ✓ | 97.4 | NR | Thick blood smear | ||||
| Malaysia | 164 | 129 patients/ Whatman 3MM | In-house | ✓ | ✓ | ✓ | ✓ | NR | NR | Limit of detection: 6 parasites/μL. | ||
| Singapore | 165 | 52 patients/ Whatman No.1 | In-house | ✓ | ✓ | 100 | 100 | Thin/thick blood smear | Limit of detection: 4 parasites/μL. | |||
| Malaysia, Myanmar, Thailand | 166 | 81 patients/ Isocode cards | In-house | ✓ | ✓ | ✓ | 94.1 (Pf); 100 (Pv) | 100 (Pf); 99.1 (Pv) | Thin/thick blood smear | 1 of 1 Po samples detected. | ||
| Thailand | 58 | 136 patients/ Whatman 3MM | Multiplex PCR | ✓ | ✓ | 100 (Pf); 92.7 (Pv) | 100 (Pf); 100 (Pv) | Consensus of three PCR assays | Specificity of all three assays lower (93.8–97%) compared with microscopy. Microscopy had 90.7–92.5% sensitivity and 91.5–100% specificity. | |||
| Nested PCR | ✓ | ✓ | 100 (Pf); 100 (Pv) | 99 (Pf); 100 (Pv) | ||||||||
| RT-PCR | ✓ | ✓ | 100 (Pf); 100 (Pv) | 100 (Pf); 100 (Pv) | ||||||||
| Saudi Arabia | 55 | 118 patients/ Whatman paper | In-house | ✓ | 73 | NR | Thin/thick blood smear | Several microscopy-negative samples were positive on DBS PCR. | ||||
| Thailand, Zimbabwe | 141 | 156 patients/ FTA card | In-house | ✓ | 97.8 | 100 | Thin/thick blood smear | Limit of detection: 10 copies/reaction. | ||||
| Iran | 56 | 75 patients/ DNA Banking Card | In-house | ✓ | ✓ | 97 | 100 | Thin/thick blood smear | Whole blood was more sensitive but less specific than DBS compared with microscopy (100% sensitivity, 95.2% specificity). | |||
| Kenya | 57 | 356 patients/ Whatman 3MM | In-house | ✓ | 100 | 79 | Thin/thick blood smear | Low specificity potentially caused by insufficient microscopy expertise.57 |
Pf = Plasmodium falciparum; Pm = P. malariae; Po = P. ovale; Pv = P. vivax; RT-PCR = real time PCR; NR = not reported.
Parasites.
Non-malarial parasites cause many neglected tropical diseases afflicting hundreds of millions of people, predominantly in resource-poor regions with limited access to diagnostic facilities.61 The potential use of filter paper to aid diagnosis and understanding of the epidemiology of these diseases is, thus, very attractive. The mapping of lymphatic filariasis and monitoring of elimination programs provide an ideal role for DBS. Three recent studies evaluated serological tests for Wuchereria bancrofti Og4C3 antigen on DBS compared with serum, giving sensitivities of > 93% and specificities of 82–100%62–64 (Table 4). An early study performed in Ghana reported a lower sensitivity (50%),65 possibly because of a difference in strain type (most other studies were performed in Asia), an assay cutoff that was set too high, or insufficient blood volume spotted onto filter paper. The CELISA (Cellabs Pty Ltd, Manly, Australia) (W. bancrofti and Brugia spp.) and Brugia Rapid (Reszon Diagnostics, Selangor, Malaysia) (Brugia spp.) tests performed on DBS eluate and compared with serum or plasma proved reasonably sensitive (71–98%).66,67 Nucleic acid testing was evaluated for DBS versus microscopy for Brugian filariasis and Loa loa and seems sensitive, particularly for the latter at 96%.68–70 African and American trypanosomiases have both been successfully diagnosed on DBS with high sensitivity and specificity,71–74 but the sample size for Trypanosoma cruzi was relatively small.73 Strict control of humidity by storing DBS in sealed plastic bags with silica gel immediately after drying may have been a key factor, resulting in the higher sensitivity reported in the work by Chappuis and others71 compared with the work by Truc and others.72
Table 4.
Summary of studies evaluating DBS for parasites other than malaria
| Disease, assay type, and country | Ref. | Number of samples/filter paper type | Test | Sensitivity (%) | Specificity (%) | Notes |
|---|---|---|---|---|---|---|
| Lymphatic filariasis: Wb, Bspp serology and dipstick antibody test | 94 patients/ Whatman 903 | In-house EIA | 92 | 77 | ||
| India | 167 | |||||
| Ghana | 65 | 1,808 patients/Og4C3 paper | Og4C3 ELISA (Wb; Tropical Biotechnology) | 50.3 | 96.4 | |
| Sri Lanka | 62 | 60 patients/ Nobotu 1 | Og4C3 ELISA (Wb) | 97 | NR | |
| India | 63 | 30 patients/ Whatman No.3 | Og4C3 ELISA (Wb) | 76.6–93.3 | 100 | Time of the day at which samples are collected impacts sensitivity. |
| India, Egypt, Haiti, Kenya, Papua New Guinea, Sri Lanka | 64 | 188 patients/Whatman No.3 | Og4C3 ELISA (Wb) | NR | NR | |
| Egypt | 66 | 81 samples/filter paper (Tropical Biotechnology) | Filariasis (Wb and Bspp) CELISA (Cellabs) | 91 (Wb); 98 (Bspp) | NR | Based on a panel of known positives. |
| Uganda | 67 | 66 patients/Whatman 3MM | Brugia Rapid (Reszon Diagnostics) | 79 | NR | Significant cross-reactivity with other filarial infections.66,67 |
| Lymphatic filariasis: B. malayi, NAAT | ||||||
| Indonesia | 68 | 36 patients/ Whatman 3MM | In-house PCR and ELISA combination | 86 | NR | PCR-ELISA produced comparable results compared with DNA Detection Test Strips (Roche, Germany). |
| Malaysia | 69 | 21 patients/ Whatman 3MM | In-house PCR | NR | NR | |
| Mansonelliasis NAAT | ||||||
| Brazil | 168 | 12 patients/ Whatman paper | In-house PCR | NR | NR | PCR was able to distinguish between O. volvulus, M. ozzardi, and M. perstans. |
| Loa loa filariasis NAAT | ||||||
| Cameroon | 70 | 68 patients/ NR | In-house PCR | 96 | NR | High specificity. No cross-reactivity with other filarial species. Limit of detection 1 microfilaria/20 μL whole blood (as DBS). |
| HAT serology (card agglutination test) | ||||||
| Sudan | 71 | 100 patients/ NR | Micro-CATT (ITM Antwerp) | 91 | NR | |
| Central African Republic, Ivory Coast | 72 | 940 patients/ Whatman No.4 | Micro-CATT (ITM Antwerp) | 89.4–95.5 | 95.5–96.6 | Truc and others72 report rapid drop in sensitivity (67.8%) after 3 days without strict humidity control of paper. Ranges reported by Truc and others72 reflect testing at two different sites. |
| Chagas disease serology | ||||||
| Brazil | 74 | 6,222 patients/ Whatman No.1 | In-house ELISA, IF, and HA | ELISA, 78.1; IF, 69.2; HA, 64.6 | ELISA, 99.7; IF, 99.4; HA, 99.6 | |
| Brazil | 73 | 24 patients/ NR | Chagas Stat-Pak (ICT; Chembio Diagnostic Systems) | 100 | 100 | Chagas Stat-Pak performed on small sample size. More sensitive and specific than large-scale evaluation with serum. |
| Echinococcosis serology | ||||||
| Argentina | 76 | 479 patients/ Whatman No.1 | In-house ELISA | NR | NR | Coltorti and others76 report sensitivity of DBS to be similar to serum. |
| Uruguay | 78 | 1,149 patients/ Whatman No.1 | In-house ELISA | NR | NR | |
| China | 77 | 2,482 patients/ Whatman No.1 | In-house ELISA | 96 | 87 | |
| Visceral leishmaniasis NAAT | ||||||
| Portugal | 75 | 24 patients/ Whatman No.2 | In-house PCR | 71–75 | NR | 15/20 positive for patients not on treatment and 17/24 if patients on treatment included. Useful as an initial screening tool. |
| Fascioliasis serology | ||||||
| Bolivia | 79 | 68 patients/ Whatman No.1 | In-house ELISA | NR | NR | Samples missed on DBS had the lowest ELISA readings. Samples stored for 10 years at 4°C were successfully detected. |
| Giardiasis serology | ||||||
| Saudi Arabia | 84 | 147 patients/ Whatman No.4 | In-house ELISA | 72–96 | 39–98 | al-Tukhi and others84 reported ranges that depended on ELISA OD reading and final eluate dilution. Guimaraes and Sogayar83 had a high rate of false positives with ELISA. |
| Brazil | 83 | 133 patients/ Whatman No.1 | In-house IF; in-house ELISA | 82; 72 | 70; 39 | |
| Cysticercosis serology | ||||||
| Brazil | 80 | 151 patients/ Whatman No.4 | Qualicode Cysticercosis ELISA kit (Immunetics Inc.) | 80 | NR | Good agreement between serum and DBS. May be a useful initial screening test. Fall in sensitivity if filter paper was not frozen after 1 week storage. Ranges were caused by samples being processed at two sites using two methods. |
| Mexico | 81 | 305 patients/ Whatman No. 311 | In-house ELISA | 39–66 | 87–96 | |
| Toxoplasmosis serology (latex agglutination) | ||||||
| United Kingdom | 82 | 273 patients/ Whatman 903 | Eiken Toxoreagent Latex Agglutination | 98.8 | 100 | |
Bssp = Brugia malayi and B. timori; CATT = Card Agglutination Test for Trypanosomiasis; HAT = Human African Trypanosomiasis; IF = Immunofluorescence; Wb = Wucheraria bancrofti.
PCR testing on DBS for visceral leishmaniasis (Leishmania infantum) in immunocompromised patients before therapy was evaluated against bone marrow microscopy in a small series of patients, yielding a sensitivity of 75%.75 PCR on DBS was significantly more sensitive than microscopy and culture of peripheral blood. Campino and others75 suggest a possible role for PCR on DBS as an initial screening test, potentially avoiding more invasive bone marrow aspiration. Seroprevalence studies for echinococcosis, fascioliasis, cysticercosis, and toxoplasmosis performed well on DBS.76–82 However, antibodies to cysticercosis decreased rapidly when stored on filter paper.81 Detection of exposure to giardiasis suffered from low specificity, possibly reflecting cross-reactivity or long-term persistence of antibodies.83,84
Bacteria.
There have been few studies evaluating the use of filter paper to diagnose or determine the seroprevalence of bacterial infections compared with viruses and parasites (Table 5).
