Abstract
Opportunistic diseases cause substantial morbidity and mortality to human immunodeficiency virus (HIV)-infected patients. Highly active antiretroviral therapy (HAART) leading to immune reconstitution is the most effective treatment of preventing opportunistic diseases. This retrospective study established an epidemiologic profile of opportunistic diseases 10 years after the introduction of HAART. The HIV antiretroviral therapy-naive patients matching inclusion criteria were included. The primary outcome was the prevalence of opportunistic diseases. From January 1, 2002 to September 30, 2010, 654 opportunistic diseases were identified in 458 patients. Pulmonary tuberculosis, herpes zoster, cerebral toxoplasmosis, oral candidiasis, and severe pneumonia accounted for 22.05%, 15.94%, 14.19%, 14.19%, and 9.39%, respectively. Cryptococcal meningitis and pneumocystosis accounted for 0.44% and 0.21%, respectively. The prevalence of opportunistic diseases in Gabon remains high. New guidelines emphasize the importance of initiating antiretroviral therapy early to reconstitute the immune system, and reduce disease risk, and treat the primary opportunistic infection of pulmonary tuberculosis.
Introduction
Human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) is a major public health concern in sub-Saharan Africa, where 70% of the 33 million people estimated to be infected by HIV in 2009 reside. Between 2002 and 2020, ∼68 million people will die of AIDS in the 45 countries most affected.1 Over the past 10 years in Gabon where the prevalence of HIV/AIDS has been ∼5.9%, the government has established a countrywide program involving ambulatory treatment centers to confront the challenges of HIV/AIDS. This program has included the introduction of highly active antiretroviral therapy (HAART) in 2001 as a standard of care.
Opportunistic diseases (ODs) are a heterogeneous group of diseases, infections, and malignancies that result in significant morbidity and mortality in HIV-infected individuals. Progressive deterioration of the immune system caused by a decline in CD4 + T cells is the main risk factor for the development of ODs. In developed countries, the introduction of HAART, which halts then reverses the decline in CD4 + T cell counts, has dramatically reduced the incidence of ODs among people living with HIV/AIDS (PLHIV/AIDS).2 There is little data concerning the epidemiologic profile of ODs in Gabon. Between 1992 and 1998, 3 years before the introduction of HAART, the authors conducted two retrospective studies to establish the frequency of ODs in the Infectious Diseases Unit of the Jeanne Ebori Foundation in Libreville, Gabon, where the majority of HIV-infected patients were managed. Pulmonary tuberculosis was the most frequent OD, responsible for 12% of all admissions.3,4 To improve diagnosis, prevention, and treatment of ODs patients, a retrospective study was conducted to establish an epidemiologic profile of ODs in PLHIV/AIDS 10 years after the introduction of HAART.
Methods
The authors conducted a retrospective study between January 1, 2002 and September 30, 2010 at the Infectious Diseases Unit, Fondation Jeanne Ebori Hospital, and the main center managing PLHIV/AIDS in Libreville, Gabon. Libreville is the capital of Gabon with an estimated population of 578,136 (one-third of the national population). There are an estimated 63,903 PLVIH in the country and the unit sees about 800 HIV-infected patients annually. The study was conducted according to good clinical practice guidelines and was approved by the National Ethics Committee and the Faculty of Medicine and Health Sciences of Libreville, Gabon (Medical Doctorate Thesis 718, 899, 2012).
