Abstract
We present health and demographic surveillance system data to assess associations with health care utilization and human immunodeficiency virus (HIV) service receipt in a high HIV prevalence area of western Kenya. Eighty-six percent of 15,302 residents indicated a facility/clinician for routine medical services; 60% reported active (within the past year) attendance. Only 34% reported a previous HIV test, and self-reported HIV prevalence was 6%. Active attendees lived only slightly closer to their reported service site (2.8 versus 3.1 km; P < 0.001) compared with inactive attendees. Multivariate analysis showed that younger respondents (< 30 years of age) and active and inactive attendees were more likely to report an HIV test compared with non-attendees; men were less likely to report HIV testing. Despite traveling farther for HIV services (median distance = 4.4 km), 77% of those disclosing HIV infection reported HIV care enrollment. Men and younger respondents were less likely to enroll in HIV care. Socioeconomic status was not associated with HIV service use. Distance did not appear to be the major barrier to service receipt. The health and demographic surveillance system data identified patterns of service use that are useful for future program planning.
Introduction
Understanding local population health care use characteristics are important in order to strategically initiate or scale-up healthcare services and allocate financial and human resources efficiently. Geographic accessibility, travel time, waiting time, age, health status, income, service costs, and education are factors shown to be associated with health care use1–9 and improvements in facility physical infrastructure, staffing, and commodity availability and implementation of free high-quality healthcare have demonstrated increases in health care utilization.10–13 Routine national surveys tend to collect data on individuals' general health care use but often lack specific data on the healthcare service location. Spatial data provide opportunities to examine geographic factors associated with healthcare facility selection.
As the global human immunodeficiency virus (HIV) pandemic enters its fourth decade, recent surveys in many affected countries in Africa have shown substantial increases in the proportion of adults that are aware of HIV, familiar with its transmission routes, and knowledgeable as to the benefits of receiving HIV care services, including antiretroviral treatment (ART).14–19 The once seemingly insurmountable logistical and financial barriers of the late 1990s and early 2000s to knowing one's HIV status and receipt of HIV clinical services have greatly decreased with the development and distribution of inexpensive rapid HIV test kits, the availability of generic antiretroviral drugs, and financial and technical support for commodity management systems, infrastructure, and human resources through international donors, (e.g., the President's Emergency Plan for AIDS Relief and the Global Fund) However, despite these advances and the increasing availability of free, quality HIV services, HIV health care utilization remains suboptimal; most country adult testing rates remain below 40%,16,18,20 and programs are challenged to increase early uptake of HIV care and ART services among HIV-infected individuals to control and reduce the spread of acquired immunodeficiency syndrome (AIDS).
Demographic and spatial factors associated with persons' health care utilization and access to HIV services can provide insight into a population's health-seeking behavior and inform future program planning and service delivery. We present an analysis of population-based survey data to assess geographic and sociodemographic indicators associated with access to healthcare services collected as part of community preparatory work prior to the initiation of community home-based HIV counseling and testing (HBCT) services.
Materials and Methods
Setting and study population.
Nyanza Province is a rural area of western Kenya characterized by high burdens of HIV (14.0–14.9% adult HIV seroprevalence), tuberculosis, and malaria.14,21–23 Since 2001, the Kenya Medical Research Institute (KEMRI) and the U.S. Centers for Disease Control and Prevention (CDC) have maintained a Health and Demographic Surveillance System (HDSS) among 220,000 residents in three districts. Household visits occur every four months to update sociodemographic and mortality data.24 Households and nearby facilities have been mapped and are updated annually by using a hand-held, differential global positioning system (Trimble Navigation Ltd., Sunnyvale, CA).25 All-cause adult mortality rates have decreased in recent years from 24.6/1,000 person-years in 2003–2004 to 16.3/1,000 person-years in 2007–2008, most likely caused by expansion of HIV services.26
By October 2007, approximately 100 Nyanza healthcare facilities were providing ART to 50,000 persons, and 10 of these facilities were located in the HDSS area. Most area healthcare facilities provided HIV testing services through stand-alone voluntary counseling and testing (VCT) centers (sVCT) or during clinical care activities, e.g., opt-out testing in antenatal care services, or through targeted provider-initiated testing and counseling (PITC) while providing other clinical services (e.g., tuberculosis treatment). HIV testing was also periodically available at mobile sites (mobile VCT [mVCT]) and conducted during designated government campaigns or through non-governmental organization activities.
The HDSS study protocol and all amendments, including cross-sectional surveys, are approved annually by the KEMRI Ethical Review Committee and the CDC Institutional Review Board. Informed consent is obtained from all adult participants and from parents or legal guardians of minors.
Data collection and analysis.
In April–December 2007, as part of preparatory activities prior to the launching of HBCT, we randomly selected 8,532 households and 21,886 adults (≥ 15 years of age) in two HDSS districts (Siaya and Gem) to receive a brief standardized interviewer-administered scannable paper questionnaire (TELEforms. Cardiff Software, Vista, CA) that captured self-reported health care use and access to HIV services. This sample was a 25% simple random sample of all HDSS households in these two districts. Informed consent was obtained from all participants, and interviews took place privately in an individual's home and collected information on location of general medical services, HIV testing history, self-reported HIV status and enrollment into HIV care. Survey data were merged with the larger HDSS longitudinal database.
Distances were calculated from household residence to health facility of reported use. Exact locations for health facilities outside the HDSS were unavailable, but were generally > 50 km and thus considered to be 50 km for analytic purposes. Socioeconomic status (SES) was evaluated by using the HDSS household SES index, which is calculated based on a weighted average of household assets using principal components analysis. This analysis provides a composite asset index score; this index is used to rank households into one of five quintiles ranging from lowest to highest. Respondents who received recent (< 12 months) service receipt from a cited medical facility were defined as active medical care attendees. Inactive medical care attendees reported > 12 months since service receipt, and non-attendees reported no medical facility services.