Table 5.
Summary of studies evaluating DBS for bacteria
| Disease, assay type, and country | Ref. | Number of samples/filter paper type | Test | Sensitivity (%) | Specificity (%) | Notes |
|---|---|---|---|---|---|---|
| Leprosy serology | ||||||
| French Polynesia | 86 | 168 patients/ Whatman No.1 | In-house ELISA | Multibacillary 96; Paucibacillary 29 | Multibacillary 96; Paucibacillary 96 | |
| India | 87 | 94 patients/ Whatman No.3 | In-house ELISA | Multibacillary 97; Paucibacillary 73 | Multibacillary 100; Paucibacillary 100 | Based on a cutoff of 1:40 (OD) |
| India | 88 | 81 patients/ Whatman No.3 | MLPA (Fujirebio); in-house ELISA | 67.7 (MLPA); 76.9 (ELISA) | 98.7 (MLPA); 83.4 (ELISA); | |
| Nepal | 85 | 200 patients/ NR | In-house ELISA | NR | NR | Earlobe capillary blood more sensitive than serum or finger-prick blood85 |
| Orientia tsutsugamushi and Rickettsia typhi (scrub typhus and murine typhus) | ||||||
| Laos | 93 | 53 scrub typhus patients; 53 murine typhus patients/ Whatman 903 | In-house ELISA | 95 IgM and 90 IgG; 91 IgM and 82 IgG | 88 IgM and 100 IgG; 100 IgM and 100 IgG | Lower antibody titers with DBS; storage at room temperature for 1 month did not affect antibody titers93,94 |
| Coxiella burnetii, Bartonella quintana and Rickettsia conorii serology | ||||||
| France | 94 | 94 patients/ Fischer Scientific paper94 | In-house ELISA | 100 | 100 | |
| Leptospirosis serology (MAT) | ||||||
| La Reunion | 90 | 52 patients/ Whatman 903 | MAT | 100 | 100 | DBS samples showed lower antibody titers compared with serum |
| Syphilis serology | ||||||
| United States | 91 | 1,098 patients/ Whatman 903 | In-house ELISA | 96 | 94 | |
| Tanzania | 92 | 1,037 patients/ Whatman 903 | Serodia TPPA (Fujirebio) | 98.3 | 100 | |
| Yaws serology | ||||||
| Papua New Guinea | 95 | 70 patients/ Whatman 903 | TPHA–Serodia TP kit (Fujirebio) | 96.5 | 100 | Results unaffected by up to 2 months storage |
| Brucella serology | ||||||
| Spain | 89 | 160 patients/ Whatman 2992 | Brucella ELISA (Virotech System Diagnostika) | NR | NR | Pearson correlation coefficient: r = 0.8 for IgM and IgG; time-consuming extraction method |
MAT = microscopic agglutination test; MLPA = Mycobacterium leprae particle agglutination; TPHA = Treponema pallidum-specific hemagglutination test; TPPA = Treponema pallidum particle agglutination test.
The success of using both serum and DBS to screen for leprosy is dependent on the bacillary burden, with multibacillary patients more readily identified.85–87 The commercially available Serodia Leprae particle agglutination test (Fujirebio, Tokyo, Japan) using DBS had 97.5% concordance with serum for patients of any bacillary burden.88 Interestingly, the sensitivity of capillary DBS taken from skin smear sites, such as the earlobe, was slightly but significantly higher compared with venous DBS and serum. This result may reflect a higher concentration of antibodies at the site of infection compared with circulating antibodies.85
Brucella antibodies were eluted from filter paper with difficulty, and correlation coefficients with serum were modest.89 However, correlation coefficients are not valid statistical tests for comparison of diagnostic methods.46 Serological tests for other bacterial pathogens, including syphilis, yaws, leptospirosis, and some rickettsial diseases, performed well on DBS and could be stored successfully for sufficient periods of time to allow transport to a laboratory for analysis.90–95
Practical Aspects And Implications Of Using Dbs Samples Compared With Traditional Methods
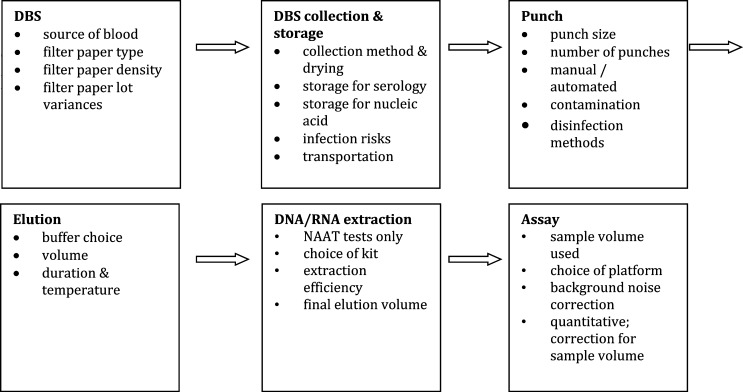
Some of the key neglected but practical aspects that should be taken into account when using DBS samples are discussed below (Figure 2).
Figure 2.
Practical aspects and implications of using DBS. NA = nucleic acid.
Filter paper.
There are many different filter paper brands available consisting of 100% cellulose, and they vary in thickness and pore size. Although many manufacturers produce cards, only two brands are US Food and Drug Administration (FDA) -approved for human whole-blood collection (Whatman 903 and PerkinElmer [Beaconsfield, UK] 226 filter papers). For the Centers for Disease Control and Prevention (CDC) newborn screening quality assurance program, each lot is checked to ensure that the relationship between spot size and whole-blood volume varies minimally.96 When comparing 903 and 226 filter papers, < 4–5% difference was detected for analytes used for neonatal screening.96 FTA Elute and FTA (Whatman; GE Healthcare, Buckinghamshire, UK) are treated filter papers that lyse cells and inactivate antibodies, viruses, and bacteria but allow NAAT assays. Assays should not be transferred between paper types without additional evaluation.
Sample collection and storage recommendations.
Manufacturers' recommendations as well as the protocols presented by Mei and others14 and the US CDC97 provide useful guides. The WHO guidelines for HIV drug resistance testing with DBS and others contain a more detailed description of how to collect DBS samples (particularly for RNA viruses).96,98,99 A number of studies also examined HIV DNA and RNA storage conditions when validating DBS methods.100–103 For serology, specific collection and storage recommendations have been produced by the CDC.97
Collecting finger- or heel-prick blood with DBS is a fast and convenient method that requires minimal training. After the DBS sample has been dried for at least 3 hours, it should be stored in a zipped bag with desiccant to reduce humidity damage. If DBS are stored in freezers, ensure that they are dried thoroughly after being brought to room temperature to avoid condensation inside the bag. The effect of long-term storage at different temperatures on diagnostic accuracy of DBS has been investigated for only a few pathogens with variable results (e.g., HCV with poor/uncertain stability35,40 versus dengue, EBV, and measles with better stability).47,49,52 Standardization of experimental methods for assessing DBS stability would help considerably.
Recording the quality and integrity of filter paper samples on arrival at the laboratory is essential, as they can vary because of incorrect blood sampling or environmental factors, such as humidity, contamination, and mold overgrowth.
The presence of nucleic acids or antibodies in venous and capillary blood may vary for different pathogens. Two studies suggest that dengue virus capillary viremia may be more prolonged than venous viremia,43,104 suggesting that it would be important, in an evaluation of NS1 assays and NAATs, that both DBS and liquid blood samples are compared using capillary blood.
Biosafety issues.
Because DBS contain dried blood, regardless of the pathogen being investigated, the samples should be processed as potentially infectious material, and health and safety regulations should be followed. However, safety and packaging requirements are simpler than for liquid blood, and DBS can be shipped as non-regulated, exempt materials.105 However, although it is believed that bacteria and viruses have reduced activity when stored as DBS samples, group A streptococci could still be cultured after elution of DBS samples, and dengue virus is still viable after 48 hours on DBS at room temperature.47,106,107 FTA paper carries the advantage of inactivating highly pathogenic organisms to allow safe transportation, with reported complete inactivation of highly pathogenic Avian Influenza Virus (AIV) 1 hour after adsorption onto FTA paper.108 However, more evaluation of the potential infectiousness of different pathogens on DBS is needed.
Contamination risks.
Manual or automated punch devices, such as handheld office punches or automated machines (like the devices used for neonatal screening), are suitable for removing paper discs from DBS. There is a potential risk of carryover contamination that can be avoided by cleaning the punch device with bleach or related products and punching sterile blank paper between samples. Recently, perforated filter paper cards have become available (Whatman and PerkinElmer), allowing the spots to be removed with a pipette tip, obviating the need for punching machines and reducing contamination risks.
Selecting an assay.
For quantitative assays, adjusting the cutoff for DBS samples compared with whole blood or serum may improve sensitivity and/or specificity, depending on the required balance between them.34 Assays that use a relatively small quantity of plasma/serum that is first diluted with sample buffer are more suitable for DBS samples than assays requiring large quantities. Attempts to keep DBS elution comparable with serum/plasma according to the manufacturer's recommendations will greatly improve the chances that results of assays on DBS and standard samples will have comparable accuracy. The quantity of serum in whole blood dried on filter paper is difficult to determine but essential for protocol development. Factors, such as hematocrit, blood volume per spot, and filter paper characteristics, contribute to different extraction yields of a DBS sample.109
Certain pathogens, such as HIV, are present in large quantities in whole blood (up to 104 copies per drop), whereas others, such as Salmonella enterica serovar Typhi and Orientia tsutsugamushi, are present at very low density. DBS as an alternative to standard samples is only possible if the pathogen is present in sufficient numbers for nucleic acid amplification.
Reporting DBS evaluation studies.
The Standards for Reporting of Diagnostic Accuracy (STARD) guidelines110 are an important starting point for assessing DBS evaluations. Many studies evaluating filter paper do not include full details on the paper type or processing, key information regarding reference standards, and use of appropriate statistical tests. In Table 6, we propose additional points to the current STARD checklist to address these issues.
Table 6.