Included were HIV-positive in- and outpatients matching the following criteria: the ability to follow-up at the study site, the presence of HIV ART-naive patients, and availability of medical records. The HAART was initiated according to national guidelines. Data on patients regarding demographics, clinical information, laboratory features, HAART regimen, and use of co-trimoxazole prophylaxis were collected using a standardized case report form. Confidentiality of data was guaranteed using anonymous identification codes. The primary outcome of interest was relative prevalence of ODs clinically diagnosed according to the presence of the following criteria: pruritic papular eruptions, superficial fungal infections, peripheral neuropathy, cutaneous herpes simplex, seborrheic dermatitis, herpes zoster, oral candidiasis, vulvovaginal candidiasis, and myositis. Pulmonary tuberculosis was diagnosed either on the basis of one or more sputum-positive samples for acid-fast bacilli on smear and/or culture with a compatible clinical assessment or by physical examination, chest-x-ray (CXR), and negative sputum microscopy in cases with a compatible medical history but with a clear response to anti-tuberculosis treatment. Extrapulmonary tuberculosis was confirmed by histological and/or microbiological findings of tissue and/or fluids from the suspected sites of infection. Cerebral toxoplasmosis was diagnosed based on computed tomography scan findings for positive toxoplasmosis serology or successful presumptive treatment. Community-acquired pneumonia was diagnosed based on medical history, physical examination, and CXR with or without a microbiological diagnosis.
Non-typhoidal salmonellosis was diagnosed based on positive cultures and esophageal candidiasis was diagnosed endoscopically. Furthermore, microsporidiosis and isosporiasis were confirmed by positive stool examination, Kaposi's sarcoma was confirmed by histological assessment of a biopsy and pneumocystosis was based on high clinical suspicion and compatible CXR, with or without positive examination of sputum induced by inhalation of hypertonic saline solution. Bronchoalveolar lavage was rarely performed. Cryptococcal meningitis was confirmed by microscopic examination of cerebrospinal fluid or by positive serum or cerebrospinal fluid cryptococcal antigen. Extrapulmonary cryptococcosis was confirmed by microbiological findings of tissue and/or fluids from the suspected site of infection. In addition, immunological failure was defined by a reduction in CD4 T-cell count compared with initial count. There was a 50% reduction compared with peak CD4 T-cell count and persistence of CD4 T cells < 100/mm3 6 months after HAART was initiated despite good treatment adherence. The HIV viral load was not performed. Finally, clinical failure was defined by at least one of the following events: clinical deterioration, occurrence of ODs, non-AIDS defining malignancies, and death. Statistical analyses were performed using Epi Info version 3.5.1.5 Variables were compared using χ2 and Fisher's exact tests as appropriate. Statistical significance was determined using a conventional P level of 0.05.
Results
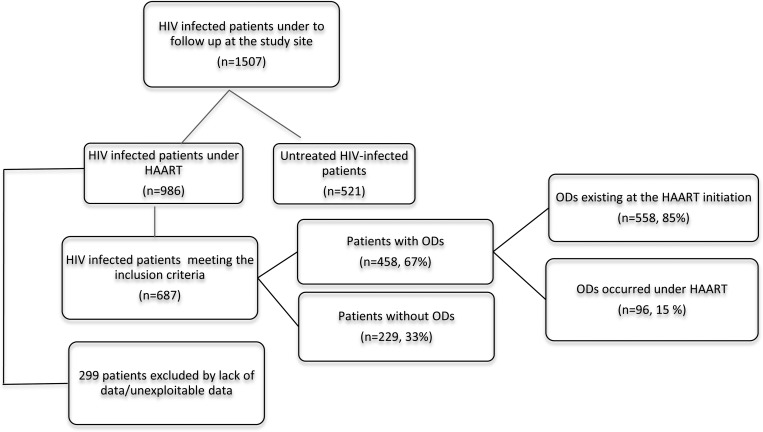
Between January 2002 and September 2010, 1507 HIV-infected patients were admitted for follow-up to the unit. Six hundred and eighty-seven patients (45.6%) matched inclusion criteria (Figure 1). Mean duration of follow-up was 2.3 years. Multi-partner sex was the most common risk factor for HIV infection and was observed in 79% of cases. Median age was 44 (27–51) years and male/female ratio was 0.75. The HIV-1 mono-infection was found in 98.5% of cases. The HIV-1 and HIV-2 co-infections accounted for 1.5% of admitted patients. Hepatitis B serology was performed in 24% of patients and HBsAg was positive in 19 of 165 (11.5%) cases. Co-trimoxazole prophylaxis for the prevention of infectious ODs was prescribed in 51.3% of patients with ODs (Tables 1 and 2); a combination of zidovudine + lamivudine + efavirenz was used in 39% of cases and stavudine + lamivudine + efavirenz in 28% of cases.