Descriptive statistics, including frequencies, percentages, medians, and ranges, were used to summarize the data. Differences were assessed using the chi-square test of independence (nominal variables) or the Wilcoxon rank sum test (continuous variables). Logistic regression was used to calculate unadjusted odds ratios (OR) and 95% confidence intervals (CIs) to describe the associations between respondent characteristics and dependent variables. To test associations with HIV testing status, variables were entered into a multiple logistic regression model using backward selection; persons reporting HIV-positive status were excluded to avoid any change in site of health care, which may have occurred after HIV diagnosis. A second model among HIV-positive persons tested factors associated with HIV care receipt also using backwards selection. SES was retained in both models to control for potential confounding and comparability purposes. For both models, we computed intraclass correlation coefficients for outcome variables to assess potential clustering by village. However, these correlation coefficients were extremely low (< 0.05). Therefore, generalized estimating equation models were not used in these analyses. Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
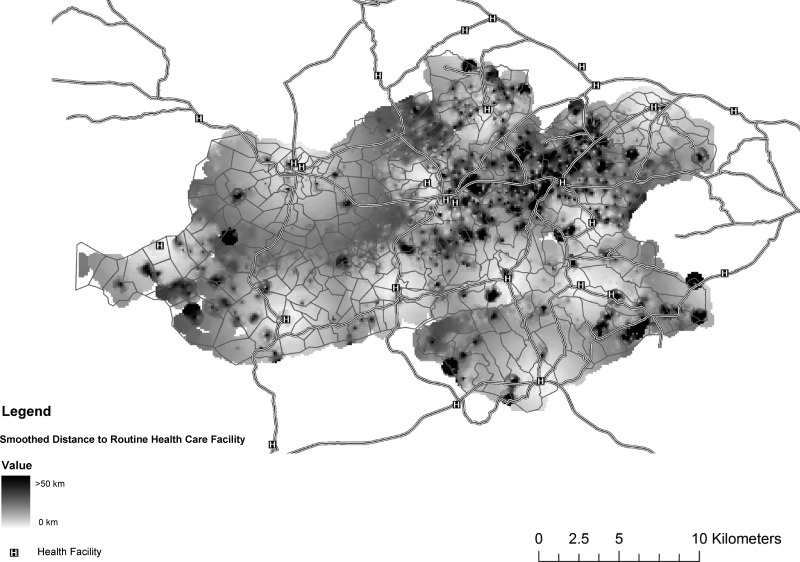
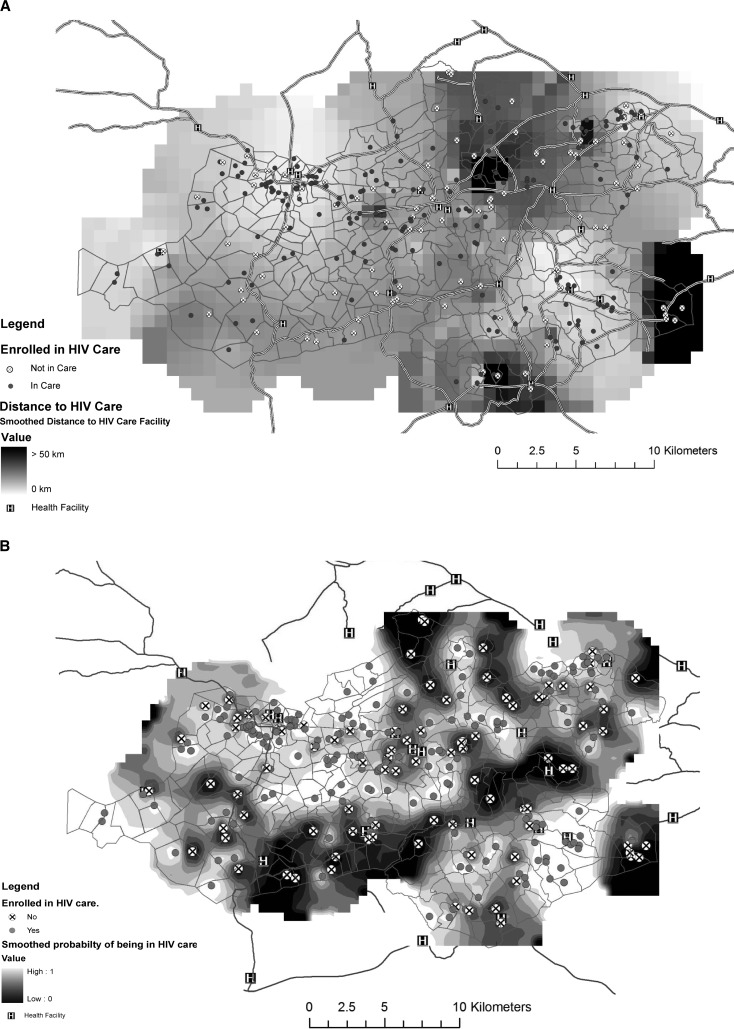
We used ArcGIS version 9.3 (ESRI, Redlands, CA) with the Spatial Analyst Extension to produce the map interpolations (Figures 1 and 4). The Inverse Density Weighting algorithm was used with quadratic weighting by the distance from the nearest neighbors to the interpolation pixel. All respondents within 1 km were used to interpolate the value of a point for distance to the nearest healthcare facility (Figure 1). For HIV care patients, respondents within 2 km were used to interpolate the values of points for the distance and probability of being in care maps (Figure 4).
Figure 1.
Travel distance for routine facility-based healthcare for health and demographic surveillance system residents, Nyanza Province, Kenya, 2007. (N = 11,268 residents with a mapped health facility)
Figure 4.
A, Travel distance for facility-based human immunodeficiency virus care for 246 health and demographic surveillance system residence, Nyanza Province, Kenya, 2007. B, Probability of being in HIV care among 321 self-reported HIV-positive respondents by HDSS residence, Nyanza Province, Kenya, 2007.
Results
Participants.
Overall, 15,302 (70%) of 21,883 selected persons were interviewed; 5,467 (25%) were not at home at the time of interview, 968 (4%) had migrated out of the area, 91 (0.4%) had died, and 55 refused to take part in the interview. Among the 15,302 respondents, 9,074 (59%) were female; median age was 35 years, range 15–104 years, and 53% were married (Table 1). Response rates were 63% and 76% for men and women, respectively. Respondents were more likely to be ≥ 30 years of age (59% versus 36%; P < 0.0001) and female (59% versus 44%; P < 0.0001) compared with non-respondents. In addition, female respondents were more likely to be older, married, or widowed; of lower SES; report higher use of healthcare services (overall and recently); and to reside slightly closer to their facility of routine care compared with their male respondent counterparts (Table 1).
Table 1.