Additional suggested Standards for Reporting of Diagnostic Accuracy (STARD) checklist points for DBS evaluation
| Concerns when using DBS | STARD checklist adjustments for DBS evaluations |
|---|---|
| Inconsistency in terminology | Make use of clear terminology (i.e., DBS, dried serum spots, dried urine spots, etc. or dried “sample type” spots). |
| Unclear or not reporting filter paper sample collection method | Sample collection: state the filter paper brand and weight used, which and how fluids were obtained and spotted onto filter paper, and the drying period before storage. |
| Unclear reporting of reference method and sample | Report the index sample and its collection, storage, and transportation details; provide detailed rationale for discordances in methods between index and reference test. |
| Unclear or not reporting storage and time between collecting and analyzing samples | Sample processing: state the time and storage conditions (humidity control and temperature) in the field, during transportation, and in the laboratory, preferably in a tabled manner. |
| Unclear or not reporting punch method and punch disinfection procedure | Report punching method with reference to source or manufacturer and punch disinfection procedure if used. |
| Unclear or not reporting how quantitative data was obtained from filter paper samples | For quantitative or numerical test results, indicate the calculation methods and rationale of the index and reference standard. |
| Unclear or not reporting the biological variability of samples and mean difference between index and reference sample | For quantitative test outcomes, report the mean and range of results for index and reference test. |
| Unclear or not reporting of diagnostic accuracy of quantitative test outcomes. | For quantitative test outcomes, estimates of diagnostic accuracy and measures of statistical uncertainty (e.g., 95% confidence intervals) by quantitative grouped ranges (e.g., 1,000–5,000 copies/mL). |
Use Of Filter Paper For Samples Other Than Whole Blood
Whole blood is the most practical sample to collect on filter paper; however, many reference assays have used other samples types (e.g., serum or plasma), and some diseases are preferably diagnosed using other specimen types (Supplemental Table D).
Evaluation of dried serum spots to detect HAV antibodies showed a sensitivity and specificity of 100% compared with liquid serum,111 and HIV ELISA had a sensitivity of 83%.112 NAATs of dried serum spots perform very well for HAV (92.3% and 100%) and HCV (100% and 100%) sensitivity and specificity, respectively, versus liquid serum.111,113 Both hepatitis viruses showed a 10-fold fall in viral load after storage for 4 weeks on paper at room temperature.111,113
Three studies used dried plasma spots and one study used dried breast milk spots compared with liquid plasma for HIV quantitative PCR.114–116 HIV RNA on filter paper was stable at room temperature for > 1 year. Dried buffy coat spots may be used as a substrate to detect HIV proviral DNA. When dried on filter paper and compared with liquid samples, there was 100% concordance between results.117
Although bone marrow is a difficult sample to obtain, it is the most sensitive substrate for diagnosis of visceral leishmaniasis. In one small study, 34 of 35 patients suspected of having the disease on clinical grounds were positive by NAAT on dried bone marrow spots. This test was more sensitive than bone marrow microscopy.118
Cutaneous and mucocutaneous samples may be scraped, aspirated, or directly impressed onto filter paper to diagnose leishmaniasis and using slit skin smears, leprosy. The sensitivity of PCR on lesions impressed onto paper for leishmaniasis ranged from 92.3% to 100% and specificity was 100% compared with PCR on tissue samples119,120; parasite speciation was also possible. Mycobacterium leprae was detected by PCR from slit skin smears on filter paper (60%) in patients with known leprosy as frequently as from slit skin smears stored in ethanol (58%).121
Sputum and saliva have been more widely examined. Only 67% of serologically positive measles patients were positive by PCR on dried saliva spots, which were inferior to whole-saliva and throat swabs.122 Detection of malaria DNA in dried saliva and dried urine spots was less sensitive than blood microscopy.123 Dried induced sputum and bronchoalveolar lavage fluid spots to identify Pneumocystis jirovecii by PCR had reported sensitivity of 67% and 90–91%, respectively, compared with microscopic examination of liquid samples.124 Dried cervical smear fluid spots were evaluated for detection of Human Papilloma Virus by PCR. Concordance of 94–100% was reported in two of three studies compared with PCR directly on smear or cytobrush samples.125–127
Dried cerebrospinal fluid (CSF) spots in children with meningitis were assayed by PCR for Streptococcus pneumoniae and Haemophilus influenzae with a sensitivity of 92% and 70% and specificity of 99% and 100%, respectively, compared with direct CSF PCR.128 The detection of cysticercosis antibodies was less successful, ranging from 52% to 63%, compared with neat CSF depending on the type of filter paper used to store CSF.81
Both stool and urine have been stored on filter paper. Vibrio cholerae could be cultured from dried stool spots after 14 days if humid conditions were maintained129 and was equivalent to standard transport medium. Viral enteric pathogens, including Norovirus, Rotavirus, and Adenovirus serotypes 40 and 41, were detected by NAAT from dried stool spots on chromatography paper, with good concordance with enzyme immunoassay (EIA) performed directly on stool.130–132 Pre-treating the paper with sodium dodecyl sulfate (SDS)/ethylenediaminetetraacetic acid (EDTA) inactivated the virus, allowing safe handling of the paper. CMV is readily detected in urine in viremic patients. Dried urine spots were reported to have 90% concordance with PCR on DNA extracted directly from urine.133
Use Of Filter Paper In Tropical Veterinary Health
Filter paper has been widely used as a specimen substrate in tropical veterinary health in both livestock and wildlife diseases. Several zoonotic diseases discussed above, including echinococcosis, brucellosis, and trypanosomiasis,134 are also important causes of mortality in other mammals. However, non-zoonotic diseases are responsible for about one-half of livestock losses worldwide.134 Poultry, swine, and cattle suffer the greatest burden of disease, with viruses and parasites being the major causes. Early warning systems are needed to detect highly pathogenic organisms, such as AIV. The difficulties of traditional sample collection methods, discussed above for humans, are equally applicable in the veterinary setting. Filter paper has played a key role in circumventing many of these challenges for veterinary medicine. Smith and Burgoyne135 discuss the problems likely to be faced with the use of filter paper (FTA) with veterinary samples. Leishmaniasis is an important zoonosis with reservoirs in canids; however, serological studies among dogs using filter paper compared with serum have given relatively poor sensitivity of 22.2% or agreement of 68.8% (k = 0.234).136,137
Discussion
Over the last 50 years, filter paper has gained an increasingly important role as a substrate for the diagnosis and surveillance of infectious diseases. Recently, this role has gone beyond diagnosis to include detection of markers of resistance, detailed genetic or serological analysis, and monitoring of therapeutic interventions, including drug levels, vaccine-induced responses, and viral loads.
Almost any clinical sample may be stored on filter paper for subsequent analysis, although finger-prick blood is the most convenient and widely used. Point-of-care tests are increasingly providing a key role in diagnosing and surveying infectious diseases in remote settings, and affordable microfluidics devices based on paper to diagnose infectious diseases are promising tools.138
Viruses, particularly HIV, have been most frequently targeted with filter paper diagnostics. Serological tests perform very well, with seven studies reporting sensitivity and specificity close to 100%. NAAT performance is more variable because of the greater instability of nucleic acids, but mostly, it reached similar diagnostic accuracy. Infant diagnoses using both RNA and DNA are feasible; however, RNA tests tend to suffer from reduced specificity. Hepatitis viruses, many of the Herpes virus family, measles, and rubella also perform well with serological tests, with sensitivities and specificities of > 90%. NAATs seem promising, although more evaluations are needed, particularly for HCV and HEV. Dengue serology performed on DBS is clearly suitable for seroprevalence studies, although it is less clear for the diagnosis of acute primary and acute secondary infections. Dengue serotyping is epidemiologically important and can also be successfully performed from DBS.43,139
DBS also play a key role in the diagnosis of parasitic infections. Detection of malaria by PCR using in-house methods is generally superior to microscopy. Most studies report sensitivities of > 94% and specificities of > 99%.56,58,140,141 Because of the prevalence of filariasis in remote settings, filter paper has been used in the diagnosis and investigation of epidemiology and response to eradication programs. Using commercially available assays, sensitivities of > 90% may be achieved.62,66 Leishmaniasis, cysticercosis, and giardiasis have proved to be less promising in the few studies that have evaluated DBS compared with a recognized gold standard.75,81,83 Serological tests for leptospirosis, treponemal infections, and some rickettsia have yielded excellent results,90,92,94 whereas others, such as brucellosis, have been less successful.89
The selection of pathogens that may perform well on filter paper is dependent on several important factors, crucially the presence and quantity of serological markers and nucleic acids in the blood at the time of sample collection, their stability on filter paper, and the elution method that maximizes test performance with DBS.
There are several key advantages of using filter paper over the traditional specimens of whole blood or serum. Many of the pathogens discussed above are most common in remote and resource-poor settings with limited access to advanced diagnostic facilities. Filter paper obviates the need for a cold chain to preserve specimens in transport to a central laboratory, thus enormously increasing the accessibility of these tests. Filter paper is generally cheap (although some of the treated papers, such as FTA, are very expensive), requires only a small sample volume, and needs minimal technical expertise to perform. These factors are likely to make sample collection more acceptable to the patient and less of a burden for the health system, and they will probably increase testing uptake.142 Filter paper is easily and safely delivered using almost any existing transportation network. Recent advances in chemically pre-treated cards have provided increased safety in handling and transporting samples.108 Filter paper has been used with multiplex serological and NAATs to diagnose combinations of Hepatitis B, C, and HIV,143,144 increasing the diagnostic potential of a single DBS.
There are, however, important difficulties and limitations in the evaluation of filter papers as diagnostic tools. A great variety of terminology has been used, and studies evaluating the same pathogen often use different methodologies encompassing almost every stage of the process from filter paper selection to final assay procedures, making comparison vexed. Some studies have used DBS without justifying that the method is accurate against a reference standard. Many filter paper varieties have been used (products are not always clearly labeled with the paper weight in grams per meter2), and sample volumes will vary; therefore, care is required when moving techniques between paper types. A consensus document on terminology and methodology would be invaluable for advancing the field of filter paper diagnostics. Surprisingly, there have been no cost-effectiveness analyses of the use of filter paper for infectious disease diagnostics.
Human and animal health are inextricably linked, but there has been very little, if any, collaboration between scientists and health workers interested in human and non-human health and filter paper diagnostics. More One Health collaboration on these techniques would benefit both fields.
High temperatures and humidity over prolonged periods severely reduce test sensitivity, particularly for NAATs, although this finding seems to vary between pathogens.35,47,145–147 Inevitably, the volume of blood per spot will be less than the volume of a whole-blood sample collected by venipuncture. DBS containing whole blood may also influence NAATs or serological assays because of the presence of inhibitors. They can, however, be overcome by DBS-specific protocols.148,149 Although some guidelines exist, there is an urgent need for more robust standardized protocols for sampling, storage, processing, and evaluating filter paper techniques. Of the studies reported in this review, 42% of them were not prospective, real-life evaluations; such studies would provide a stronger evidence base to support recommendations. Additionally, most studies used pipettes to spot venous blood onto filter paper, giving a greater consistency in blood volume than direct application of blood to paper. However, this consistency is unlikely to be achieved with field samples. A number of studies did not report sensitivity and specificity, and several studies inappropriately used correlation coefficients.46 The inclusion of additional reported items to improve accuracy and completeness of filter paper studies could greatly improve consistency and clinical use of the results (Table 6).