Figure 1.
Profile of the study population.
Table 1.
Factors associated with ODs in the study population*
| Variables | Patients with ODs (N = 458) | Patients without ODs (N = 229) | P |
|---|---|---|---|
| Sex-ratio (M/F) | 215/243 | 81/148 | 0.0039 |
| Age brackets (years) | 0.7109 | ||
| 0–19 | 13 | 8 | |
| 20–49 | 280 | 145 | |
| > 50 | 165 | 76 | |
| Circumstances of diagnosis | 0.0000 | ||
| Clinical suspicion | 369 | 128 | |
| Voluntary HIV counseling and testing | 53 | 69 | |
| Others | 36 | 32 | |
| Observance of treatment | 0.0289 | ||
| 100–95% | 60 | 41 | |
| 94–80% | 116 | 71 | |
| < 80% | 282 | 117 | |
| Biological findings | 0.0349 | ||
| Hemoglobin levels ≤ 10 g/dL | 117 | 42 | |
| Hemoglobin levels > 10 g/dL | 341 | 187 | |
| CDC classification system for HIV | 0.00001 | ||
| Category A | 119 | 97 | |
| Categories B and C | 339 | 132 | |
| Baseline CD4 cells count (/mm3) | 0.0000 | ||
| ≤ 200 | 273 | 69 | |
| > 200 | 185 | 160 | |
| Hepatitis co-infections | |||
| Hepatitis B: positive/negative HBsAg | 15/106 | 4/40 | 0.5575 |
| Hepatitis C: positive/negative serology | 2/65 | 2/33 | 0.5023 |
| Outcome | 0.0110 | ||
| Cotrimoxazole prophylaxis | 235 (51.3%) | 125 (54.58%) | 0.4 |
| Died | 6 | 0 | |
| Survived | 173 | 110 | |
| Missing outcome | 279 | 119 |
ODs = opportunistic diseases; CDC = Centers for Disease Control and Prevention; HIV = human immunodeficiency virus;
Table 2.
Prevalence of opportunistic diseases (ODs) among the 458 patients (over the 687 included) who presented one or more episode(s) of ODs*
| Our study (2002–2010) | Okome-Nkoumou and others 20003 (1992–1996) | Okome-Nkoumou 20064 (1994–1998) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Opportunistic diseases | Total (n) | Percentage (n/654 ODs) | Prevalence (n/458 patients) | Number (n) | Percentage (n/380 ODs) | Prevalence (n/351) | Number (n) | Percentage (n/221 ODs) | Prevalence (n/457 patients) |
| Bacterial infections | 194 | 29.66% | 42.35% | 117 | 30.78% | 33.33% | 138 | 62.44% | 30.20% |
| Pulmonary tuberculosis | 101 | 15.44% | 22.05% | 43 | 11.31% | 12.26% | 53 | 23.98% | 11.60% |
| Extrapulmonary tuberculosis | 13 | 1.98% | 2.84% | 8 | 2.10% | 2.27% | |||
| Severe community acquired pneumonia | 43 | 6.58% | 9.39% | 2 | 0.53% | 0.6% | |||
| Nontyphoidal salmonellosis | 37 | 5.66% | 8.07% | 64 | 16.84% | 18.2% | 49 | 22.17% | 10.72% |
| Bacteremia | 36 | 16.29% | 7.88% | ||||||
| Viral infections | 80 | 12.23% | 17.47% | 65 | 17.10% | 18.5% | 23 | 10.41% | 5.03% |
| Herpes zoster | 73 | 11.16% | 15.94% | 65 | 17.10% | 18.5% | 22 | 9.95% | 4.81% |
| Chronic cutaneous herpes simplex | 7 | 1.07% | 1.53% | 1 | 0.46% | 0.22% | |||
| Fungal infections | 112 | 17.12% | 24.45% | 130 | 34.21% | 37% | 22 | 9.95% | 4.81% |
| Oral candidiasis | 65 | 9.94% | 14.19% | 105 | 27.63% | 30% | 22 | 9.95% | 4.81% |
| Vulvovaginal candidiasis | 4 | 0.61% | 0.87% | ||||||
| Esophageal candidiasis | 38 | 5.81% | 8.30% | 25 | 6.58% | 7% | |||
| Severe superficial mycoses | 5 | 0.76% | 1.09% | ||||||
| Protozoal infections | 81 | 12.38% | 17.68% | 16 | 4.21% | 4.6% | 17 | 7.69% | 3.72% |
| Cerebral toxoplasmosis | 65 | 9.94% | 14.19% | 8 | 2.10% | 2.3% | 10 | 4.52% | 2.19% |
| Intestinal microsporidiosis | 11 | 1.68% | 2.40% | 1 | 0.46% | 0.22% | |||
| Intestinal isosporosis | 2 | 0.31% | 0.44% | 2 | 0.53% | 0.6% | 4 | 1.81% | 0.87% |