Demographic profile and utilization of healthcare services by sex, Nyanza Province, Kenya
| Characteristic | Total, n = 15,302 (%) | Male, n = 6,228 (%) | Female, n = 9,074 (%) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age category, years | < 0.0001 | |||
| 15–20 | 3,372 (22) | 1,802 (29) | 1,570 (17) | |
| 21–30 | 3,104 (20) | 1,216 (20) | 1,888 (21) | |
| 31–40 | 2,364 (15) | 842 (14) | 1,522 (18) | |
| 41–50 | 2,119 (14) | 686 (11) | 1,433 (15) | |
| > 50 | 4,343 (28) | 1,682 (27) | 2,661 (29) | |
| Marital status | < 0.0001 | |||
| Married | 8,158 (53) | 3,198 (51) | 4,960 (61) | |
| Single | 5,779 (38) | 2,892 (46) | 2,887 (32) | |
| Widowed | 1,365 (9) | 138 (2) | 1,227 (14) | |
| Socioeconomic status quintiles | < 0.0001 | |||
| Lowest | 2,114 (15) | 707 (12) | 1,407 (16) | |
| Low | 2,471 (17) | 926 (16) | 1,545 (18) | |
| Middle | 2,777 (19) | 1,113 (19) | 1,664 (19) | |
| High | 3.228 (22) | 1,339 (23) | 1,889 (22) | |
| Highest | 3,774 (26) | 1,715 (30) | 2,059 (24) | |
| Healthcare use | ||||
| Accessed healthcare services/advice | 13,911 (91) | 5,541 (89) | 8,370 (92) | 0.001 |
| Reported use of medical facility/doctor | 13,228 (86) | 5,234 (84) | 7,994 (88) | < 0.0001 |
| Reported recent (< 12 months) medical care/advice | 9,138 (60) | 3,320 (53) | 5,818 (64) | < 0.0001 |
| Median distance from residence to healthcare facility of routine care (km) | 2.9 | 3.0 | 2.8 | 0.005 |
Health care utilization.
More than 90% of participants indicated a specific individual/location for routine healthcare services or advice, including a health facility, doctor, pharmacy, traditional healer. A total of 13,228 (86%) cited a medical facility or doctor; the median distance traveled for health facility care was 2.9 km (range 0.2–50 km) (Table 1 and Figure 1). A total of 9,138 (60%) respondents were active medical care attendees and were more likely to be female, younger, and slightly wealthier compared with inactive or non-attendees (Table 2). In addition, active medical attendees were more likely to be aware of HIV deaths in their village and be more receptive to and knowledgeable about HIV interventions. Compared with inactive medical attendees, active attendees were also slightly more likely to live closer to their routine healthcare facility.
Table 2.
Demographics, knowledge, and access to HIV testing services among 15,298 persons by health care utilization group, Nyanza Province, Kenya*
| Characteristic | Active medical care attendees, n = 9,138 (%) | Inactive medical care attendees (≥ 12 months), n = 4,083 (%) | Non-attendees, n = 2,077 (%) | P |
|---|---|---|---|---|
| Demographics | ||||
| Male sex | 3,320 (36) | 1,908 (47) | 996 (48) | < 0.0001 |
| < 30 years of age | 4,023 (44) | 1,530 (37) | 681 (33) | < 0.0001 |
| Married | 3,387 (37) | 1,591 (39) | 801 (38) | < 0.0001 |
| SES quintiles | n = 8,568 | n = 3,851 | n = 1,942 | < 0.0001 |
| Lowest | 1,097 (13) | 572 (15) | 444 (23) | |
| Low | 1,464 (17) | 669 (17) | 337 (17) | |
| Middle | 1,664 (19) | 759 (20) | 354 (18) | |
| High | 1,978 (23) | 855 (22) | 394 (20) | |
| Highest | 2,365 (28) | 996 (26) | 413 (21) | |
| Mapped healthcare facility | n = 7,713 | n = 3,549 | – | < 0.001 |
| Median distance from residence to healthcare facility of routine care (km) | 2.8 | 3.1 | ||
| HIV knowledge and perceptions | ||||
| Knew someone in village who died of HIV | 3,261 (36) | 1,303 (32) | 567 (27) | |
| Supports HIV testing for pregnant woman | n = 9,124 | n = 4,076 | n = 2,069 | < 0.0001 |
| Yes | 8,728 (96) | 3,819 (94) | 1,827 (88) | |
| Supports HIV testing for male partners of pregnant women | n = 9,119 | n = 4,072 | n = 2,067 | < 0.0001 |
| Yes | 8,390 (92) | 3,619 (89) | 1,698 (82) | |
| Would accept HBCT | n = 9,045 | n = 4,029 | n = 2,029 | < 0.0001 |
| Yes | 8,004 (88) | 3,466 (86) | 1,664 (82) | |
| Knows about ARV use in PMTCT | n = 9,110 | n = 4,077 | n = 2,063 | < 0.0001 |
| Yes | 6,040 (66) | 2,541 (62) | 1,120 (54) | |
| Would circumcise sons | n = 6,151 | n = 2,732 | n = 1,632 | < 0.0001 |
| Yes | 1,552 (25) | 599 (22) | 234 (17) | |
| Would get circumcised if circumcision were free (uncircumcised men only) | n = 3,007 | n = 1,717 | n = 897 | < 0.0001 |
| Yes | 966 (32) | 475 (28) | 176 (20) | |
| HIV testing | ||||
| Knew where to receive HIV testing | 7,536 (82) | 3,128 (77) | 1,311 (63) | < 0.0001 |
| Reported a previous HIV test | 3,815 (42) | 1,077 (26) | 328 (16) | < 0.0001 |
| Reported a positive HIV test result | 297 (8) | 18 (2) | 6 (2) | < 0.0001 |
| Location of testing | n = 3,761 | n = 1,071 | n = 317 | < 0.0001 |
| Nearby health facility | 1,860 (49) | 452 (42) | 95 (30) | |
| Nearby mobile VCT | 831 (22) | 297 (28) | 119 (38) | |
| Nearby stand-alone VCT | 113 (3) | 38 (4) | 16 (5) | |
| Far away health facility | 449 (12) | 131 (12) | 44 (14) | |
| Far away mobile VCT | 140 (4) | 58 (5) | 16 (5) | |
| Far away stand-alone VCT | 259 (7) | 62 (6) | 18 (6) | |
| Home | 109 (3) | 33 (3) | 9 (3) | |
| Timing of last HIV test (months) | n = 3,808 | n = 1,073 | n = 325 | < 0.0001 |
| < 12 | 2,721 (71) | 525 (49) | 186 (57) | |
| 12–23 | 692 (18) | 337 (31) | 84 (26) | |
| ≥ 24 | 395 (10) | 211 (20) | 55 (17) | |
| Reasons for never testing† | n = 5,310 | n = 3,001 | n = 1,747 | < 0.0001 |
| Unnecessary/no reason/too busy | 3,528 (61) | 1,961 (65) | 1,200 (69) | |
| Lack of knowledge (test or testing site) | 1,067 (20) | 638 (21) | 442 (25) | |
| Logistics (transport, cost, disabled) | 710 (13) | 377 (12) | 219 (12) | |
| Afraid of results/illness | 592 (11) | 264 (10) | 112 (6) | |
| Embarrassed | 341 (6) | 176 (6) | 81 (5) | |
| Stigma/confidentiality concerns | 143 (3) | 71 (2) | 34 (2) | |
Excluded four respondents who indicated a site of routine care, but did not indicate timing of last visit.
Exceeds 100% because some respondents may have indicated > 1 reasons for not testing.