Our review has important limitations. We only included studies published in English, excluded related subjects, such as filter paper assays of drug resistance and viral loads, excluded in-house assays for those diseases with well-recognized commercially available assays, and did not do a detailed assessment of veterinary use of filter paper (this assessment would require a literature review in its own right).
This work is a first attempt to summarize the subject of filter paper diagnostics in tropical diseases. We highlight the many advantages that filter paper offers over traditional samples and discuss the associated limitations and difficulties. Consensus should be reached regarding the methodology and terminology used to better advance this important diagnostic tool. Filter paper has been shown to be a valuable asset in increasing accessibility, making affordable, robust, sensitive, and specific diagnostic testing available to patients in remote settings. Its use in surveillance of neglected tropical diseases targeted for elimination and potentially, veterinary pathogens makes DBS an important tool in international health.
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to colleagues at Mahosot Hospital, Vientiane, especially Dr. Rattanaphone Phetsouvanh and the directors, for their support and advice.
Footnotes
Financial support: Financial support was provided by the Wellcome Trust of Great Britain and UBS Optimus Foundation.
Authors' addresses: Pieter W. Smit, Terveyden ja hyvinvoinnin laitos, Helsinki, Finland, E-mail: pieterwsmit@gmail.com. Ivo Elliott, Department of Infectious Diseases, Nottingham City Hospital, Nottingham, United Kingdom, E-mail: ivo@tropmedres.ac. Rosanna W. Peeling and David Mabey, London School of Hygiene and Tropical Medicine, London, United Kingdom, E-mails: Rosanna.peeling@lshtm.ac.uk and David.mabey@lshtm.ac.uk. Paul N. Newton, Centre for Tropical Medicine, Nuffield Department of Medicine, Churchill Hospital, University of Oxford, Oxford, United Kingdom, London School of Hygiene and Tropical Medicine, London, United Kingdom, E-mail: paul@tropmedres.ac.
Reprint requests: Paul N. Newton. Lao-Oxford-Mahosot Hospital–Wellcome Trust Research Unit, Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao People's Democratic Republic, E-mail: paul@tropmedres.ac.
References
- 1.Wheat PF. History and development of antimicrobial susceptibility testing methodology. J Antimicrob Chemother. 2001;48((Suppl 1)):1–4. doi: 10.1093/jac/48.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 2.Chediak A. The diagnosis of syphilis on a dessicated and defibrinated blood drop. Rev Med Cubana. 1932;43:953–956. [Google Scholar]
- 3.Zimmermann E. Die Trockblutprobe auf Syphilis; ein Beitrag zu ihrer Vereinfachung. Munch Med Wochenschr. 1939;2:1732–1733. [Google Scholar]
- 4.Joe LK. A simple inexpensive and efficient method of preparing dysentery, typhoid and paratyphoid feces for dispatch to the laboratory. Ned Tijdschr Geneeskd. 1950;94:1246–1254. [PubMed] [Google Scholar]
- 5.Anderson RI, Sadun EH, Williams JS. A technique for the use of minute amounts of dried blood in the fluorescent antibody test for schistosomiasis. Exp Parasitol. 1961;11:111–116. doi: 10.1016/0014-4894(61)90014-5. [DOI] [PubMed] [Google Scholar]
- 6.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–343. [PubMed] [Google Scholar]
- 7.Solomon AW, Engels D, Bailey RL, Blake IM, Brooker S, Chen JX, Chen JH, Churcher TS, Drakeley CJ, Edwards T, Fenwick A, French M, Gabrielli AF, Grassly NC, Harding-Esch EM, Holland MJ, Koukounari A, Lammie PJ, Leslie J, Mabey DC, Rhajaoui M, Secor WE, Stothard JR, Wei H, Willingham AL, Zhou XN, Peeling RW. A diagnostics platform for the integrated mapping, monitoring, and surveillance of neglected tropical diseases: rationale and target product profiles. PLoS Negl Trop Dis. 2012;6:e1746. doi: 10.1371/journal.pntd.0001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbi M, Binda S, Caroppo S. Diagnosis of congenital CMV infection via dried blood spots. Rev Med Virol. 2006;16:385–392. doi: 10.1002/rmv.517. [DOI] [PubMed] [Google Scholar]
- 9.Bertagnolio S, Parkin NT, Jordan M, Brooks J, Garcia-Lerma JG. Dried blood spots for HIV-1 drug resistance and viral load testing: a review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 2010;12:195–208. [PubMed] [Google Scholar]
- 10.Buckton AJ. New methods for the surveillance of HIV drug resistance in the resource poor world. Curr Opin Infect Dis. 2008;21:653–658. doi: 10.1097/QCO.0b013e3283186d1a. [DOI] [PubMed] [Google Scholar]
- 11.Hamers RL, Smit PW, Stevens W, Schuurman R, Rinke de Wit TF. Dried fluid spots for HIV type-1 viral load and resistance genotyping: a systematic review. Antivir Ther. 2009;14:619–629. [PubMed] [Google Scholar]
- 12.Johannessen A, Troseid M, Calmy A. Dried blood spots can expand access to virological monitoring of HIV treatment in resource-limited settings. J Antimicrob Chemother. 2009;64:1126–1129. doi: 10.1093/jac/dkp353. [DOI] [PubMed] [Google Scholar]
- 13.Keevil BG. The analysis of dried blood spot samples using liquid chromatography tandem mass spectrometry. Clin Biochem. 2011;44:110–118. doi: 10.1016/j.clinbiochem.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 2001;131:1631S–1636S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- 15.Parker SP, Cubitt WD. The use of the dried blood spot sample in epidemiological studies. J Clin Pathol. 1999;52:633–639. doi: 10.1136/jcp.52.9.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiscus SA, Cheng B, Crowe SM, Demeter L, Jennings C, Miller V, Respess R, Stevens W. HIV-1 viral load assays for resource-limited settings. PLoS Med. 2006;3:e417. doi: 10.1371/journal.pmed.0030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snijdewind IJ, van Kampen JJ, Fraaij PL, van der Ende ME, Osterhaus AD, Gruters RA. Current and future applications of dried blood spots in viral disease management. Antiviral Res. 2012;93:309–321. doi: 10.1016/j.antiviral.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Burnett JE. Dried blood spot sampling: practical considerations and recommendation for use with preclinical studies. Bioanalysis. 2011;3:1099–1107. doi: 10.4155/bio.11.68. [DOI] [PubMed] [Google Scholar]
- 19.UNAIDS-WHO . Report on the Global AIDS Epidemic. 2008. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp Available at. Accessed May 1, 2013. [Google Scholar]
- 20.Sherman GG, Stevens G, Jones SA, Horsfield P, Stevens WS. Dried blood spots improve access to HIV diagnosis and care for infants in low-resource settings. J Acquir Immune Defic Syndr. 2005;38:615–617. doi: 10.1097/01.qai.0000143604.71857.5d. [DOI] [PubMed] [Google Scholar]
- 21.Boillot F, Peeters M, Kosia A, Delaporte E. Prevalence of the human immunodeficiency virus among patients with tuberculosis in Sierra Leone, established from dried blood spots on filter paper. Int J Tuberc Lung Dis. 1997;1:493–497. [PubMed] [Google Scholar]
- 22.Leelawiwat W, Young NL, Chaowanachan T, Ou CY, Culnane M, Vanprapa N, Waranawat N, Wasinrapee P, Mock PA, Tappero J, McNicholl JM. Dried blood spots for the diagnosis and quantitation of HIV-1: stability studies and evaluation of sensitivity and specificity for the diagnosis of infant HIV-1 infection in Thailand. J Virol Methods. 2009;155:109–117. doi: 10.1016/j.jviromet.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Nsojo A, Aboud S, Lyamuya E. Comparative evaluation of amplicor HIV-1 DNA test, version 1.5, by manual and automated dna extraction methods using venous blood and dried blood spots for HIV-1 DNA pcr testing. Tanzan J Health Res. 2010;12:229–235. doi: 10.4314/thrb.v12i4.58621. [DOI] [PubMed] [Google Scholar]
- 24.Stevens W, Erasmus L, Moloi M, Taleng T, Sarang S. Performance of a novel human immunodeficiency virus (HIV) type 1 total nucleic acid-based real-time PCR assay using whole blood and dried blood spots for diagnosis of HIV in infants. J Clin Microbiol. 2008;46:3941–3945. doi: 10.1128/JCM.00754-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patton JC, Akkers E, Coovadia AH, Meyers TM, Stevens WS, Sherman GG. Evaluation of dried whole blood spots obtained by heel or finger stick as an alternative to venous blood for diagnosis of human immunodeficiency virus type 1 infection in vertically exposed infants in the routine diagnostic laboratory. Clin Vaccine Immunol. 2007;14:201–203. doi: 10.1128/CVI.00223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lofgren SM, Morrissey AB, Chevallier CC, Malabeja AI, Edmonds S, Amos B, Sifuna DJ, von Seidlein L, Schimana W, Stevens WS, Bartlett JA, Crump JA. Evaluation of a dried blood spot HIV-1 RNA program for early infant diagnosis and viral load monitoring at rural and remote healthcare facilities. AIDS. 2009;23:2459–2466. doi: 10.1097/QAD.0b013e328331f702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S, Erickson B, Mak WB, Salituro J, Abravaya K. A novel RealTime HIV-1 Qualitative assay for the detection of HIV-1 nucleic acids in dried blood spots and plasma. J Virol Methods. 2011;178:216–224. doi: 10.1016/j.jviromet.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Stevens WS, Noble L, Berrie L, Sarang S, Scott LE. Ultra-high-throughput, automated nucleic acid detection of human immunodeficiency virus (HIV) for infant infection diagnosis using the Gen-Probe Aptima HIV-1 screening assay. J Clin Microbiol. 2009;47:2465–2469. doi: 10.1128/JCM.00317-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lilian RR, Bhowan K, Sherman GG. Early diagnosis of human immunodeficiency virus-1 infection in infants with the NucliSens EasyQ assay on dried blood spots. J Clin Virol. 2010;48:40–43. doi: 10.1016/j.jcv.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization . WHO Recommendations on the Diagnosis of HIV Infection in Infants and Children. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 31.Noda S, Eizuru Y, Minamishima Y, Ikenoue T, Mori N. Detection of human T-cell lymphotropic virus type 1 infection by the polymerase chain reaction using dried blood specimens on filter papers. J Virol Methods. 1993;43:111–122. doi: 10.1016/0166-0934(93)90094-8. [DOI] [PubMed] [Google Scholar]