| Cryptococcal meningitis | 2 | 0.31% | 0.44% | 6 | 1.58% | 1.7% | 2 | 0.90 | 0.44% |
| Pneumocystosis | 1 | 0.14% | 0.21% | ||||||
| Maligancies | 9 | 1.37% | 1.96% | 22 | 5.79% | 6.3% | 9 | 4.07% | 1.97% |
| Kaposi's sarcoma | 9 | 1.37% | 1.96% | 22 | 5.79% | 6.3% | 9 | 4.07% | 1.97% |
| Other | 178 | 27.24% | 38.86% | 30 | 7.89% | 8.5% | 12 | 5.43% | 2.63% |
| Prurigo nodularis | 166 | 25.39% | 36.24% | 30 | 7.89% | 8.5% | 12 | 5.43% | 2.63% |
| Severe seborrheic dermatitis | 5 | 0.77% | 1.09% | ||||||
| Peripheral neuropathy | 3 | 0.46% | 0.65% | ||||||
| Myosistis | 4 | 0.62% | 0.87% | ||||||
| Total | 654 | 100% | |||||||
The two similar Gabonese previous studies are included.
Of 654 patients diagnosed with ODs (558 before and 96 following HAART), 458 were identified (1.4 OD/patient) (Table 2). Between 2002 and 2010, the annual incidence of OD remained high, with peaks in 2003 (81 cases) and 2008 (67 cases). Among patients with infectious ODs, 22.05% were the result of pulmonary tuberculosis, 15.94% caused by herpes zoster, 14.19% caused by cerebral toxoplasmosis, 14.19% as a result of oral candidiasis, and 9.39% caused by severe community-acquired pneumonia. Cryptococcal meningitis and pneumocystosis were rare, accounting for only 0.44% and 0.21% of patients, respectively. Prevalence of pruritic papular eruption was 36.24% and Kaposi's sarcoma 1.96%. Lymphomas were not reported (Table 2). The HAART led to a median increase of 494/mm3 CD4 cells. Six patients died. The percentage of missing outcome data was high (61%).
Discussion
The HAART was introduced in Gabon in 2001 and consequently between 2001 and 2009, the rate of HIV infection decreased by 25%.1 The authors of this study conducted a retrospective review during that period with the objective of describing the spectrum of ODs in the management of HIV-infected patients in the referral unit in Libreville, Gabon. The authors previously conducted two retrospective studies in Libreville to evaluate the prevalence of ODs in a population of HIV-infected patients (Table 2).
Pulmonary tuberculosis is a leading cause of death in HIV-infected patients.6 As a result, pulmonary tuberculosis was found to be a common illness in HIV-infected patients receiving HAART in Libreville, Gabon, and was associated with a high prevalence (22.05%). A similar prevalence of 26.1% was found in Bamako, Mali, in a population of 115 HIV-infected patients of which 91.3% had a prevalence of < CD4 T cells of 200/mm3 between January 2004 and December 2005.7
In terms of prevalence, non-typhoidal salmonellosis was the third bacterial OD found in the current study with a prevalence of 8.07 versus 18.2% between 1992 and 19963 and 8.07 versus 10.72% between 1994 and 1998.4 The HIV infection with advanced immunosuppression is a major risk factor for invasive non-typhoidal salmonellosis disease in Africa.8 Salmonella enterica serovar, Salmonella typhimurim, and Salmonella enterica serovar enteritidis are the leading causes of non-typhoidal salmonellosis in Africa. The rapid emergence of multidrug-resistant strains can make treatment difficult and recurrence of invasive non-typhoidal is common.8 Third generation cephalosporins and fluoroquinolones are the most effective treatments but remain expensive for the sub-Saharan African population.