HIV = human immunodeficiency virus; SES = socioeconomic status; HBCT = home-based counseling and testing; ARV = antiretroviral drug; PMTCT = prevention of mother to child transmission; VCT = voluntary counseling and testing.
HIV testing.
Despite well over half of each health care utilization group (active, inactive, or non-attendees) reporting knowing where to obtain HIV testing, only 5,220 (34%, 95% CI = 33–35%) had ever received an HIV test, and active medical attendees were significantly more likely to have been tested, have a positive result, and have been tested in the past 12 months (Table 2). In addition, although a health facility located near their place of residence was the most common location of HIV testing for active or inactive medical attendees, mVCT services were more frequently used by non-attendees. Reasons for not receiving HIV testing were similar across groups with the majority perceiving that testing was unnecessary, followed by a lack of knowledge about an aspect of the test/testing procedure, and logistical issues. Stigma constituted < 5% of the cited barriers by any use group.
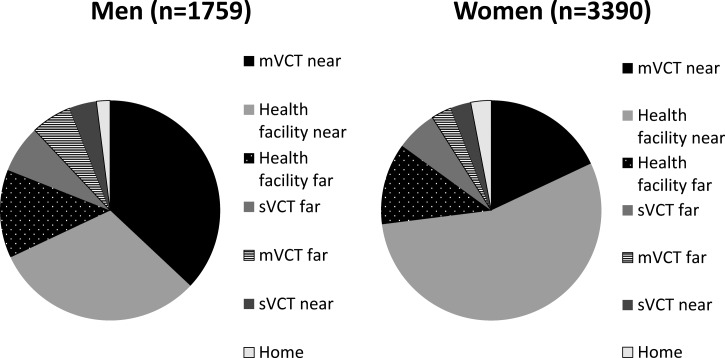
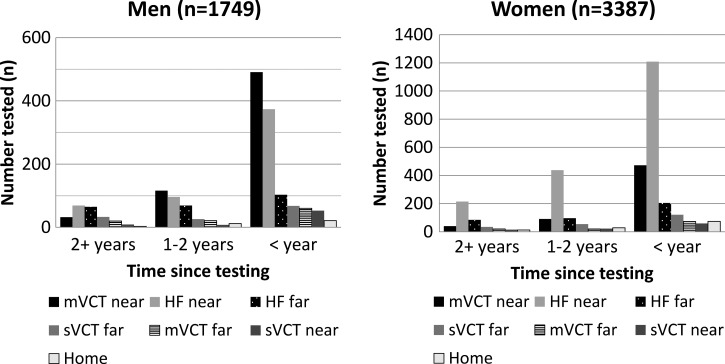
Nearby health facilities and mVCT were the most popular testing venues, but the location of HIV testing varied by sex, age, and duration of time since the last test. Among men who reported a HIV testing location, 644 (37%, 95% CI = 34–39%) tested through nearby mVCT services compared with only 603 (18%; 95% CI = 16–19%) of women testing through mVCT (Figure 2 ). More than half (55%, 95% CI = 53–57%) of women reported being tested at a nearby health facility. Most (3,432, 66%) persons tested were tested in the past year. Among the 5,136 persons reporting testing location and duration of time since the last test, the proportion reporting testing at nearby health facilities and nearby mVCT services increased from 12% and 6% > 2 years ago to 66% and 78%, respectively (Figure 3 ). Nearby sVCT services accounted for a small (approximately 3%) proportion of overall and annual HIV testing uptake, and the proportion of HIV testing (health facility, mVCT, and sVCT) received at faraway locations decreased over time to only 22% among those tested in the past year versus 42% for those tested > 2 years ago (OR = 0.63; 95% CI = 0.58–0.68, P < 0.0001).
Figure 2.
Location of human immunodeficiency virus testing among 5,149* tested health and demographic surveillance system residents, by sex, Nyanza Province, Kenya, 2007. *71 persons (29 men and 42 women) did not report a testing location. mVCT = mobile voluntary counseling and testing; sVCT = stand-alone voluntary counseling and testing.
Figure 3.
Human immunodeficiency virus test location and time of testing among 5,136* health and demographic surveillance system residents, by sex, Nyanza Province, Kenya. 2007. *Persons with testing location and timing of test data. mVCT = mobile voluntary counseling and testing; sVCT = stand-alone voluntary counseling and testing; HF = health facility.
In the multivariate model, persons < 30 years of age who were married, knew someone who died of HIV, knew where to receive HIV testing, and cited active/inactive medical care versus no care were significantly more likely to report receiving HIV testing (Table 3). Men and those widowed were significantly less likely to receive testing. SES and distance were not associated with receipt of HIV testing.
Table 3.
Univariate and multivariate associations with receipt of HIV testing among 14,961 HDSS respondents, Nyanza Province, Kenya*
| Variable | No. (%) tested | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | Adjusted OR (95% CI) | P | ||
| < 30 years of age | 2,494 (41) | 1.81 (1.70–1.95) | < 0.0001 | 2.18 (2.00–2.38) | < 0.0001 |
| Male sex | 1,693 (28) | 0.67 (0.62–0.72) | < 0.0001 | 0.57 (0.53–0.62) | < 0.0001 |
| Marital status | |||||
| Married | 3,020 (38) | 1.48 (1.37–1.59) | < 0.0001 | 1.76 (1.60–1.92) | < 0.0001 |
| Widowed | 220 (17) | 0.49 (0.42–0.57) | 0.79 (0.66–0.94) | ||
| Single | 1,659 (29) | 1.0 | 1.0 | ||
| Knew HIV-infected person who died in village | 2,014 (40) | 1.67 (1.56–1.80) | < 0.0001 | 1.51 (1.39–1.63) | < 0.0001 |
| Knew where to go for testing | 4,686 (40) | 9.77 (8.46–11.28) | < 0.0001 | 8.17 (7.05–9.46) | < 0.0001 |
| SES quintiles | |||||
| Lowest | 835 (28) | 0.74 (0.66–0.82) | < 0.0001 | 1.02 (0.91–1.15) | 0.43 |
| Low | 734 (31) | 0.84 (0.75–0.93) | 0.97 (0.86–1.10) | ||
| Middle | 940 (34) | 1.00 (0.90–1.11) | 1.06 (0.94–1.18) | ||
| High | 1,111 (35) | 1.04 (0.94–1.14) | 1.08 (0.97–1.20) | ||
| Highest | 1,279 (35) | 1.0 | 1.0 | ||
| Medical attendance | |||||
| Active | 3,615 (39) | 3.93 (3.38–4.57) | < 0.0001 | 2.64 (2.25–3.09) | < 0.0001 |
| Inactive | 1,070 (26) | 2.17 (1.85–2.55) | 1.65 (1.39–1.95) | ||
| Non-attendence | 214 (14) | 1.0 | 1.0 | ||
| Increasing distance (1 km) to site of routine medical care | 1.00 (0.99–1.01) | 0.34 | |||
Persons reporting an HIV-positive test result were excluded.