- 32.Parker SP, Taylor MB, Ades AE, Cubitt WD, Peckham C. Use of dried blood spots for the detection and confirmation of HTLV-I specific antibodies for epidemiological purposes. J Clin Pathol. 1995;48:904–907. doi: 10.1136/jcp.48.10.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Croom HA, Richards KM, Best SJ, Francis BH, Johnson EI, Dax EM, Wilson KM. Commercial enzyme immunoassay adapted for the detection of antibodies to hepatitis C virus in dried blood spots. J Clin Virol. 2006;36:68–71. doi: 10.1016/j.jcv.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Judd A, Parry J, Hickman M, McDonald T, Jordan L, Lewis K, Contreras M, Dusheiko G, Foster G, Gill N, Kemp K, Main J, Murray-Lyon I, Nelson M. Evaluation of a modified commercial assay in detecting antibody to hepatitis C virus in oral fluids and dried blood spots. J Med Virol. 2003;71:49–55. doi: 10.1002/jmv.10463. [DOI] [PubMed] [Google Scholar]
- 35.Tuaillon E, Mondain AM, Meroueh F, Ottomani L, Picot MC, Nagot N, Van de Perre P, Ducos J. Dried blood spot for hepatitis C virus serology and molecular testing. Hepatology. 2010;51:752–758. doi: 10.1002/hep.23407. [DOI] [PubMed] [Google Scholar]
- 36.Gil A, Gonzalez A, Dal-Re R, Dominguez V, Astasio P, Aguilar L. Detection of antibodies against hepatitis A in blood spots dried on filter paper. Is this a reliable method for epidemiological studies? Epidemiol Infect. 1997;118:189–191. doi: 10.1017/s0950268896007297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melgaco JG, Pinto MA, Rocha AM, Freire M, Gaspar LP, Lima SM, Cruz OG, Vitral CL. The use of dried blood spots for assessing antibody response to hepatitis A virus after natural infection and vaccination. J Med Virol. 2011;83:208–217. doi: 10.1002/jmv.21973. [DOI] [PubMed] [Google Scholar]
- 38.Mendy M, Kirk GD, van der Sande M, Jeng-Barry A, Lesi OA, Hainaut P, Sam O, McConkey S, Whittle H. Hepatitis B surface antigenaemia and alpha-foetoprotein detection from dried blood spots: applications to field-based studies and to clinical care in hepatitis B virus endemic areas. J Viral Hepat. 2005;12:642–647. doi: 10.1111/j.1365-2893.2005.00641.x. [DOI] [PubMed] [Google Scholar]
- 39.Villar LM, de Oliveira JC, Cruz HM, Yoshida CFT, Lampe E, Lewis-Ximenez LL. Assessment of dried blood spot samples as a simple method for detection of hepatitis B virus markers. J Med Virol. 2011;83:1522–1529. doi: 10.1002/jmv.22138. [DOI] [PubMed] [Google Scholar]
- 40.Solmone M, Girardi E, Costa F, Pucillo L, Ippolito G, Capobianchi MR. Simple and reliable method for detection and genotyping of hepatitis C virus RNA in dried blood spots stored at room temperature. J Clin Microbiol. 2002;40:3512–3514. doi: 10.1128/JCM.40.9.3512-3514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuno G, Gomez I, Gubler DJ. An ELISA procedure for the diagnosis of dengue infections. J Virol Methods. 1991;33:101–113. doi: 10.1016/0166-0934(91)90011-n. [DOI] [PubMed] [Google Scholar]
- 42.Balmaseda A, Saborio S, Tellez Y, Mercado JC, Perez L, Hammond SN, Rocha C, Kuan G, Harris E. Evaluation of immunological markers in serum, filter-paper blood spots, and saliva for dengue diagnosis and epidemiological studies. J Clin Virol. 2008;43:287–291. doi: 10.1016/j.jcv.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Matheus S, Meynard J-B, Lacoste V, Morvan J, Deparis X. Use of capillary blood samples as a new approach for diagnosis of dengue virus infection. J Clin Microbiol. 2007;45:887–890. doi: 10.1128/JCM.02063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran TNT, de Vries PJ, Hoang LP, Phan GT, Le HQ, Tran BQ, Vo CMT, Nguyen NV, Kager PA, Nagelkerke N, Groen J. Enzyme-linked immunoassay for dengue virus IgM and IgG antibodies in serum and filter paper blood. BMC Infect Dis. 2006;6:13. doi: 10.1186/1471-2334-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herrera Rdl C, Cabrera MV, Garcia S, Gilart M. IgM antibodies to dengue virus in dried blood on filter paper. Clin Chim Acta. 2006;367:204–206. doi: 10.1016/j.cca.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 46.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 47.Prado I, Rosario D, Bernardo L, Alvarez M, Rodriguez R, Vazquez S, Guzman MG. PCR detection of dengue virus using dried whole blood spotted on filter paper. J Virol Methods. 2005;125:75–81. doi: 10.1016/j.jviromet.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Grivard P, Le Roux K, Laurent P, Fianu A, Perrau J, Gigan J, Hoarau G, Grondin N, Staikowsky F, Favier F, Michault A. Molecular and serological diagnosis of Chikungunya virus infection. Pathol Biol (Paris) 2007;55:490–494. doi: 10.1016/j.patbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Riddell MA, Leydon JA, Catton MG, Kelly HA. Detection of measles virus-specific immunoglobulin m in dried venous blood samples by using a commercial enzyme immunoassay. J Clin Microbiol. 2002;40:5–9. doi: 10.1128/JCM.40.1.5-9.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riddell MA, Byrnes GB, Leydon JA, Kelly HA. Dried venous blood samples for the detection and quantification of measles IgG using a commercial enzyme immunoassay. Bull World Health Organ. 2003;81:701–707. [PMC free article] [PubMed] [Google Scholar]
- 51.Uzicanin A, Lubega I, Nanuynja M, Mercader S, Rota P, Bellini W, Helfand R. Dried blood spots on filter paper as an alternative specimen for measles diagnostics: detection of measles immunoglobulin M antibody by a commercial enzyme immunoassay. J Infect Dis. 2011;204:S564–S569. doi: 10.1093/infdis/jir088. [DOI] [PubMed] [Google Scholar]
- 52.Fachiroh J, Prasetyanti PR, Paramita DK, Prasetyawati AT, Anggrahini DW, Haryana SM, Middeldorp JM. Dried-blood sampling for Epstein-Barr virus immunoglobulin G (IgG) and IgA serology in nasopharyngeal carcinoma screening. J Clin Microbiol. 2008;46:1374–1380. doi: 10.1128/JCM.01368-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Binda S, Caroppo S, Dido P, Primache V, Veronesi L, Calvario A, Piana A, Barbi M. Modification of CMV DNA detection from dried blood spots for diagnosing congenital CMV infection. J Clin Virol. 2004;30:276–279. doi: 10.1016/j.jcv.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Dowd JB, Aiello AE, Chyu L, Huang YY, McDade TW. Cytomegalovirus antibodies in dried blood spots: a minimally invasive method for assessing stress, immune function, and aging. Immun Ageing. 2011;8:3. doi: 10.1186/1742-4933-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Harthi SA, Jamjoom MB. PCR assay in malaria diagnosis using filter paper samples from Jazan region, Saudi Arabia. J Egypt Soc Parasitol. 2008;38:693–706. [PubMed] [Google Scholar]
- 56.Ataei S, Nateghpour M, Hajjaran H, Edrissian GH, Foroushani AR. High specificity of semi-nested multiplex PCR using dried blood spots on DNA banking card in comparison with frozen liquid blood for detection of Plasmodium falciparum and Plasmodium vivax. J Clin Lab Anal. 2011;25:185–190. doi: 10.1002/jcla.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wangai LN, Karau MG, Njiruh PN, Sabah O, Kimani FT, Magoma G, Kiambo N. Sensitivity of microscopy compared to molecular diagnosis of P. falciparum: implications on malaria treatment in epidemic areas in Kenya. Afr J Infect Dis. 2011;5:1–6. doi: 10.4314/ajid.v5i1.66504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boonma P, Christensen PR, Suwanarusk R, Price RN, Russell B, Lek-Uthai U. Comparison of three molecular methods for the detection and speciation of Plasmodium vivax and Plasmodium falciparum. Malar J. 2007;6:124. doi: 10.1186/1475-2875-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 60.Beshir K, Sutherland CJ, Merinopoulos I, Durrani N, Leslie T, Rowland M, Hallett RL. Amodiaquine resistance in Plasmodium falciparum malaria in Afghanistan is associated with the pfcrt SVMNT allele at codons 72 to 76. Antimicrob Agents Chemother. 2010;54:3714–3716. doi: 10.1128/AAC.00358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization . Working to Overcome the Global Impact of Neglected Tropical Diseases. Geneva: World Health Organization; 2011. [Google Scholar]
- 62.Itoh M, Gunawardena NK, Qiu XG, Weerasooriya MV, Kimura E. The use of whole blood absorbed on filter paper to detect Wuchereria bancrofti circulating antigen. Trans R Soc Trop Med Hyg. 1998;92:513–515. doi: 10.1016/s0035-9203(98)90896-3. [DOI] [PubMed] [Google Scholar]
- 63.Hoti SL, Elango A, Radjame K, Yuvaraj J, Pani SP. Detection of day blood filarial antigens by Og4C3 ELISA test using filter paper samples. Natl Med J India. 2002;15:263–266. [PubMed] [Google Scholar]
- 64.Wattal S, Dhariwal AC, Ralhan PK, Tripathi VC, Regu K, Kamal S, Lal S. Evaluation of Og4C3 antigen ELISA as a tool for detection of bancroftian filariasis under lymphatic filariasis elimination programme. J Commun Dis. 2007;39:75–84. [PubMed] [Google Scholar]
- 65.Gyapong JO, Omane-Badu K, Webber RH. Evaluation of the filter paper blood collection method for detecting Og4C3 circulating antigen in bancroftian filariasis. Trans R Soc Trop Med Hyg. 1998;92:407–410. doi: 10.1016/s0035-9203(98)91068-9. [DOI] [PubMed] [Google Scholar]