Herpes zoster was the most frequent viral opportunistic infection found in the current study with a prevalence of 15.94%. This prevalence was higher in Gabon between 1992 and 1996 (18.5%)3 and lower between 1994 and 1998 (4.81%).4 In a Malian study, a low prevalence of 2.6% was found.7 Cerebral toxoplasmosis is a major cause of morbidity and mortality, especially in resource-poor settings. It is also a common neurological complication in some countries despite the availability of HAART and effective prophylaxis. Toxoplasmosis has been historically considered one of the most important opportunistic infections detected in HIV/AIDS patients. Prevalence has been found to be related to ethnicity, certain risk factors, and reactivation of toxoplasmosis. The current study found a prevalence of 14.19% versus 2.19% between 1994 and 19984; this increase was probably the result of better access to cerebral imaging.
The HIV-related cryptococcal meningitis remains a major opportunistic infection in sub-Saharan Africa and is responsible for 13–44% of all deaths in HIV-infected patients.9 Most episodes of cryptococcal meningitis in HIV-infected patients occur during deep immunosuppression (CD4 cell counts < 100/mm3) and are probably the result of reactivation of latent infections.10 The prevalence of cryptococcal infection in the current study was 0.44%. These results were similar to the authors' two previous studies that showed a prevalence of 0.44–1.7%3,4; similarly, a weak prevalence of cryptococcal meningitis has been reported in studies in Mali7 and Ivory Coast,11 accounting for 3.1% and 2.5% of ODs, respectively.
Kaposi's sarcoma is the most common malignancy affecting HIV-infected patients with a total incidence in cohorts of HIV-infected patients ranging from 25–35%.12,13 Moreover, Kaposi's sarcoma may be associated with an increased risk of developing ODs and showing shorter durations from HIV seroconversion to the first AIDS-defining event.14 The prevalence of Kaposi's sarcoma in the current study was 1.96%, which is comparable to the pre-HAART era in Libreville, Gabon. Kaposi's sarcoma might have been underreported caused by a lack of available tools for the diagnosis of extracutaneous manifestations.
The current study had several limitations. First, there were a high number of patients lost to follow-up (61% of patients with ODs) and missing data as a result of the retrospective design that might have impaired the incidence of OD and outcome analyses. Furthermore, considering the malignancies involved (AIDS- and non-AIDS-defining cancers), the current study reported only nine cases of Kaposi's sarcoma. These findings could be explained by a dysfunctional Gabonese health care system and lack of diagnostic tools as well as high mortality rates associated with theses untreated diseases. These findings could also explain the absence of disseminated Mycobacterium avium complex and cytomegalovirus infections. Finally, the current study was not designed to directly measure the impacts of HAART on OD incidence nor of immune reconstitution inflammatory syndrome on OD incidence.
Conclusions
Gabon remains an area of high prevalence of HIV/AIDS infection with a national seroprevalence of 5.9% (7.1% in Libreville). The ODs remain a major health concern in sub-Saharan Africa where limitations in medical resources result in diagnostic and treatment obstacles. Immune restoration under HAART represents the most effective preventive treatment.
Almost 10 years after the deployment of HAART, the overall prevalence of ODs in Gabon remains high: two-thirds of patients presented with ODs during follow-up. However, the landscape has changed, particularly regarding infectious ODs. Multidisciplinary approaches including the deployment of HAART are necessary for reducing the effect of ODs on patients with HIV/AIDS. Efforts require the development of more effective programs to help identify patients earlier and link and include them as part of appropriate and effective care approaches. An effective team of health care professionals must be made available to deliver high quality care once patients with HIV are identified and persuaded to seek treatment. Future studies are needed to evaluate the impact of HAART on HIV/AIDS epidemic in Gabon.