HDSS = health and demographic surveillance system.
HIV care.
A total of 4,987 (96%) of the 5,220 persons who reported previous HIV testing disclosed their results, yielding a 6% self-reported HIV prevalence. Among the 321 HIV-infected residents, the median age was 37 years, the age range was 16–77 years, 70% were female, 55% were married, and 15% were widowed. More than half (203, 63%) were given a diagnosis in the past year, and health facilities accounted for >70% of HIV testing/diagnosis for men and women.
At the time of the interview, 246 (77%) of the self-reported HIV-infected respondents reported receiving HIV care services, and 47% were receiving ART. These HIV-infected respondents traveled farther for their healthcare services compared with other respondents. They reported traveling a median of 4.4 km for HIV care (range = 0.13–50 km) and 3.6 km for routine care versus 2.8 km for routine care reported by other respondents (P < 0.0001). “One-stop shopping” was common; 195 (79%) respondents reported attending the same facility for routine and HIV care. This finding was reflected in higher use of routine services at the main district hospital by HIV-infected respondents compared with other respondents (47% versus 23%; P < 0.0001). HIV care coverage was relatively evenly distributed across the area with small pockets of low coverage through the mid-area and coincided with areas requiring greater travel distances to reach HIV care services (Figure 4). Surprisingly, among the 75 persons not enrolled in HIV care, 72 (96%) reported a healthcare facility for routine healthcare and 59 (79%) were active medical attendees.
In a multivariate model adjusting for age, sex, marital status, test date, disclosure, and SES, persons who received their HIV testing at a healthcare facility and who lived slightly farther from their reported facility for routine general care were more likely to be enrolled into HIV care (Table 4). Men and younger persons (< 30 years of age) were less likely to be enrolled in care. Marital status, SES, duration of time since HIV testing, and disclosure of test results were not associated with enrollment into care. Compared with women in care, men in care were more likely to be married (70% versus 47%; P = 0.007), > 30 years of age (90% versus 75%; P = 0.009), and have been tested > 24 months ago (25% versus 13%; P = 0.02). Although, men in care were also more likely to be receiving ART (58% versus 49%) compared with women, this finding was not statistically significant.
Table 4.
Univariate and multivariate associations of enrollment in HIV care among 321 self-reported HIV-infected HDSS respondents, Nyanza Province, Kenya
| Variable | No. in care (%) | OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|---|
| Male sex | 63 (66) | 0.46 (0.27–0.79) | 0.005 | 0.30 (0.16–0.56) | < 0.0001 |
| < 30 years of age | 52 (63) | 0.40 (0.23–0.70) | 0.001 | 0.30 (0.16–0.56) | < 0.0002 |
| Marital status | |||||
| Married | 130 (73) | 0.66 (0.36–1.22) | 0.18 | – | – |
| Widowed | 42 (82) | 1.14 (0.47–2.75) | |||
| Single | 74 (80) | 1.0 | |||
| SES quintiles | |||||
| Lowest | 49 (77) | 1.04 (0.47–2.34) | 0.88 | 1.02 (0.43–2.42) | 0.89 |
| Low | 55 (81) | 1.35 (0.59–3.09) | 1.42 (0.59–3.45) | ||
| Middle | 34 (72) | 0.84 (0.36–1.96) | 0.89 (0.35–2.22) | ||
| High | 58 (76) | 1.03 (0.48–2.23) | 1.06 (0.46–2.44) | ||
| Highest | 50 (76) | 1.0 | 1.0 | ||
| Timing of last HIV testing (months) | |||||
| < 12 | 157 (77) | 0.85 (0.40–1.84) | 0.69 | – | – |
| 12–23 | 49 (72) | 0.64 (0.27–1.54) | |||
| ≥ 24 | 40 (80) | 1.0 | |||
| HIV tested at healthcare facility | 179 (77) | 2.05 (1.17–3.59) | 0.01 | 2.70 (1.45–5.04) | 0.002 |
| Disclosed test results to someone | 179 (77) | 1.18 (0.67–2.08) | 0.56 | – | – |
| Knew someone who died of HIV in the village | 120 (77) | 1.03 (0.61–1.73) | 0.91 | – | – |
| Median distance from residence to healthcare facility of routine care (km) | 4.38 (in care); 2.47 (not in care) | 1.07 (1.06–1.16) | 0.06 | 1.10 (1.01–1.20) | 0.03 |
HDSS = health and demographic surveillance system.
Discussion
Our findings in this population-based survey show that HDSS residents' access to health services was high, as 86% of rural HDSS residents reported a medical facility or doctor for routine medical services, and 60% reported recent (<12 months) medical service. By using household and healthcare facility location data, we were able to determine residents' individual travel distances for reported routine and HIV care and evaluate distance for associations with access to general and HIV-specific clinical services. Although, women and active medical attendees were statistically more likely to live closer to their source of routine care, programmatically this difference was small, and neither logistic regression models nor map data indicated that increasing distance to health facility reduced the probability of receiving HIV testing or enrollment into care and treatment of area residents. SES was not associated with HIV testing receipt or enrollment in HIV care. These findings suggest that uptake of services may be largely influenced by individual perceptions of the importance of health care and knowledge as modified by the effects of age and sex.
Although, more than two-thirds of respondents knew where to get tested, only 34% reported a previous HIV test, a figure consistent with national statistics in 2007.21 Men were significantly less likely to report HIV testing and, if a result was positive, less likely to enroll in HIV care. These findings are consistent with other studies in Africa in which male HIV testing rates and enrollment figures consistently lag behind those of women.27–32 Although facility-based testing through antenatal clinics and other programs has contributed substantially to increasing women's HIV testing coverage, similar increases among men have not been noted because men constitute a smaller proportion of overall daily health facility attendance tending to only seek care when severely ill.33 In addition, providers may not emphasize the importance of testing to their male patients outside of high-probability diagnostic settings (tuberculosis clinics, in-patient wards), and many facility-based male testing initiatives are often promoted through antenatal care testing, which may be difficult logistically, socially, or culturally to attract large numbers of men.34
The main reason provided for not testing, regardless of health care utilization group, was that it was either unnecessary or the person was too busy. Thus, in our population, in which 84% of men reported a clinician or medical facility for routine medical care and 53% had received clinical services within the past 12 months, effective implementation of routine opt-out PITC through voluntary medical male circumcision programs and other outpatient clinical services could be a cost-effective strategy35 and substantially expand male testing coverage36 and uptake of HIV care and treatment services.37 Consequently, the complementary implementation of non-facility-based HIV testing strategies demonstrated to have strong linkage to care and treatment services (e.g., mVCT with point of care CD4 cell testing38 or incentivized testing39) could be more efficiently directed to male and younger populations not otherwise reached through general medical services.