- 66.Weil GJ, Curtis KC, Fischer PU, Won KY, Lammie PJ, Joseph H, Melrose WD, Brattig NW. A multicenter evaluation of a new antibody test kit for lymphatic filariasis employing recombinant Brugia malayi antigen Bm-14. Acta Trop. 2011;120:S19–S22. doi: 10.1016/j.actatropica.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer P, Bonow I, Supali T, Ruckert P, Rahmah N. Detection of filaria-specific IgG4 antibodies and filarial DNA, for the screening of blood spots for Brugia timori. Ann Trop Med Parasitol. 2005;99:53–60. doi: 10.1179/136485905X13339. [DOI] [PubMed] [Google Scholar]
- 68.Kluber S, Supali T, Williams SA, Liebau E, Fischer P. Rapid PCR-based detection of Brugia malayi DNA from blood spots by DNA Detection Test Strips. Trans R Soc Trop Med Hyg. 2001;95:169–170. doi: 10.1016/s0035-9203(01)90148-8. [DOI] [PubMed] [Google Scholar]
- 69.Rahmah N, Nurulhasanah O, Norhayati S, Zulkarnain I, Norizan M. Comparison of conventional versus real-time PCR detection of Brugia malayi DNA from dried blood spots from school children in a low endemic area. Trop Biomed. 2010;27:54–59. [PubMed] [Google Scholar]
- 70.Fink DL, Kamgno J, Nutman TB. Rapid molecular assays for specific detection and quantitation of Loa loa microfilaremia. PLoS Negl Trop Dis. 2011;5:8. doi: 10.1371/journal.pntd.0001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chappuis F, Pittet A, Bovier PA, Adams K, Godineau V, Hwang SY, Magnus E, Büscher P. Field evaluation of the CATT/Trypanosoma brucei gambiense on blood-impregnated filter papers for diagnosis of human African trypanosomiasis in southern Sudan. Trop Med Int Health. 2002;7:942–948. doi: 10.1046/j.1365-3156.2002.00956.x. [DOI] [PubMed] [Google Scholar]
- 72.Truc P, Lejon V, Magnus E, Jamonneau V, Nangouma A, Verloo D, Penchenier L, Buscher P. Evaluation of the micro-CATT, CATT/Trypanosoma brucei gambiense, and LATEX/T b gambiense methods for serodiagnosis and surveillance of human African trypanosomiasis in West and Central Africa. Bull World Health Organ. 2002;80:882–886. [PMC free article] [PubMed] [Google Scholar]
- 73.Luquetti AO, Ponce C, Ponce E, Esfandiari J, Schijman A, Revollo S, Anez N, Zingales B, Ramgel-Aldao R, Gonzalez A, Levin MJ, Umezawa ES, Da Silveira JF. Chagas' disease diagnosis: a multicentric evaluation of Chagas Stat-Pak, a rapid immunochromatographic assay with recombinant proteins of Trypanosoma cruzi. Diagn Microbiol Infect Dis. 2003;46:265–271. doi: 10.1016/s0732-8893(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 74.Zicker F, Smith PG, Luquetti AO, Oliveira OS. Mass screening for Trypanosoma cruzi infections using the immunofluorescence, ELISA and haemagglutination tests on serum samples and on blood eluates from filter-paper. Bull World Health Organ. 1990;68:465–471. [PMC free article] [PubMed] [Google Scholar]
- 75.Campino L, Cortes S, Pires R, Oskam L, Abranches P. Detection of Leishmania in immunocompromised patients using peripheral blood spots on filter paper and the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 2000;19:396–398. doi: 10.1007/s100960050503. [DOI] [PubMed] [Google Scholar]
- 76.Coltorti E, Guarnera E, Larrieu E, Santillan G, Aquino A. Seroepidemiology of human hydatidosis: use of dried blood samples on filter paper. Trans R Soc Trop Med Hyg. 1988;82:607–610. doi: 10.1016/0035-9203(88)90527-5. [DOI] [PubMed] [Google Scholar]
- 77.Bartholomot B, Vuitton DA, Harraga S, Shi DZ, Giraudoux P, Barnish G, Wang YH, MacPherson CNL, Craig PS. Combined ultrasound and serologic screening for hepatic alveolar Echinococcosis in central China. Am J Trop Med Hyg. 2002;66:23–29. doi: 10.4269/ajtmh.2002.66.23. [DOI] [PubMed] [Google Scholar]
- 78.Cohen H, Paolillo E, Bonifacino R, Botta B, Parada L, Cabrera P, Snowden K, Gasser R, Tessier R, Dibarboure L, Wen H, Allan JC, Soto de Alfaro H, Rogan MT, Craig PS. Human cystic echinococcosis in a Uruguayan community: a sonographic, serologic, and epidemiologic study. Am J Trop Med Hyg. 1998;59:620–627. doi: 10.4269/ajtmh.1998.59.620. [DOI] [PubMed] [Google Scholar]
- 79.Strauss W, O'Neill SM, Parkinson M, Angles R, Dalton JP. Short report: diagnosis of human fascioliasis: detection of anti-cathepsin L antibodies in blood samples collected on filter paper. Am J Trop Med Hyg. 1999;60:746–748. doi: 10.4269/ajtmh.1999.60.746. [DOI] [PubMed] [Google Scholar]
- 80.Peralta RHS, Macedo HW, Vaz AJ, Machado LR, Peralta Jose M. Detection of anti-cysticercus antibodies by ELISA using whole blood collected on filter paper. Trans R Soc Trop Med Hyg. 2001;95:35–36. doi: 10.1016/s0035-9203(01)90324-4. [DOI] [PubMed] [Google Scholar]
- 81.Fleury A, Bouteille B, Garcia E, Marquez C, Preux PM, Escobedo F, Sotelo J, Dumas M. Neurocysticercosis: validity of ELISA after storage of whole blood and cerebrospinal fluid on paper. Trop Med Int Health. 2001;6:688–693. doi: 10.1046/j.1365-3156.2001.00767.x. [DOI] [PubMed] [Google Scholar]
- 82.Parker SP, Cubitt WD. Modified latex agglutination test for antibodies to Toxoplasma gondii in eluates from Guthrie cards. J Clin Pathol. 1992;45:907–909. doi: 10.1136/jcp.45.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guimaraes S, Sogayar MIL. Detection of anti-Giardia lamblia serum antibody among children of day care centers. Rev Saude Publica. 2002;36:63–68. doi: 10.1590/s0034-89102002000100010. [DOI] [PubMed] [Google Scholar]
- 84.al-Tukhi MH, Ackers JP, al-Ahdal MN, Peters W. Enzyme-linked immunosorbent assay for the detection of anti-Giardia specific immunoglobulin G in filter paper blood samples. Trans R Soc Trop Med Hyg. 1993;87:36–38. doi: 10.1016/0035-9203(93)90412-j. [DOI] [PubMed] [Google Scholar]
- 85.Butlin CR, Soares D, Neupane KD, Failbus SS, Roche PW. IgM anti-phenolic glycolipid-I antibody measurements from skin-smear sites: correlation with venous antibody levels and the bacterial index. Int J Lepr Other Mycobact Dis. 1997;65:465–468. [PubMed] [Google Scholar]
- 86.Chanteau S, Plichart R, Boutin JP, Roux J, Cartel JL. Finger-prick blood collection and computer-assisted enzyme-linked immunosorbent assay for large-scale serological studies on leprosy. Trans R Soc Trop Med Hyg. 1989;83:414–416. doi: 10.1016/0035-9203(89)90523-3. [DOI] [PubMed] [Google Scholar]
- 87.Dhandayuthapani S, Anandan D, Vasanthi B, Bhatia VN. Use of eluates of filter paper blood spots in ELISA for the serodiagnosis of leprosy. Indian J Med Res. 1989;89:150–157. [PubMed] [Google Scholar]
- 88.Sekar B, Anandan D. Evaluation of Mycobacterium leprae particle agglutination test, using eluates of filter paper blood spots. Lepr Rev. 1992;63:117–124. [PubMed] [Google Scholar]
- 89.Takkouche B, Iglesias J, Alonso-Fernandez JR, Fernandez-Gonzalez C, Gestal-Otero JJ. Detection of Brucella antibodies in eluted dried blood: a validation study. Immunol Lett. 1995;45:107–108. doi: 10.1016/0165-2478(94)00247-o. [DOI] [PubMed] [Google Scholar]
- 90.Desvars A, Gigan J, Hoarau G, Gerardin P, Favier F, Michault A. Short report: Seroprevalence of human leptospirosis in Reunion Island (Indian Ocean) assessed by microscopic agglutination test on paper disc-absorbed whole blood. Am J Trop Med Hyg. 2011;85:1097–1099. doi: 10.4269/ajtmh.2011.11-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stevens R, Pass K, Fuller S, Wiznia A, Noble L, Duva S, Neal M. Blood spot screening and confirmatory tests for syphilis antibody. J Clin Microbiol. 1992;30:2353–2358. doi: 10.1128/jcm.30.9.2353-2358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coates GL, Guarenti L, Parker SP, Willumsen JF, Tomkins AM. Evaluation of the sensitivity and specificity of a Treponema pallidum dried blood spot technique for use in the detection of syphilis. Trans R Soc Trop Med Hyg. 1998;92:44. doi: 10.1016/s0035-9203(98)90949-x. [DOI] [PubMed] [Google Scholar]
- 93.Phetsouvanh R, Blacksell SD, Jenjaroen K, Day NP, Newton PN. Comparison of indirect immunofluorescence assays for diagnosis of scrub typhus and murine typhus using venous blood and finger prick filter paper blood spots. Am J Trop Med Hyg. 2009;80:837–840. [PMC free article] [PubMed] [Google Scholar]
- 94.Fenollar F, Raoult D. Diagnosis of rickettsial diseases using samples dried on blotting paper. Clin Diagn Lab Immunol. 1999;6:483–488. doi: 10.1128/cdli.6.4.483-488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Backhouse JL, Lee MH, Nesteroff SI, Hudson BJ, Hamilton PA. Modified indirect hemagglutination test for detection of treponemal antibodies in finger-prick blood. J Clin Microbiol. 1992;30:561–563. doi: 10.1128/jcm.30.3.561-563.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mei JV, Zobel SD, Hall EM, De Jesus VR, Adam BW, Hannon WH. Performance properties of filter paper devices for whole blood collection. Bioanalysis. 2010;2:1397–1403. doi: 10.4155/bio.10.73. [DOI] [PubMed] [Google Scholar]
- 97.Centre for Disease Control and Prevention . Serologic Assays for Human Immunodeficiency Virus Antibody in Dried-Blood Specimens Collected on Filter Paper. Atlanta, GA: US CDC; 2002. [Google Scholar]
- 98.Corran PH, Cook J, Lynch C, Leendertse H, Manjurano A, Griffin J, Cox J, Abeku T, Bousema T, Ghani AC, Drakeley C, Riley E. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 2008;7:195. doi: 10.1186/1475-2875-7-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.World Health Organization . WHO Manual for HIV Drug Resistance Testing Using Dried Blood Spot Specimens. Geneva. Switzerland: World Health Organization; 2012. [Google Scholar]
- 100.Hearps AC, Ryan CE, Morris LM, Plate MM, Greengrass V, Crowe SM. Stability of dried blood spots for HIV-1 drug resistance analysis. Curr HIV Res. 2010;8:134–140. doi: 10.2174/157016210790442740. [DOI] [PubMed] [Google Scholar]