Footnotes
Authors' addresses: Madeleine Okome-Nkoumou, Vincent Guiyedi, Magloire Ondounda, Nora Efire, and Mireille Dibo, Faculty of Medicine and Health Sciences, University of Health Sciences - Infectious Diseases and Tropical Medicine, Libreville, Gabon, E-mails: okomenkoumou@gmail.com, guidyvin@hotmail.com, magondounda@yahoo.fr, nora_sylvana@yahoo.fr, and mireillemys81@yahoo.fr. Philippe Clevenbergh, Union's office in Myanmar - Infectious Diseases, Naypyidaw, Myanmar, E-mail: pclevenbergh@theunion.org. Arnaud Dzeing-Ella, Centre Hospitalier de Denain, Service des Maladies Infectieuses et Tropicales, 25 bis rue Jean Jaurès, 59723 Denain cedex, France, E-mails: dzeingarnaud@yahoo.fr or dzeingarnaud@gmail.com.
References
- 1.UNAIDS Global Report: UNAIDS Report on the Global AIDS Epidemic 2010. 2010. http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf Available at. Accessed July 8, 2011.
- 2.Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, Hogg E, Komarow L. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS ONE. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okome-Nkoumou M, Mbounja-Loclo ME, Kombila M. Spectrum of opportunistic infections in subjects infected with HIV at Libreville, Gabon. Sante. 2000;10:329–337. [PubMed] [Google Scholar]
- 4.Okome-Nkoumou M, Boguikouma JB, Kombila M. Opportunistic diseases in HIV-infected patients at the Jeanne Ebori Foundation in Libreville, Gabon. Med Trop. 2006;66:167–171. [PubMed] [Google Scholar]
- 5.CDC Epi Info. http://wwwn.cdc.gov/epiinfo/ Available at. Accessed February 15, 2012.
- 6.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 7.Oumar AA, Diallo S, Kaba MK, Cisse IA, Tounkara A. Prevalence des infections opportunistes au cours du SIDA en milieu hospitalier de Bamako, Mali. Louv Med. 2008;127:12–17. [Google Scholar]
- 8.Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux E, Zijlstra EE, Heyderman RS, Hart CA, Molyneux ME. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46:963–969. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21:2119–2129. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol. 1999;37:3204–3209. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant AD, Djomand G, Smets P, Kadio A, Coulibaly M, Kakou A, Maurice C, Whitaker JP, Sylla-Koko F, Bonard D, Wiktor SZ, Hayes RJ, De Cock KM, Greenberg AE. Profound immunosuppression across the spectrum of opportunistic disease among hospitalized HIV-infected adults in Abidjan, Cote d'Ivoire. AIDS. 1997;11:1357–1364. doi: 10.1097/00002030-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Lundgren JD, Melbye M, Pedersen C, Rosenberg PS, Gerstoft J. Changing patterns of Kaposi's sarcoma in Danish acquired immunodeficiency syndrome patients with complete follow-up. The Danish Study Group for HIV Infection (DASHI) Am J Epidemiol. 1995;141:652–658. doi: 10.1093/oxfordjournals.aje.a117481. [DOI] [PubMed] [Google Scholar]
- 13.Peters BS, Beck EJ, Coleman DG, Wadsworth MJ, Mcguinness O, Harris JR, Pinching AJ. Changing disease patterns in patients with AIDS in a referral centre in the United Kingdom: the changing face of AIDS. BMJ. 1991;302:203–207. doi: 10.1136/bmj.302.6770.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lifson AR, Darrow WW, Hessol NA, O'Malley PM, Barnhart JL, Jaffe HW, Rutherford GW. Kaposi's sarcoma in a cohort of homosexual and bisexual men. Epidemiology and analysis for cofactors. Am J Epidemiol. 1990;131:221–231. doi: 10.1093/oxfordjournals.aje.a115492. [DOI] [PubMed] [Google Scholar]