Many HIV care attendees indicated the same district, sub-district, or other nearby facility for their general and HIV care. This preference for facilities that can provide HIV care and ART in addition to general care may explain why HIV care attendees reported traveling significantly farther for medical services (routine and HIV care) compared with other respondents. However, because these distance differences were relatively small and maps did not identify large pockets of underserved area, it does not appear that distance was a major barrier to enrollment in HIV care. Although other studies have indicated a significant distance-decay effect in health care use with increasing distance for pediatric sick visits,6 acute infections,1 pediatric immunizations,40 infant deliveries,41 and ART,42 it is unclear if this holds true for HIV services currently in this adult population and region. Kenya has rapidly decentralized HIV care services to lower level facilities, and although comprehensive HIV care services, including ART, are not available at all healthcare facilities, they are much more available than in prior years. Findings from our study and others43,44 suggest that slightly longer travel distances for HIV care in this geographic area may be perceived more as inconvenient as opposed to impossible.
Persons enrolled in HIV care were more likely to be female, older, and identified through healthcare facility–based HIV testing, suggesting that they may have been identified through routine PITC testing approaches or were infected earlier and had more advanced cases of HIV/AIDS at the time of diagnosis. Younger persons may be healthier, and healthier persons (perceived healthier or with a higher CD4 cell count) often fail to access HIV care and treatment services.32 Testing approaches (e.g., sVCT, mVCT, HBCT and self-testing) designed to appeal to men and younger and/or healthier persons that take place in nonmedical sites often lack inherent referral systems, facilitation, or follow-up to ensure HIV care and treatment enrollment for newly identified HIV-infected persons.45,46 Even an effective PITC program that expands testing coverage may not result in improved uptake in care and treatment services without effective referral systems.37,47,48
This study had a number of limitations. First, although population HIV prevalence was underestimated with an absolute difference of 5–8%44,49 in our survey sample that relied on self-report, we estimate that few persons may have known their HIV-positive status and chose not to disclose it. Unfortunately, most (85–88%) HIV-positive respondents44,49 would have never been tested and had undiagnosed HIV infection. Second, self-reporting of HIV status most likely also resulted in a higher proportion of HIV-positive persons reporting enrollment into HIV care because persons more comfortable in disclosing their status to an interviewer and/or others are probably more likely to enroll in care. In addition, social desirability bias may have influenced responses indicating enrollment in care and disclosure. Third, distances to healthcare facilities were missing for 15% of respondents and it is possible that those persons may have differed in other characteristics. However, we examined models with missing as a separate category and determined that these persons were not demographically different than those with distance available. Fourth, our 30% non-response rate limited analysis of adolescent or young adult data and the external validity of the data. Finally, because was a cross-sectional study, we can only identify associations of HIV services and cannot establish temporal sequence of events or causality.
Analysis of HDSS data provide important information about health care utilization patterns in this rural population, which could assist in the configuration and coordination of public health delivery systems to ensure maximum healthcare coverage and operational efficiencies and influence resource allocation decisions. For example, given that a substantial proportion of residents access area facilities, the provision of routine, quality facility-based HIV testing to all active medical care attendees could increase the proportion of the population tested to at least 70% within a year, optimize HIV care and ART enrollment, and link persons to other health programs (e.g., male circumcision, reproductive services). Area sVCT sites should be assessed to determine if they are cost-effective or if resources would be better directed towards the expansion of facility-based testing and other approaches specifically targeting population gaps in coverage. Repeated analysis of HDSS data over time would be useful to track and evaluate the effects of changes in resource allocation, thereby creating a feedback mechanism to inform subsequent funding, policy, personnel deployment, and infrastructure development decisions.
ACKNOWLEDGMENTS
We thank the HDSS residents for contributions to the study; the study staff for contributions and implementation of the questionnaire; and Vitalis Ogola, Amek Nyaguara, and Jan Moore for assistance with data collection, interpretation, graphics, and review and comments on the manuscript. KEMRI/CDC HDSS is a member of the INDEPTH Network. This manuscript is published with the approval of the Director of KEMRI. Data were partially presented at the Fifth International AIDS Conference on HIV Pathogenesis, Prevention, and Treatment, July 19–22, 2009, Cape Town, South Africa (abstract WEPED232).
Disclaimer: The findings and conclusions of this work are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This study was supported by the President's Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention under the terms of cooperative agreement no. 5U19CI000323-05.
Authors' addresses: Marta-Louise Ackers, Division of Global HIV/AIDS, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: mda6@cdc.gov. Allen Hightower (retired), E-mail: awh1953@gmail.com. David Obor, Lilian Ngere, and Kayla F. Laserson, Kenya Medical Research Institute/Centers for Disease Control and Prevention Research and Public Health Collaboration, Kisumu, Kenya, E-mails: dobor@kemricdc.org, lngere@kemricdc.org, and klaserson@cdc.gov. Peter Ofware, African Medical and Research Foundation, Nairobi, Kenya, E-mail: peter.ofware@amref.org.