- 101.Li CC, Beck IA, Seidel KD, Frenkel LM. Persistence of human immunodeficiency virus type 1 subtype B DNA in dried-blood samples on FTA filter paper. J Clin Microbiol. 2004;42:3847–3849. doi: 10.1128/JCM.42.8.3847-3849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Monleau M, Montavon C, Laurent C, Segondy M, Montes B, Delaporte E, Boillot F, Peeters M. Evaluation of different RNA extraction methods and storage conditions of dried plasma or blood spots for human immunodeficiency virus type 1 RNA quantification and PCR amplification for drug resistance testing. J Clin Microbiol. 2009;47:1107–1118. doi: 10.1128/JCM.02255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Michaud V, Gil P, Kwiatek O, Prome S, Dixon L, Romero L, Le Potier MF, Arias M, Couacy-Hymann E, Roger F, Libeau G, Albina E. Long-term storage at tropical temperature of dried-blood filter papers for detection and genotyping of RNA and DNA viruses by direct PCR. J Virol Methods. 2007;146:257–265. doi: 10.1016/j.jviromet.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 104.Kuno G. Review of the factors modulating dengue transmission. Epidemiol Rev. 1995;17:321–335. doi: 10.1093/oxfordjournals.epirev.a036196. [DOI] [PubMed] [Google Scholar]
- 105.Centres for Disease Control and Prevention . Shipping Guidelines for Dried-Blood Spot Specimens. Atlanta, GA: US CDC; 2012. [Google Scholar]
- 106.Reitmeyer JC, Ewert A, Crawford MA, Reitmeyer GR, Mock L. Survival of group A streptococci in dried human blood. J Med Microbiol. 1993;38:61–63. doi: 10.1099/00222615-38-1-61. [DOI] [PubMed] [Google Scholar]
- 107.Evengard B, Ehrnst A, von Sydow M, Pehrson PO, Lundbergh P, Linder E. Effect of heat on extracted HIV viral infectivity and antibody activity using the filter paper technique of blood sampling. AIDS. 1989;3:591–595. doi: 10.1097/00002030-198909000-00006. [DOI] [PubMed] [Google Scholar]
- 108.Abdelwhab EM, Luschow D, Harder TC, Hafez HM. The use of FTA filter papers for diagnosis of avian influenza virus. J Virol Methods. 2011;174:120–122. doi: 10.1016/j.jviromet.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 109.Adam BW, Alexander JR, Smith SJ, Chace DH, Loeber JG, Elvers LH, Hannon WH. Recoveries of phenylalanine from two sets of dried-blood-spot reference materials: Prediction from hematocrit, spot volume, and paper matrix. Clin Chem. 2000;46:126–128. [PubMed] [Google Scholar]
- 110.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Desbois D, Roque-Afonso AM, Lebraud P, Dussaix E. Use of dried serum spots for serological and molecular detection of hepatitis A virus. J Clin Microbiol. 2009;47:1536–1542. doi: 10.1128/JCM.02191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barin F, Plantier JC, Brand D, Brunet S, Moreau A, Liandier B, Thierry D, Cazein F, Lot F, Semaille C, Desenclos JC. Human immunodeficiency virus serotyping on dried serum spots as a screening tool for the surveillance of the AIDS epidemic. J Med Virol. 2006;78((Suppl 1)):S13–S18. doi: 10.1002/jmv.20600. [DOI] [PubMed] [Google Scholar]
- 113.Abe K, Konomi N. Hepatitis C virus RNA in dried serum spotted onto filter paper is stable at room temperature. J Clin Microbiol. 1998;36:3070–3072. doi: 10.1128/jcm.36.10.3070-3072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ayele W, Schuurman R, Messele T, Dorigo-Zetsma W, Mengistu Y, Goudsmit J, Paxton WA, de Baar MP, Pollakis G. Use of dried spots of whole blood, plasma, and mother's milk collected on filter paper for measurement of human immunodeficiency virus type 1 burden. J Clin Microbiol. 2007;45:891–896. doi: 10.1128/JCM.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cassol S, Gill MJ, Pilon R, Cormier M, Voigt RF, Willoughby B, Forbes J. Quantification of human immunodeficiency virus type 1 RNA from dried plasma spots collected on filter paper. J Clin Microbiol. 1997;35:2795–2801. doi: 10.1128/jcm.35.11.2795-2801.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brambilla D, Jennings C, Aldrovandi G, Bremer J, Comeau AM, Cassol SA, Dickover R, Jackson JB, Pitt J, Sullivan JL, Butcher A, Grosso L, Reichelderfer P, Fiscus SA. Multicenter evaluation of use of dried blood and plasma spot specimens in quantitative assays for human immunodeficiency virus RNA: measurement, precision, and RNA stability. J Clin Microbiol. 2003;41:1888–1893. doi: 10.1128/JCM.41.5.1888-1893.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rossi de Gasperis M, Caione MD, Concato C, Fiscarelli E, Di Pietro N, Salotti V, Putignani L, Menichella D, Callea F. Quantitative recovery of proviral HIV-1 DNA from leukocytes by the Dried Buffy Coat Spot method for real-time PCR determination. J Virol Methods. 2010;170:121–127. doi: 10.1016/j.jviromet.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 118.Alam MZ, Shamsuzzaman AKM, Kuhls K, Schonian G. PCR diagnosis of visceral leishmaniasis in an endemic region, Mymensingh district, Bangladesh. Trop Med Int Health. 2009;14:499–503. doi: 10.1111/j.1365-3156.2009.02254.x. [DOI] [PubMed] [Google Scholar]
- 119.Boggild AK, Valencia BM, Espinosa D, Veland N, Ramos AP, Arevalo J, Llanos-Cuentas A, Low DE. Detection and species identification of Leishmania DNA from filter paper lesion impressions for patients with American cutaneous leishmaniasis. Clin Infect Dis. 2010;50:e1–e6. doi: 10.1086/648730. [DOI] [PubMed] [Google Scholar]
- 120.Fata A, Khamesipour A, Mohajery M, Hosseininejad Z, Afzalaghaei M, Berenji F, Ganjbakhsh M, Akhavan AA, Eskandari E, Amin-Mohammadi A. Whatman paper (FTA cards) for storing and transferring Leishmania DNA for PCR examination. Iran J Parasitol. 2009;4:37–42. [Google Scholar]
- 121.Aye KS, Matsuoka M, Kai M, Kyaw K, Win AA, Shwe MM, Thein M, Htoo MM, Htoon MT. FTA card utility for PCR detection of Mycobacterium leprae. Jpn J Infect Dis. 2011;64:246–248. [PubMed] [Google Scholar]
- 122.Chibo D, Riddell MA, Catton MG, Birch CJ. Applicability of oral fluid collected onto filter paper for detection and genetic characterization of measles virus strains. J Clin Microbiol. 2005;43:3145–3149. doi: 10.1128/JCM.43.7.3145-3149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mharakurwa S, Simoloka C, Thuma PE, Shiff CJ, Sullivan DJ. PCR detection of Plasmodium falciparum in human urine and saliva samples. Malar J. 2006;5:103. doi: 10.1186/1475-2875-5-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nuchprayoon S, Saksirisampant W, Jaijakul S, Nuchprayoon I. FlindersTechnology Associates (FTA) filter paper-based DNA extraction with polymerase chain reaction (PCR) for detection of Pneumocystis jirovecii from respiratory specimens of immunocompromised patients. J Clin Lab Anal. 2007;21:382–386. doi: 10.1002/jcla.20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gustavsson I, Lindell M, Wilander E, Strand A, Gyllensten U. Use of FTA card for dry collection, transportation and storage of cervical cell specimen to detect high-risk HPV. J Clin Virol. 2009;46:112–116. doi: 10.1016/j.jcv.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 126.Kailash U, Hedau S, Gopalkrishna V, Katiyar S, Das BC. A simple ‘paper smear’ method for dry collection, transport and storage of cervical cytological specimens for rapid screening of HPV infection by PCR. J Med Microbiol. 2002;51:606–610. doi: 10.1099/0022-1317-51-7-606. [DOI] [PubMed] [Google Scholar]
- 127.Banura C, Franceschi S, van Doorn LJ, Wabwire-Mangen F, Mbidde EK, Weiderpass E. Detection of cervical human papillomavirus infection in filter paper samples: a comparative study. J Med Microbiol. 2008;57:253–255. doi: 10.1099/jmm.0.47520-0. [DOI] [PubMed] [Google Scholar]
- 128.Peltola H, Roine I, Leinonen M, Kuisma L, Mata AG, Arbo A, Goyo J, Saukkoriipi A. Diagnosis of Streptococcus pneumoniae and Haemophilus influenzae type b meningitis by identifying DNA from cerebrospinal fluid-impregnated filter paper strips. Pediatr Infect Dis J. 2010;29:111–114. doi: 10.1097/inf.0b013e3181b4f041. [DOI] [PubMed] [Google Scholar]
- 129.Page AL, Alberti KP, Guenole A, Mondongue V, Lonlas Mayele S, Guerin PJ, Quilici ML. Use of filter paper as a transport medium for laboratory diagnosis of cholera under field conditions. J Clin Microbiol. 2011;49:3021–3023. doi: 10.1128/JCM.00514-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wollants E, Maes P, Thoelen I, Vanneste F, Rahman M, Van Ranst M. Evaluation of a norovirus sampling method using sodium dodecyl sulfate/EDTA-pretreated chromatography paper strips. J Virol Methods. 2004;122:45–48. doi: 10.1016/j.jviromet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 131.Rahman M, Goegebuer T, De Leener K, Maes P, Matthijnssens J, Podder G, Azim T, Van Ranst M. Chromatography paper strip method for collection, transportation, and storage of rotavirus RNA in stool samples. J Clin Microbiol. 2004;42:1605–1608. doi: 10.1128/JCM.42.4.1605-1608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zlateva KT, Maes P, Rahman M, Van Ranst M. Chromatography paper strip sampling of enteric adenoviruses type 40 and 41 positive stool specimens. Virol J. 2005;2:6. doi: 10.1186/1743-422X-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nozawa N, Koyano S, Yamamoto Y, Inami Y, Kurane I, Inoue N. Real-time PCR assay using specimens on filter disks as a template for detection of cytomegalovirus in urine. J Clin Microbiol. 2007;45:1305–1307. doi: 10.1128/JCM.02502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.The International Bank for Reconstruction and Development/The World Bank and The TAFS Forum . World Livestock Disease Atlas: A Quantitative Analysis of Global Animal Health Data (2006–2009) Washington, DC: World Bank; 2011. http://documents.worldbank.org/curated/en/2011/11/15812714/world-livestock-disease-atlas-quantitative-analysis-global-animal-health-data-2006-2009 Available at. [Google Scholar]