References
- 1.Muller I, Smith T, Mellor S, Rare L, Genton B. The effect of distance from home on attendance at a small rural health centre in Papua New Guinea. Int J Epidemiol. 1998;27:878–884. doi: 10.1093/ije/27.5.878. [DOI] [PubMed] [Google Scholar]
- 2.Buor D. Analysing the primacy of distance in the utilization of health services in the Ahafo-Ano South district, Ghana. Int J Health Plann Manage. 2003;18:293–311. doi: 10.1002/hpm.729. [DOI] [PubMed] [Google Scholar]
- 3.Burton DC, Flannery B, Onyango B, Larson C, Alaii J, Zhang X, Hamel MJ, Breiman RF, Feikin DR. Healthcare-seeking behaviour for common infectious disease-related illnesses in rural Kenya: a community-based house-to-house survey. J Health Popul Nutr. 2011;29:61–70. doi: 10.3329/jhpn.v29i1.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danso-Appiah A, De Vlas SJ, Bosompem KM, Habbema JD. Determinants of health-seeking behaviour for schistosomiasis-related symptoms in the context of integrating schistosomiasis control within the regular health services in Ghana. Trop Med Int Health. 2004;9:784–794. doi: 10.1111/j.1365-3156.2004.01267.x. [DOI] [PubMed] [Google Scholar]
- 5.Danso-Appiah A, Stolk WA, Bosompem KM, Otchere J, Looman CW, Habbema JD, de Vlas SJ. Health seeking behaviour and utilization of health facilities for schistosomiasis-related symptoms in Ghana. PLoS Negl Trop Dis. 2010;4:e867. doi: 10.1371/journal.pntd.0000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feikin DR, Nguyen LM, Adazu K, Ombok M, Audi A, Slutsker L, Lindblade KA. The impact of distance of residence from a peripheral health facility on pediatric health utilisation in rural western Kenya. Trop Med Int Health. 2009;14:54–61. doi: 10.1111/j.1365-3156.2008.02193.x. [DOI] [PubMed] [Google Scholar]
- 7.Harris B, Goudge J, Ataguba JE, McIntyre D, Nxumalo N, Jikwana S, Chersich M. Inequities in access to health care in South Africa. J Public Health Policy. 2011;32((Suppl 1)):S102–S123. doi: 10.1057/jphp.2011.35. [DOI] [PubMed] [Google Scholar]
- 8.Nteta TP, Mokgatle-Nthabu M, Oguntibeju OO. Utilization of the primary health care services in the Tshwane Region of Gauteng Province, South Africa. PLoS ONE. 2010;5:e13909. doi: 10.1371/journal.pone.0013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao J, Murray AT, Agadjanian V, Hayford SR. Geographic influences on sexual and reproductive health service utilization in rural Mozambique. Appl Geogr. 2012;32:601–607. doi: 10.1016/j.apgeog.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson D, Gouws E, Sach M, Karim SS. Effect of removing user fees on attendance for curative and preventive primary health care services in rural South Africa. Bull World Health Organ. 2001;79:665–671. [PMC free article] [PubMed] [Google Scholar]
- 11.Xu K, Evans DB, Kadama P, Nabyonga J, Ogwal PO, Nabukhonzo P, Aguilar AM. Understanding the impact of eliminating user fees: utilization and catastrophic health expenditures in Uganda. Soc Sci Med. 2006;62:866–876. doi: 10.1016/j.socscimed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Bhattarai A, Ali AS, Kachur SP, Martensson A, Abbas AK, Khatib R, Al-Mafazy AW, Ramsan M, Rotllant G, Gerstenmaier JF, Molteni F, Abdulla S, Montgomery SM, Kaneko A, Bjorkman A. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgert CR, Bigogo G, Adazu K, Odhiambo F, Buehler J, Breiman RF, Laserson K, Hamel MJ, Feikin DR. Impact of implementation of free high-quality health care on health facility attendance by sick children in rural western Kenya. Trop Med Int Health. 2011;16:711–720. doi: 10.1111/j.1365-3156.2011.02752.x. [DOI] [PubMed] [Google Scholar]
- 14.Kenya National Bureau of Statistics (KNBS) and ICF Macro . Kenya Demographic and Health Survey 2008–09. Calverton, MD: ICF Macro; 2010. [Google Scholar]
- 15.Lifson AR, Demissie W, Tadesse A, Ketema K, May R, Yakob B, Metekia M, Slater L, Shenie T. HIV/AIDS stigma-associated attitudes in a rural Ethiopian community: characteristics, correlation with HIV knowledge and other factors, and implications for community intervention. BMC Int Health Hum Rights. 2012;12:6. doi: 10.1186/1472-698X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinreb AA, Stecklov G. Social inequality and HIV-testing: comparing home- and clinic-based testing in rural Malawi. Demogr Res. 2009;21:627–646. [Google Scholar]
- 17.de Beer IH, Gelderblom HC, Schellekens O, Gaeb E, van Rooy G, McNally A, Wit FW, Tobias Rde W. University students and HIV in Namibia: an HIV prevalence survey and a knowledge and attitude survey. J Int AIDS Soc. 2012;15:9. doi: 10.1186/1758-2652-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimbabwe National Statistics Agency and ICF Macro . Zimbabwe Demographic and Health Survey 2010–2011. Calverton, MD: ICF Macro; 2012. [Google Scholar]
- 19.National Bureau of Statistics (NBS) Tanzania and ICF Macro . Tanzania Demographic and Health Survey 2010. Dar es Salaam, Tanzania: NBS and ICF Macro; 2011. [Google Scholar]
- 20.World Health Organization, UNICEF . Global HIV/AIDS Response, Progress Report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 21.National AIDS and STI Congrol Programme (NASCOP) Kenya AIDS Indicator Survey 2007 (KAIS 2007) Final Report. Nairobi, Kenya: NASCOP; 2009. [Google Scholar]
- 22.Division of Leprosy, Tuberculosis, and Lung Disease (DLTLD) Annual Report 2007. Nairobi, Kenya: DLTLD; 2007. [Google Scholar]
- 23.Division of Malaria Control, Kenya National Bureau of Statistics (KNMS), ICF Macro . 2010 Kenya Malaria Indicator Survey. Nairobi, Kenya: KNBS and ICF Macro; 2011. [Google Scholar]
- 24.Odhiambo FO, Laserson KF, Sewe M, Hamel MJ, Feikin DR, Adazu K, Ogwang S, Obor D, Amek N, Bayoh N, Ombok M, Lindblade K, Desai M, Ter Kuile F, Phillips-Howard P, van Eijk AM, Rosen D, Hightower A, Ofware P, Muttai H, Nahlen B, Decock K, Slutsker L, Breiman RF, Vulule JM. Profile: the KEMRI/CDC Health and Demographic Surveillance System–Western Kenya. Int J Epidemiol. 2012;41:977–987. doi: 10.1093/ije/dys108. [DOI] [PubMed] [Google Scholar]
- 25.Hightower AW, Ombok M, Otieno R, Odhiambo R, Oloo AJ, Lal AA, Nahlen BL, Hawley WA. A geographic information system applied to a malaria field study in western Kenya. Am J Trop Med Hyg. 1998;58:266–272. doi: 10.4269/ajtmh.1998.58.266. [DOI] [PubMed] [Google Scholar]
- 26.Gargano JW, Laserson K, Muttai H, Odhiambo F, Orimba V, Adamu-Zeh M, Williamson J, Sewe M, Nyabiage L, Owuor K, Broz D, Marston B, Ackers M. The adult population impact of HIV care and antiretroviral therapy (ART) in a resource poor setting, 2003–2008. AIDS. 2012;26:1545–1554. doi: 10.1097/QAD.0b013e328353b7b9. [DOI] [PubMed] [Google Scholar]