- 135.Smith LM, Burgoyne LA. Collecting, archiving and processing DNA from wildlife samples using FTA databasing paper. BMC Ecol. 2004;4:4. doi: 10.1186/1472-6785-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Figueiredo FB, Madeira MF, Nascimento LD, Abrantes TR, Mouta-Confort E, Passos SR, Schubach TM. Canine visceral leishmaniasis: study of methods for the detection of IgG in serum and eluate samples. Rev Inst Med Trop Sao Paulo. 2010;52:193–196. doi: 10.1590/s0036-46652010000400005. [DOI] [PubMed] [Google Scholar]
- 137.Kalayou S, Tadelle H, Bsrat A, Abebe N, Haileselassie M, Schallig HD. Serological evidence of Leishmania donovani infection in apparently healthy dogs using direct agglutination test (DAT) and rk39 dipstick tests in Kafta Humera, north-west Ethiopia. Transbound Emerg Dis. 2011;58:255–262. doi: 10.1111/j.1865-1682.2011.01209.x. [DOI] [PubMed] [Google Scholar]
- 138.Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem. 2010;82:3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 139.Matheus S, Chappert JL, Cassadou S, Berger F, Labeau B, Bremand L, Winicki A, Huc-Anais P, Quenel P, Dussart P. Virological surveillance of dengue in Saint Martin and Saint Barthelemy, French West Indies, using blood samples on filter paper. Am J Trop Med Hyg. 2012;86:159–165. doi: 10.4269/ajtmh.2012.11-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mangold KA, Manson RU, Koay ES, Stephens L, Regner M, Thomson RB, Jr, Peterson LR, Kaul KL. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–2440. doi: 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamamura M, Makimura K, Ota Y. Evaluation of a new rapid molecular diagnostic system for Plasmodium falciparum combined with DNA filter paper, loop-mediated isothermal amplification, and melting curve analysis. Jpn J Infect Dis. 2009;62:20–25. [PubMed] [Google Scholar]
- 142.Craine N, Parry J, O'Toole J, D'Arcy S, Lyons M. Improving blood-borne viral diagnosis; clinical audit of the uptake of dried blood spot testing offered by a substance misuse service. J Viral Hepat. 2009;16:219–222. doi: 10.1111/j.1365-2893.2008.01061.x. [DOI] [PubMed] [Google Scholar]
- 143.Lukacs Z, Dietrich A, Ganschow R, Kohlschutter A, Kruithof R. Simultaneous determination of HIV antibodies, hepatitis C antibodies, and hepatitis B antigens in dried blood spots—a feasibility study using a multi-analyte immunoassay. Clin Chem Lab Med. 2005;43:141–145. doi: 10.1515/CCLM.2005.023. [DOI] [PubMed] [Google Scholar]
- 144.De Crignis E, Re MC, Cimatti L, Zecchi L, Gibellini D. HIV-1 and HCV detection in dried blood spots by SYBR Green multiplex real-time RT-PCR. J Virol Methods. 2010;165:51–56. doi: 10.1016/j.jviromet.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 145.Solomon SS, Solomon S, Rodriguez II, McGarvey ST, Ganesh AK, Thyagarajan SP, Mahajan AP, Mayer KH. Dried blood spots (DBS): a valuable tool for HIV surveillance in developing/tropical countries. Int J STD AIDS. 2002;13:25–28. doi: 10.1258/0956462021924578. [DOI] [PubMed] [Google Scholar]
- 146.Cachafeiro A, Sherman GG, Sohn AH, Beck-Sague C, Fiscus SA. Diagnosis of human immunodeficiency virus type 1 infection in infants by use of dried blood spots and an ultrasensitive p24 antigen assay. J Clin Microbiol. 2009;47:459–462. doi: 10.1128/JCM.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patton JC, Coovadia AH, Meyers TM, Sherman GG. Evaluation of the ultrasensitive human immunodeficiency virus type 1 (HIV-1) p24 antigen assay performed on dried blood spots for diagnosis of HIV-1 infection in infants. Clin Vaccine Immunol. 2008;15:388–391. doi: 10.1128/CVI.00265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mercader S, Featherstone D, Bellini WJ. Comparison of available methods to elute serum from dried blood spot samples for measles serology. J Virol Methods. 2006;137:140–149. doi: 10.1016/j.jviromet.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 149.Gohring K, Dietz K, Hartleif S, Jahn G, Hamprecht K. Influence of different extraction methods and PCR techniques on the sensitivity of HCMV-DNA detection in dried blood spot (DBS) filter cards. J Clin Virol. 2010;48:278–281. doi: 10.1016/j.jcv.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 150.Castro AC, Borges LG, Souza Rda S, Grudzinski M, D'Azevedo PA. Evaluation of the human immunodeficiency virus type 1 and 2 antibodies detection in dried whole blood spots (DBS) samples. Rev Inst Med Trop Sao Paulo. 2008;50:151–156. doi: 10.1590/s0036-46652008000300004. [DOI] [PubMed] [Google Scholar]
- 151.Chaillet P, Zachariah R, Harries K, Rusanganwa E, Harries AD. Dried blood spots are a useful tool for quality assurance of rapid HIV testing in Kigali, Rwanda. Trans R Soc Trop Med Hyg. 2009;103:634–637. doi: 10.1016/j.trstmh.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 152.Lakshmi V, Sudha T, Bhanurekha M, Dandona L. Evaluation of the Murex HIV Ag/Ab Combination assay when used with dried blood spots. Clin Microbiol Infect. 2007;13:1134–1136. doi: 10.1111/j.1469-0691.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 153.Mashange W, Soko W, Gomo E. Validation of a simple and cheap gelatin particle agglutination test for human immunodeficiency virus using dried blood spot samples. Cent Afr J Med. 2003;49:5–8. [PubMed] [Google Scholar]
- 154.Sarge-Njie R, Schim Van Der Loeff M, Ceesay S, Cubitt D, Sabally S, Corrah T, Whittle H. Evaluation of the dried blood spot filter paper technology and five testing strategies of HIV-1 and HIV-2 infections in West Africa. Scand J Infect Dis. 2006;38:1050–1056. doi: 10.1080/00365540600801645. [DOI] [PubMed] [Google Scholar]
- 155.Knuchel MC, Jullu B, Shah C, Tomasik Z, Stoeckle MP, Speck RF, Nadal D, Mshinda H, Boni J, Tanner M, Schupbach J. Adaptation of the ultrasensitive HIV-1 p24 antigen assay to dried blood spot testing. J Acquir Immune Defic Syndr. 2007;44:247–253. doi: 10.1097/QAI.0b013e31802c3e67. [DOI] [PubMed] [Google Scholar]
- 156.Mwapasa V, Cachafeiro A, Makuta Y, Beckstead DJ, Pennell ML, Chilima B, Mwagomba B, Fiscus SA, Kwiek JJ. Using a simplified human immunodeficiency virus type 1 p24 antigen assay to diagnose pediatric HIV-infection in Malawi. J Clin Virol. 2010;49:299–302. doi: 10.1016/j.jcv.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Patton JC, Sherman GG, Coovadia AH, Stevens WS, Meyers TM. Ultrasensitive human immunodeficiency virus type 1 p24 antigen assay modified for use on dried whole-blood spots as a reliable, affordable test for infant diagnosis. Clin Vaccine Immunol. 2006;13:152–155. doi: 10.1128/CVI.13.1.152-155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kebe K, Ndiaye O, Diop Ndiaye H, Mbakob Mengue P, Guindo PMM, Diallo S, Leye N, Gueye SB, Gaye Diallo A, Toure Kane C, Mboup S. RNA versus DNA (NucliSENS EasyQ HIV-1 v1.2 versus Amplicor HIV-1 DNA test v1.5) for early diagnosis of HIV-1 infection in infants in Senegal. J Clin Microbiol. 2011;49:2590–2593. doi: 10.1128/JCM.02402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kerr RJ, Player G, Fiscus SA, Nelson JA. Qualitative human immunodeficiency virus RNA analysis of dried blood spots for diagnosis of infections in infants. J Clin Microbiol. 2009;47:220–222. doi: 10.1128/JCM.01521-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nugent CT, Dockter J, Bernardin F, Hecht R, Smith D, Delwart E, Pilcher C, Richman D, Busch M, Giachetti C. Detection of HIV-1 in alternative specimen types using the APTIMA HIV-1 RNA Qualitative Assay. J Virol Methods 159. 2009:10–14. doi: 10.1016/j.jviromet.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burke DS, Chatiyanonda K, Anandrik S. Improved surveillance of Japanese encephalitis by detection of virus-specific IgM in desiccated blood specimens. Bull World Health Organ. 1985;63:1037–1042. [PMC free article] [PubMed] [Google Scholar]
- 162.Long GW, Fries L, Watt GH, Hoffman SL. Polymerase chain reaction amplification from Plasmodium falciparum on dried blood spots. Am J Trop Med Hyg. 1995;52:344–346. doi: 10.4269/ajtmh.1995.52.344. [DOI] [PubMed] [Google Scholar]
- 163.Singh B, Cox-Singh J, Miller AO, Abdullah MS, Snounou G, Rahman HA. Detection of malaria in Malaysia by nested polymerase chain reaction amplification of dried blood spots on filter papers. Trans R Soc Trop Med Hyg. 1996;90:519–521. doi: 10.1016/s0035-9203(96)90302-8. [DOI] [PubMed] [Google Scholar]
- 164.Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg. 1999;60:687–692. doi: 10.4269/ajtmh.1999.60.687. [DOI] [PubMed] [Google Scholar]
- 165.Tham JM, Lee SH, Tan TM, Ting RC, Kara UA. Detection and species determination of malaria parasites by PCR: comparison with microscopy and with ParaSight-F and ICT malaria Pf tests in a clinical environment. J Clin Microbiol. 1999;37:1269–1273. doi: 10.1128/jcm.37.5.1269-1273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mangold KA, Manson RU, Koay ESC, Stephens L, Regner M, Thomson RB, Peterson LR, Kaul KL. Real-Time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–2440. doi: 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Santhanam S, Kumar H, Sethumadhavan KV, Chandrasekharan A, Jain DC, Malhotra A, Ghosh TK, Weil GJ. Detection of Wuchereria bancrofti antigen in serum and finger prick blood samples by enzyme immunoassay: field evaluation. Trop Med Parasitol. 1989;40:440–444. [PubMed] [Google Scholar]
- 168.Tang THT, Lopez-Velez R, Lanza M, Shelley AJ, Rubio JM, Luz SLB. Nested PCR to detect and distinguish the sympatric filarial species Onchocerca volvulus, Mansonella ozzardi and Mansonella perstans in the Amazon Region. Mem Inst Oswaldo Cruz. 2010;105:823–828. doi: 10.1590/s0074-02762010000600016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.