- 27.Wachira J, Kimaiyo S, Ndege S, Mamlin J, Braitstein P. What is the impact of home-based HIV counseling and testing on the clinical status of newly enrolled adults in a large HIV care program in western Kenya? Clin Infect Dis. 2012;54:275–281. doi: 10.1093/cid/cir789. [DOI] [PubMed] [Google Scholar]
- 28.Tabana H, Doherty T, Swanevelder S, Lombard C, Jackson D, Zembe W, Naik R. Knowledge of HIV status prior to a community HIV counseling and testing intervention in a rural district of South Africa: results of a community based survey. BMC Infect Dis. 2012;12:73. doi: 10.1186/1471-2334-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snow RC, Madalane M, Poulsen M. Are men testing? Sex differentials in HIV testing in Mpumalanga Province, South Africa. AIDS Care. 2010;22:1060–1065. doi: 10.1080/09540120903193641. [DOI] [PubMed] [Google Scholar]
- 30.Macpherson P, Lalloo DG, Choko AT, Mann GH, Squire SB, Mwale D, Manda E, Makombe SD, Desmond N, Heyderman R, Corbett EL. Suboptimal patterns of provider initiated HIV testing and counselling, antiretroviral therapy eligibility assessment and referral in primary health clinic attendees in Blantyre, Malawi. Trop Med Int Health. 2012;1:507–517. doi: 10.1111/j.1365-3156.2011.02946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braitstein P, Boulle A, Nash D, Brinkhof MW, Dabis F, Laurent C, Schechter M, Tuboi SH, Sprinz E, Miotti P, Hosseinipour M, May M, Egger M, Bangsberg DR, Low N. Antiretroviral Therapy in Lower Income Countries Study Group Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health (Larchmt) 2008;17:47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- 32.Kigozi IM, Dobkin LM, Martin JN, Geng EH, Muyindike W, Emenyonu NI, Bangsberg DR, Hahn JA. Late-disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009;52:280–289. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chirawu P, Langhaug L, Mavhu W, Pascoe S, Dirawo J, Cowan F. Acceptability and challenges of implementing voluntary counselling and testing (VCT) in rural Zimbabwe: evidence from the Regai Dzive Shiri Project. AIDS Care. 2010;22:81–88. doi: 10.1080/09540120903012577. [DOI] [PubMed] [Google Scholar]
- 34.Byamugisha R, Astrom AN, Ndeezi G, Karamagi CA, Tylleskar T, Tumwine JK. Male partner antenatal attendance and HIV testing in eastern Uganda: a randomized facility-based intervention trial. J Int AIDS Soc. 2011;14:43. doi: 10.1186/1758-2652-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obure CD, Vassall A, Michaels C, Terris-Prestholt F, Mayhew S, Stackpool-Moore L, Warren C. Integra Research Team Watts C. Optimising the cost and delivery of HIV counselling and testing services in Kenya and Swaziland. Sex Transm Infect. 2012;88:498–503. doi: 10.1136/sextrans-2012-050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steen TW, Seipone K, Gomez Fde L, Anderson MG, Kejelepula M, Keapoletswe K, Moffat HJ. Two and a half years of routine HIV testing in Botswana. J Acquir Immune Defic Syndr. 2007;44:484–488. doi: 10.1097/QAI.0b013e318030ffa9. [DOI] [PubMed] [Google Scholar]
- 37.Nsigaye R, Wringe A, Roura M, Kalluvya S, Urassa M, Busza J, Zaba B. From HIV diagnosis to treatment: evaluation of a referral system to promote and monitor access to antiretroviral therapy in rural Tanzania. J Int AIDS Soc. 2009;12:31. doi: 10.1186/1758-2652-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Govindasamy D, van Schaik N, Kranzer K, Wood R, Mathews C, Bekker LG. Linkage to HIV care from a mobile testing unit in South Africa by different CD4 count strata. J Acquir Immune Defic Syndr. 2011;58:344–352. doi: 10.1097/QAI.0b013e31822e0c4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nglazi MD, van Schaik N, Kranzer K, Lawn SD, Wood R, Bekker LG. An incentivized HIV counseling and testing program targeting hard-to-reach unemployed men in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2012;59:e28–e34. doi: 10.1097/QAI.0b013e31824445f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ndirangu J, Barnighausen T, Tanser F, Tint K, Newell ML. Levels of childhood vaccination coverage and the impact of maternal HIV status on child vaccination status in rural KwaZulu-Natal, South Africa. Trop Med Int Health. 2009;14:1383–1393. doi: 10.1111/j.1365-3156.2009.02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore BM, Alex-Hart BA, George IO. Utilization of health care services by pregnant mothers during delivery: a community based study in Nigeria. East Afr J Public Health. 2011;8:49–51. [PubMed] [Google Scholar]
- 42.Cooke GS, Tanser FC, Barnighausen TW, Newell ML. Population uptake of antiretroviral treatment through primary care in rural South Africa. BMC Public Health. 2010;10:585. doi: 10.1186/1471-2458-10-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatcher AM, Turan JM, Leslie HH, Kanya LW, Kwena Z, Johnson MO, Shade SB, Bukusi EA, Doyen A, Cohen CR. Predictors of linkage to care following community-based HIV counseling and testing in rural Kenya. AIDS Behav. 2011;16:1295–1307. doi: 10.1007/s10461-011-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medley A, Ackers M, Amolloh M, Owuor P, Muttai H, Audi B, Sewe M, Laserson K. Early uptake of HIV clinical care After testing HIV-positive during home-based testing and counseling in western Kenya. AIDS Behav. 2012;17:224–234. doi: 10.1007/s10461-012-0344-5. [DOI] [PubMed] [Google Scholar]
- 45.Walensky RP, Bassett IV. HIV self-testing and the missing linkage. PLoS Med. 2011;8:e1001101. doi: 10.1371/journal.pmed.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torian LV, Wiewel EW, Liu KL, Sackoff JE, Frieden TR. Risk factors for delayed initiation of medical care after diagnosis of human immunodeficiency virus. Arch Intern Med. 2008;168:1181–1187. doi: 10.1001/archinte.168.11.1181. [DOI] [PubMed] [Google Scholar]
- 47.Topp SM, Chipukuma JM, Chiko MM, Wamulume CS, Bolton-Moore C, Reid SE. Opt-out provider-initiated HIV testing and counselling in primary care outpatient clinics in Zambia. Bull World Health Organ. 2011;89:328–35A. doi: 10.2471/BLT.10.084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferguson L, Grant AD, Watson-Jones D, Kahawita T, Ong'ech JO, Ross DA. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop Med Int Health. 2012;17:564–580. doi: 10.1111/j.1365-3156.2012.02958.x. [DOI] [PubMed] [Google Scholar]
- 49.Dalal W, Feikin DR, Amolloh M, Ransom R, Burke H, Lugalia F, Ouma A, Laserson KF, Mermin J, Breiman RF, Bunnell R. Home-based HIV testing and counseling in rural and urban Kenyan communities. J Acquir Immune Defic Syndr. 2013;62:e47–e54. doi: 10.1097/QAI.0b013e318276bea0. [DOI] [PubMed] [Google Scholar]